Abstract
Induced pluripotent stem (iPS) cells are reprogrammed from somatic cells through ectopic expression of stem cell-specific transcription factors, including Oct4, Nanog, Sox2, Lin28, Klf4, and c-Myc. Although iPS cells are similar to embryonic stem (ES) cells in their pluripotency, their inherited defects, such as insertion mutagenesis, employment of oncogenes, and low efficiency, associated with the reprogramming procedure have hindered their clinical application. A study has shown that valproic acid (VPA) treatment can significantly enhance the reprogramming efficiency and avoid the usage of oncogenes. To understand how VPA can enhance pluripotency, we stably transfected an Oct4 promoter driven luciferase reporter (Oct4-1.9k-Luc) into P19 embryonic carcinoma (EC) cells and C2C12 myoblasts and examined their response to VPA. We found that VPA could both activate Oct4 promoter and rescue its inhibition by retinoic acid (RA). In C2C12 myoblasts, VPA treatment also enhanced endogenous Oct4 expression but repressed that of MyoD. Furthermore, both RARα over-expression and mutation of a proximal hormone response element (HRE) blocked the activation effect of VPA on Oct4 promoter, implying that VPA may exert its activation effect through factors targeting this HRE. Taken together, these observations identify a molecular mechanism by which VPA directly regulate Oct4 expression to ensure the acquirement and maintenance of pluripotency. J. Cell. Biochem. 110: 995–1004, 2010. © 2010 Wiley-Liss, Inc.
Stem or differentiated cells mediated tissue regeneration holds great promise for patients on whom traditional treatments have failed. To date, one of the major hurdles to the success of this therapy is the lack of legal sources of safe donor cells. Although hundreds of human embryonic stem cell lines have been established since 1998 [Thomson et al., 1998; Rao and Civin, 2006], their usage in this therapy might still be unpractical due to their immune rejection by the host. An alternative way to avoid immune rejection is using autologous cells, either stem or somatic, from the patients. If the patient's somatic cells, which are in large amount and easy to get, can be induced to trans-differentiate into precursors of target cell types in vitro, then both the cell number and immune rejection problems can be avoided.
In 2006, Takahashi and Yamanaka 2006 discovered that over-expression of Oct4, Sox2, Klf4, and c-Myc can induce pluripotent stem cells (iPS cell) from mouse embryonic and adult fibroblasts. They also demonstrated that the same factors could induce iPS cells from human fibroblasts [Takahashi et al., 2007]. They further found that germline-competent iPS cells could be induced if they were selected with Nanog expression [Okita et al., 2007]. So far, their seminal works have been reproduced by many laboratories worldwide [Wernig et al., 2007; Yu et al., 2007; reviewed in Lowry and Plath, 2008] and provide strong support for the development of customized autologous stem cells from patients suffering from genetic diseases. The factors used in these studies include Oct4, Sox2, Nanog, Klf4, c-Myc, and Lin28. Since both c-Myc and Klf4 are strong oncogenes, their inclusion in the expression cocktail cause serious tumorigenesis problem and thus should be avoided. These studies strongly demonstrated that reprogramming somatic cells to stem cells is feasible and it can be achieved by simply introducing 4 stem cell-specific factors into somatic cells.
To date, several methods, including retroviral and adenoviral transduction and plasmid transfection, have been employed to over-express these iPS factors [Lowry et al., 2008; Okita et al., 2008; Stadtfeld et al., 2008; Huangfu et al., 2008a,b]. Among them, retroviral transduction seems to give the highest efficiency (0.1%). Both retroviral transduction and plasmid transfection raised the concern of insertion mutagenesis and introduction of ecotpic DNA. Adenoviral transduction avoids the insertion mutagenesis, but unfortunately, the efficiency achieved by this approach is relatively low. Therefore, new approaches need to be taken to avoid the problem of insertion mutagenesis and increase the iPS induction efficiency.
Huangfu et al. 2008a have found that including valproic acid (VPA), a histone deacetylase inhibitor, highly increased the efficiency to 1% if Oct4, Sox2, and Klf4 were expressed retrovirally. They also found that human fibroblasts could be reprogrammed to iPS cells by only expressing Oct4 and Sox2 in the presence of VPA [Huangfu et al., 2008b], albeit the efficiency is low (0.001%). Their study has pointed out an important direction for increasing the efficiency and safety of somatic cell reprogramming: expressing fewer factors by the facilitation of small pluripotency-promoting compounds.
Skeletal muscle constitutes about 40% of adult body weight and is an easy accessible tissue. It is highly plastic and can regenerate readily upon physical and chemical damages. Its plasticity and regeneration ability attribute largely to the existence of large number of stem cells in this tissue [Seale and Rudnicki, 2000]. This muscular stem cell, called satellite cell, has been identified for decades and its method of isolation is also well established [Mauro, 1961]. Taking advantage of its large amount of stem cells and easy accessibility, skeletal muscle cells can serve as a reliable source of autologous stem and differentiated cells. Apart from this, over the last two decades, embryonic and genetic studies have unveiled key signaling pathways and regulatory genes that are involved in various stages of myogenesis. Therefore, myogenic lineage provides a unique paradigm for studying stem to differentiated cell transitions, as well as the acquisition of cellular identity [Tajbakhsh, 2005].
Here we have taken the advantage of the well defined process of myogenesis to elucidate the mechanism by which VPA promotes somatic cell reprogramming.
To test whether VPA enhanced pluripotency was due to increased expression of Oct4, we stably transfected an Oct4 promoter driven luciferase reporter (Oct4-1.9k-Luc) into P19 EC cells and C2C12 myoblasts. We found that VPA could both activate Oct4 promoter and rescue its inhibition by retinoic acid (RA). VPA treatment also enhanced endogenous Oct4 expression but repressed that of MyoD in C2C12 myoblasts. These observations suