Three-dimensional co-culture facilitates the differentiation of embryonic stem cells into mature cardiomyocytes†
Dong-Bo Ou and Yong He contributed equally to this work.
Abstract
The cardiomyocyte (CM) differentiation of embryonic stem cells (ESCs) is routinely cultured as two-dimensional (2D) monolayer, which doesn't mimic in vivo physiological environment and may lead to low differentiated level of ESCs. Here, we develop a novel strategy that enhances CM differentiation of ESCs in collagen matrix three-dimensional (3D) culture combined with indirect cardiac fibroblasts co-culture. ESCs were cultured in hanging drops to form embryoid bodies (EBs) and then applied on collagen matrix. The EBs were indirectly co-cultured with cardiac fibroblasts by the hanging cell culture inserts (PET 1 µm). The molecular expressions and ultrastructural characteristics of ESC-derived CMs (ESCMs) were analyzed by real time RT-PCR, immunocytochemistry, and Transmission Electron Microscopy (TEM). We found that the percentage of beating EBs with cardiac fibroblasts co-culture was significantly higher than that without co-culture after differentiation period of 8 days. Type I collagen used as 3D substrates enhanced the late-stage CM differentiation of ESCs and had effect on ultrastructural mature of ESCMs in late-stage development. The combined effects of 3D and co-culture that mimic in vivo physiological environment further improved the efficiency of CM differentiation from ESCs, resulting in fiber-like structures of cardiac cells with organized sarcomeric structure in ESCMs. This novel 3D co-culture system emphasizes the fact that the ESC differentiation is actively responding to cues from their environment and those cues can drive phenotypic control, which provides a useful in vitro model to investigate CM differentiation of stem cells. J. Cell. Biochem. 112: 3555–3562, 2011. © 2011 Wiley Periodicals, Inc.
There is growing interest in the utilization of embryonic stem cells (ESCs) derived cardiomyocytes (CMs) for cell and tissue replacement therapies. However, in many cases simple differentiating factor fails to efficiently direct the differentiation of ESCs into lineage-specific cells. Pluripotency and lineage-specific differentiation of ESCs are intricate biological processes that are coordinately regulated by a complex set of factors and epigenetic regulators. More researches to understand differentiation mechanism or develop appropriate culture protocols to improve the differentiation efficiency are needed.
The development of three-dimensional (3D) culture systems that support the self-renewal and differentiation of ESCs would be useful for the investigation of cardiomyogenic differentiation. Previously most in vitro differentiation models for ESCs was based on two-dimensional (2D) monolayer, even though the embryoid bodies (EBs) from ESCs have 3D configuration that has potential to form all three embryonic germ layers [Thomson et al., 1998]. These 2D cultured models don't mimic the in vivo physiological environment and may lead to low differentiated efficiency. The importance of 3D environment from synthetic or natural extracellular matrix for ESC self-renewal and differentiation has been proposed [Levenberg et al., 2003; Kim et al., 2005]. Significant differences were found in the differentiation efficiency of ESCs when they were respectively cultured in 2D and 3D environment [Liu et al., 2006]. 3D extracellular matrix not only influences self-renewal of ESCs but also plays an important role in differentiation of ESCs. Collagen as 3D matrix has been used to support ESCs to differentiate into beating CMs [Battista et al., 2005]. Recently, collagen-coated 3D scaffold that could significantly enhance the population of cardiac-lineage cells from ESCs has been developed [Huang et al., 2010], but additional studies such as employing purification techniques to enrich the population of CMs are needed.
Previous studies demonstrated that controlling the stem cell microenvironment can influence cell differentiation. Chen et al. [2007] had studied the effect of cell–cell and cell–extracellular matrix interactions on ESC differentiation. He found that ESCs were dependent on endogenous extracellular matrix for their survival and differentiation in spite of the presence of exogenous extracellular matrix. As the differentiating process of ESCs is close to the development of the embryonic heart [Banach et al., 2003], factors that contribute to essential functions during early embryogenesis are expected to be involved in the formation of EBs [Behfar et al., 2002]. However, the precise growth factor combinations that could enhance cell differentiation in ESCs are unclear [Schuldiner et al., 2000]. Therefore, ESCs should be co-cultured with special cells or cultured in conditioned media in order to promote cell differentiation. For example, when ESCs are co-cultured with visceral-endoderm-like (END-2) cells, the majority (>90%) of ESC-derived CMs (ESCMs) have a phenotype similar to that of fetal ventricular cells [Mummery et al., 2003]. The efficiency of CM differentiation in ESCs can be readily enhanced by a culture medium that has been conditioned by END-2 cells [Graichen et al., 2008]. Similarly, when medium conditioned by mouse embryo fibroblasts is used, the homogeneity of beating EBs can be significantly improved [Burridge et al., 2007].
ESCs in 3D differentiation are superior to those in 2D differentiation. Local microenvironments are considered to be the key inducers in directing the site-specific differentiation from ESCs. Nevertheless, combined effects of 3D and co-culture that mimic in vivo physiological environment on EB growth and CM differentiation from ESCs have not been systematically studied yet. Here, based on our previous work [Ou et al., 2009], we propose a new methodology to enhance the CM differentiation of ESCs on a 3D collagen matrix by indirect cardiac fibroblasts co-culture to generate CMs from ESCs. We sought to determine that the differentiation of ESCs is actively responding to cues from their environment and that those cues can drive phenotypic control and CM differentiation.
MATERIALS AND METHODS
Undifferentiated ESCs
Mouse CGR8 ESCs, kindly provided by Prof. Duanqing Pei (Chinese Academy of Sciences), were cultured without feeder cells in Dulbecco's modified Eagle's minimal essential medium (DMEM, Gibco, Invitrogen Corporation, Grand Island, NY) supplemented with 15% fetal bovine serum (Gibco), 0.1 mmol/L non-essential amino acids (Sigma, St. Louis, MO), 0.1 mmol/L β-mercaptoethanol, penicillin (100 U/ml), streptomycin (100 µg/ml), and 100 U/ml leukemia inhibitory factor (LIF) (Chemicon International Inc., Temecula, CA). LIF is the essential media supplement used to support self-renewal and maintain potency of ESCs. In this study, LIF is only used for the culture of undifferentiated ESCs.
ESCs Differentiation
Cells were cultured in hanging drops (400 cells/20 µl) for 3 days to form uniform-sized EBs for differentiation. The EBs were then transferred and cultured in suspension in cell culture flasks (BD Bioscience) with differentiation medium for 2∼3 days. Then EBs were transferred to the 6- or 12-well plates and continuously cultured in differentiation medium. The medium for differentiation was DMEM (Gibco) supplemented with 15% fetal bovine serum (Gibco), 0.1 mmol/L non-essential amino acids (Sigma), 0.1 mmol/L β-mercaptoethanol, penicillin (100 U/ml), streptomycin (100 µg/ml), and 0.1 mmol/L ascorbic acid (Sigma). To induce CM differentiation, 0.1 mmol/L ascorbic acid was added in differentiation medium [Takahashi et al., 2003].
Preparation of Murine Embryonic Cardiac Fibroblasts
Cardiac fibroblasts were obtained from enzymatically isolated crude cellular fractions from neonate rat ventricle as described previously [Fahrenbach et al., 2007]. Animal experiments were approved by the Fourth Military Medical University on the Use and Care of Animals according to National Institutes of Health Guidelines. One-day-old Sprague–Dawley rats (Animals Laboratories, Fourth Military Medical University) were euthanized by injection of pentobarbital (80 mg/kg). The hearts were quickly excised and washed with normal Tyrode solution. Ventricles were trimmed free of atria and major blood vessels, minced, and then placed in 0.1% collagenase (Sigma) solution. After 20 min enzyme digestions, the released cells were filtered, washed, and allowed to settle for 25 min in a Percoll gradient to separate non-myocytes from myocytes. The obtained fibroblasts were cultured and passaged in DMEM (Gibco) that is supplemented with 10% FBS (Gibco) so as to gain sufficient numbers for co-culture.
3D Indirect Co-Culture System
Type I collagen gels used as 3D substrates were prepared by a method described previously for 3D culture [Chen et al., 2007]. In brief, collagen gels from rat tail type I collagen (Roche Diagnostics, Basel, Switzerland) were mixed with 10% 10 × DMEM, 15% fetal calf serum, 1% penicillin/streptomycin (Invitrogen), and ultrapure water to reach a final concentration of 1 mg/ml; pH was neutralized by titration with NaOH. About 1 or 0.25 ml collagen gels were added to each well of 6- or 12-well plates and allowed to solidify for 20 min at 37°C. The thickness of collagen matrix was about 1 mm. EBs were separately transferred to the plates and cultured in above differentiation medium.
For co-culture, the cardiac fibroblasts isolated from the hearts of 1-day-old Sprague–Dawley rats were cultured to mimic the cardiac microenvironment. In particular, the cardiac fibroblasts were seeded on 12-well hanging cell culture inserts (PET membranes with 1 µm pores) (Millicell; Millipore, Bedford, MA) to prevent direct contact with the underlayer EBs. The above differentiation medium was changed every day. As previously described by Maltsev et al. [1994], early-stage differentiation (shortly after the initiation of contractions) was day (8 + 3), intermediate-stage differentiation was day (8 + 8), and late-stage differentiation was day (8 + 16).
Immunocytochemistry
Immunocytochemistry was performed when spontaneous contractions could be clearly observed in EBs. EB outgrowths or single were mechanically dissected, enzymatically dissociated by 0.25% trypsin-EDTA (0.25% Trypsin and 0.05% EDTA), and then were plated on glass coverslips for 24∼48 h. Cells were fixed in 4% paraformaldehyde for 30 min, permeabilized with 0.25% Triton X-100 for 15 min, and blocked in 5% normal goat serum (NGS) for 10 min. Subsequently, cells were incubated with the primary antibody in a humidified chamber at 37°C for 2 h. Rabbit anti-α-actinin antibodies and rabbit anti-cardiac troponin I (cTnI) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were added at dilutions of 1:150 and 1:400, respectively. After washed with 0.4% Triton X-100 and PBS, cells were incubated at 37°C for 3 h to the corresponding fluorescent secondary antibodies at a dilution of 1:20. For confocal microscopy, the primary antibodies are mouse anti-α-actinin antibodies and rabbit anti-connexin 43 (CX43) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), which were added at dilutions of 1:150 and 1:400, respectively. After having them washed with 0.4% Triton X-100 and PBS, cells were incubated at 37°C for 4 h to two corresponding fluorescent secondary antibodies at a dilution of 1:20, respectively. DAPI staining was used to identify nuclei. Analysis was performed using a confocal microscope (FV1000, Olympus, Japan).
Reverse Transcription-Polymerase Chain Reaction (PCR)
Semi-quantitative reverse transcription (RT)-PCR for Oct4, GATA4, Nkx2.5, ANF, CX43, and GAPDH was performed using standard procedures. Total RNA was prepared using Trizol reagent (Invitrogen). First strand cDNA was synthesized from 1 µg of total RNA, in a total volume of 20 µl, using oligo (dT)18 primer and a RevetAidTM First Strand cDNA Synthesis Kit. The RT-PCR was performed with GAPDH mRNA as a normalizing internal control. The resulting cDNA (50 ng) was amplified by PCR using specific primers. Primers used were (5′–3′) Oct4 (X52437): GTGGAGGAAGCCGACAACAA (forward), CAGAGCAGTGACGGGAACAGA (reverse); GATA4 (MA082897): CGGAAGCCCAAGAACCTGAATA (forward), TTGCTGGAGTTACCGCTGGAG (reverse); Nkx2.5 (MA096274): AAGACCCTCGGGCGGATAA (forward), CCATCCGTCTCGGCTTTGT (reverse); ANF (MA075498): GGGGGTAGGATTGACAGGAT (forward), AGCTGCGTGACACACCACAAG (reverse); CX43 (NM_010288): TGCCGCAATTACAACAAGCAAG (forward), TCCACGGGAACGAAATGAACAC (reverse); GAPDH (MA050371): TGTGTCCGTCGTGGATCTGA (forward), TTGCTGTTGAAGTCGCAGGAG (reverse). Thermal cycling (in 25 µl) was performed as follows: A 3 min denaturation at 94°C, 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, and a final extension for 6 min at 72°C. PCR products were resolved by electrophoresis on 1.5% agarose gels. They were visualized by UV transillumination and photographed. Semi-quantitative analysis was done by Alphaview 1.3 software (Alpha Lnnotech Inc.).
For quantitative analysis on GATA4, ANF, and CX43 expressions, real-time PCR using above primers was performed as described previously [Chen et al., 2007]. Briefly, real-time RT-PCR amplification reactions were performed in a final volume of 20 µl containing the same amount of RT product (50 ng cDNA), 10 µl of 2× iQSYBR-green mix (Takara, Japan), 300 nmol of forward and reverse primers using the LineGene 9660 real-time PCR Detection System (Bioer, China). The thermal cycling conditions comprised 95°C for 10 s, 1 min at the corresponding annealing temperature, 53°C for 10 s, and 72°C for 40 s. These settings were applied for 50 cycles. Specificity of amplification was determined by DNA melting curve during gradual temperature increments (0.5°C). The qPCR results were performed with corresponding GAPDH mRNA as a normalizing internal control. The changes in gene expression levels were compared with those of undifferentiated ESCs. The fold change is expressed as mean ± SEM.
Transmission Electron Microscopy (TEM)
EBs were mechanically dissected as a whole, washed with PBS, fixed for 3 h in 2.5% glutaraldehyde at 4°C, post-fixed in PBS containing 1% osmium tetroxide, dehydrated in ascending concentrations of ethanol, and then infiltrated and embedded in polybed 812 epoxy resin. Ultrathin (60–90 nm) sections were stained with 2% uranyl acetate, followed by 1% lead citrate and examined under a transmission electron microscope (JEM-2000EX, JAPAN).
Statistics
The ESC differentiation experiments were performed at least three times. All values are presented as mean ± SEM. Statistical significance for two comparisons was evaluated by the Student's paired or unpaired t test (two-tail). One-way ANOVA followed by Newman Keuls test was used for multiple comparisons. Differences with P < 0.05 were considered statistically significant.
RESULTS
CM Differentiation of ESCs
Under differentiation conditions, ESCs consistently aggregated and formed EBs by hanging drop culture. After being cultured in suspension for 3 days, the EBs were transferred to the plates and they continued to differentiate. To induce the CM differentiation of ESCs, 0.1 mmol/L ascorbic acid (Sigma) was added as differentiation-inducing agent. The rhythmically contracting areas consisting of 10–200 CMs were first detected in EBs on day 8 of differentiation, suggesting the occurrence of CM differentiation in ESCs. The number of EBs with contracting areas was determined for a period of observation of 24 days of differentiation. Figure 1 shows the culture of ESCs and the differentiation of ESCs into CMs. Note that the different differentiation efficiency of ESCs actively responds to cues from their environment. The percentage of EBs with contracting areas in cardiac fibroblasts co-culture was significantly higher than that without co-culture. Type I collagen used as 3D substrates did influence the differentiation rate in late stage. Combined effects of 3D and co-culture further improved the efficiency of CM differentiation from ESCs.
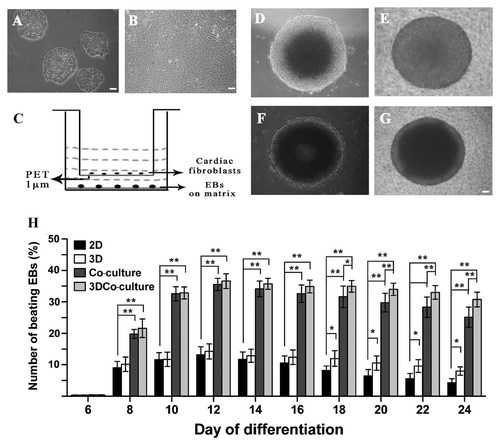
The culture of ESCs and the differentiation of ESCs into CMs. A: The clones of undifferentiated ESCs. B: The culture of cardiac fibroblasts isolated from the hearts of 1-day-old Sprague–Dawley rats. C: The 3D indirect co-culture system used in experiments: A facile 3D cell expansion and stem cell differentiation system with continuous medium conditioning while preventing mixing of stem cells and co-culture cells by hanging culture inserts. D: Six-day-old EBs in 2D culture, EBs were cultured as monolayer cells on plastic. E: Six-day-old EBs in 3D culture. EBs were seeded on collagen matrix (thickness: 1 mm) coated 6- or 12-well plates, and then they grew and developed in the collagen matrix. F: six-day-old EBs in 2D co-culture. EBs were cultured on plastic with hanging insert of cardiac fibroblasts in indirect co-culture (hanging inserts removed when photographed). G: Six-day-old EBs in 3D co-culture. EBs were cultured on collagen matrix with hanging insert of cardiac fibroblasts in indirect co-culture (hanging inserts removed when photographed). H: The percentage of EBs with contracting areas in each group during culture period. Compared with general 2D culture, the percentage of EBs with contracting areas in 3D culture was significantly higher after day 18 (P < 0.05). Compared with general co-culture, the percentage of EBs with contracting areas in 3D co-culture was significantly higher after day 18 (P < 0.05 and P < 0.01). Throughout differentiation course, the percentages of EBs with contracting areas in general co-culture and 3D co-culture were markedly higher than that in general 2D culture (P < 0.01). *P < 0.05; **P < 0.01; Scale bar = 100 µm.
Expression of Cardiac Specific Genes
We compared the differentiation efficiencies among general (2D) culture, 3D culture, 2D co-culture, and 3D co-culture to determine that the differentiation of ESCs is actively responding to different environment. Semi-quantitative RT-PCR was performed on the undifferentiated ESCs and 20-day-old EBs (Fig. 2A). Oct4, as an ESC marker, was only expressed in the undifferentiated ESCs. During ESC differentiation, the expression of Oct4 was reduced over a time course. The cardiac markers, such as GATA4, Nkx2.5, ANF, and CX43 were detected in 2D culture, 3D culture, 2D co-culture, and 3D co-culture, but we found that there were differences in GATA4, Nkx2.5, ANF, and CX43 expressions when EBs were cultured in various environments. Immunostaining of α-actinin and cTnI was performed to confirm the presence of CMs (Fig. 2B). We found that α-actinin (+) cells and cTnI (+) cells were more prominent in the 3D co-culture environment. These results were interesting, so we further determine the expressions of GATA4, ANF, and CX43 during differentiation period using a real-time PCR.
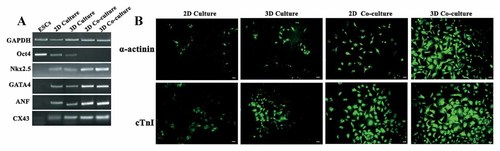
The expression of cardiac markers in ESCs. A: Semi-quantitative RT-PCR on undifferentiated ESCs and 20-day-old EBs was performed with the indicated specific primers. B: Immunostaining of α-actinin and cTnI in 2D culture, 3D culture, 2D co-culture, and 3D co-culture. Cells from EBs were incubated with primary antibodies followed by FITC-conjugated secondary antibodies (green), respectively. Scale bar = 50 µm.
Real-time PCR results demonstrated that there were significant differences in GATA4, ANF, and CX43 expressions responding to various culture environments and time course. Co-culture with cardiac fibroblast has an effect that enhanced GATA4, ANF, and CX43 expressions during differentiating course, while 3D culture enhanced GATA4, ANF, and CX43 expressions in late stage of differentiating course (Fig. 3).
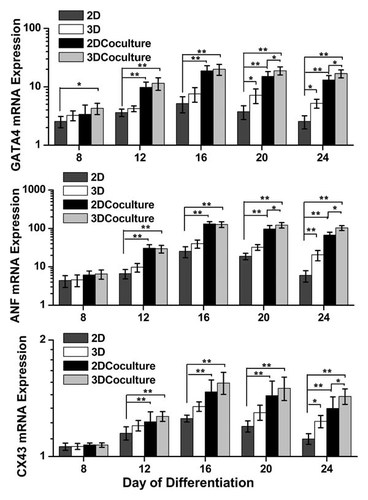
Quantitative analysis on GATA4, ANF, and CX43 expressions during differentiation period using a real-time PCR. A: GATA4 gene expression: GATA4 expression in 3D culture significantly increased after day 20, compared to that in 2D culture (P < 0.05). In 2D co-culture, GATA4 expression was significantly different from that in 2D culture after day 12 (P < 0.01). GATA4 expression in 3D co-culture was significantly different from that in 2D culture after day 8 (P < 0.05). B: ANF gene expression: Similar to GATA4 expression, ANF expression was observed at day 8 and the expression increased by 3D culture after day 20 (P < 0.05). ANF expressions in both 2D co-culture and 3D co-culture were significantly different from that in 2D culture after day 12 (P < 0.01). C: CX43 gene expression: CX43 expression was detected during differentiation period, but there were significant differences between 2D culture and 2D co-culture at day 24 (P < 0.05). CX43 expressions in both 2D co-culture and 3D co-culture were significantly different from that in 2D culture after day 12 (P < 0.01). *P < 0.05; **P < 0.01.
To characterize the CMs further, we carried out immunofluorescent staining for the detection of sarcomeric protein and junctional protein at a higher magnification using a confocal microscopy (Fig. 4). Staining with anti-α-actinin shows some unorganized myofilaments in general co-culture group (Fig. 4A1), while well-organized sarcomeric myofilaments in cytoplasmic patterns in 3D co-culture (Fig. 4A2). CX43 staining (Fig. 4, B1 and B2) indicated the presence of gap junctions. The morphology phenotypes were similar to the highly organized, parallel bundles in cells from biopsies of heart.
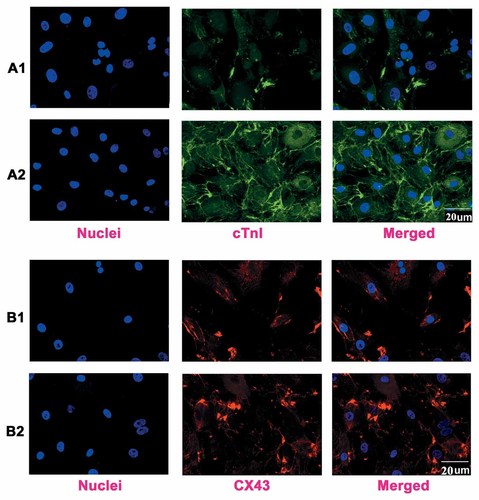
Expression of cardiac specific proteins in ESCMs at 20 days differentiation. A: Cells isolated from beating outgrowths of EBs were incubated with primary antibodies cTnI followed by FITC-conjugated secondary antibodies (green): A1, general co-culture group; A2, 3D co-culture group. B: Cells isolated from beating outgrowths of EBs were incubated with primary antibodies CX43 followed by Cy3-conjugated secondary antibodies (red): B1, general co-culture group; B2, 3D co-culture group. Nuclei in the same field were stained with DAPI (blue). Merged figures were made by FV10-ASW Systems. Scale bar = 20 µm.
Ultrastructural Analysis of ESCMs
In general (2D) co-culture (Fig. 5A, C), the myofilaments in some areas appeared as loose arrays. The precursor of Z bands (Z bodies) could be found in some of the cells. Tiny punctate contacts were sometimes observed between muscle cells, and the sarcomeres in general co-culture were less evident than those in 3D co-culture. In 3D co-culture (Fig. 5B, D), the cells exhibited the typical cardiac morphological features including sarcomeres, abundant glycogen, and mitochondria and junctional complexes.
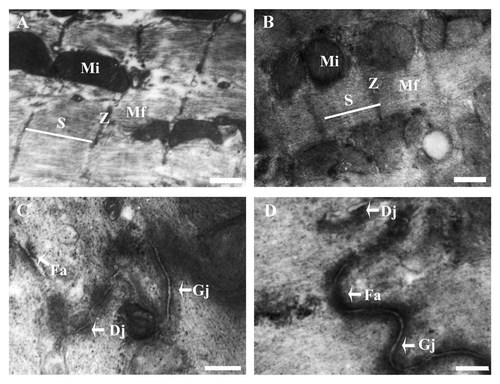
Ultrastructural analysis on ESCMs. A: The presence of sarcomeric structures, mitochondria and glycogen granules in general co-culture. B: The presence of sarcomeric structures, mitochondria and glycogen granules in 3D co-culture. C: The nascent junctional complexes were observed in general co-culture. D: The nascent junctional complexes were observed in 3D co-culture. Z, Z-bands, Mf: Myofibrils, S: Sarcomere, Mi: Mitochondrion, Gj: Gap junction, Dj: Desmosome junction, Fa: Fascia adherence. A, B: Scale bar = 500 nm; (C, D): Scale bar = 200 nm.
DISCUSSION
Previous reports have demonstrated that the ESCs can differentiate into CMs under in vitro conditions, but the CM differentiation of ESCs are routinely cultured as 2D monolayer, which does not mimic the in vivo physiological environment. The differentiation of ESCs inevitably results in a heterogeneous mixture of cell types that present in EBs. It would be more difficult to transform them into a homogenous population of CMs or to stop their differentiation at the functional CM stage. Here we examine the effect of cardiac fibroblasts on CM differentiation of ESCs in a 3D culture model based on a collagen matrix. We aim to develop a novel in vitro culture model to investigate cardiomyogenic differentiation and enhance differentiation efficiency from stem cells.
For the sake of easy operation, we cultured murine cardiac fibroblasts from rats, rather than from mice, to form mimicking cardiac microenvironment in vitro. In the indirect co-culture system, the cardiac fibroblasts were seeded on 12-well hanging cell culture inserts to prevent direct contact with the underlayer EBs. Both the EBs and cardiac fibroblasts grew in the same medium but they could be easily separated. Cardiac fibroblasts with proliferative potential isolated from neonatal cardiac cells can provide adequate numbers for co-culture applications.
In vivo cardiac fibroblasts usually regulate the synthesis and degradation of collagen and fibronectin under physiological conditions that provide structural support for the heart [Corda et al., 2000]. Cardiac fibroblasts also play a more active role in modulating the electrophysiology of the working myocardium [Camelliti et al., 2005]. It is reported that transplantation of stem cells into heart resulted in cardiac cells, which reveals that CM differentiation of ESCs requires a paracrine pathway in the heart [Behfar et al., 2002]. This indicates that cardiac fibroblasts or other cardiac cells play an important role in facilitating CM differentiation of ESCs. Kim et al. [2010] previous reported that non-CMs in EBs contain most cell types present in the embryonic heart and those non-CMs can influence CM differentiation and improve maturation of ESCMs. In this study, the significant differences in the percentage of EBs with contracting areas can be observed without versus with cardiac fibroblasts co-culture. Cardiac fibroblasts as a cellular source of signals that results in ESC differentiating toward CMs play an essential role in facilitating CM differentiation from ESCs. Careful stepwise analysis on ESC differentiation and mimicking endogenous signals from cardiac fibroblasts is most likely to increase the efficiencies of ESCs as well as other pluripotent stem cells to differentiate toward CM lineages.
The role of 3D material in control and guidance of cell development and commitment into complex and mature CMs was investigated. Collagen is the main component of native extracellular matrix. The cells interact with collagen through integrin binding-mediated interactions, which plays a significant role in directing cellular behavior and function. Type I collagen, a natural biomaterial, is a major component of the extracellular matrix of the normal heart [Kehat et al., 2004]. In this study, type I collagen gels were used as 3D substrates to support 3D-differentiation of mouse ESCs into CM. Type I collagen did influence the differentiation rate, and CM differentiation of ESCs on 3D substrates appears superior to that on 2D culture. When 3D culture combined with co-culture, the efficiency of CM differentiation of ESCs was further improved. Gene expression experiments in ESCMs from 20-day-old EBs revealed that the expressions of Nkx2.5, GATA4, ANF, and CX43 were responding to various culture environments and time courses. By comparison with the EB samples, the significantly more intense results from real-time PCR supported and extended the conclusions. Nkx2.5 is an important marker gene used to confirm the existence of cardiac precursors in EBs [Takahashi et al., 2003]. GATA4 is present in the precardiac mesoderm and subsequently in the endocardial and myocardial layers of the heart tube and developing heart [Molkentin et al., 1997]. Atrial natriuretic factor (ANF) is considered to be a marker of chamber (atrial or ventricular) working myocardium [Houweling et al., 2002]. In 3D culture environment, we found that GATA4 and ANF expressions were increased in the late stage of differentiation. Cx43, the major cardiac gap junction protein, was detected during differentiation period, but the increased expression of CX43 in 3D culture environment was only found at day 24 of differentiation. These results suggested the important role of 3D environment in facilitating the late-stage CM differentiation of ESCs.
In 3D culture environment, we found that immunostaining of α-actinin and cTnI were more prominent. To further characterize the CMs in late stage, we carried out confocal microscopy for staining with anti-α-actinin and anti-CX43 at a higher magnification. Well-organized sarcomeric myofilaments and junctional protein in cytoplasmic patterns were found in 3D co-culture. To reveal the effect of 3D culture environment on ultrastructural characteristics of CMs, TEM was performed. We found that the cells in 3D co-culture exhibited the typical cardiac morphological features including sarcomeres, glycogen, and mitochondria and junctional complexes. 3D culture environment has effect on ultrastructural mature of CMs in later stage of development.
CONCLUSIONS
Our data suggest that the combination of 3D and co-culture that mimic in vivo physiological environment further improved the efficiency of CM differentiation from ESCs and resulted in mature phenotype. These effects were dependent on fibroblasts providing soluble factors and collagen matrix used as 3D substrates, which allows 3D-development of EBs that promotes ultrastructural organization of CMs into maturate structures. This study is an advance in ESCs culture methods and gives insights into stem cell differentiation mechanisms, for it provides possibility to efficiently control and restrict the differentiation pathways and thereby generate cultures enriched in lineage-specific cells in vitro.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (No. 81070161). The authors thank Prof. Yan Jin from the Research and Development Center for Tissue Engineering, Fourth Military Medical University for valuable discussions and helpful suggestions.




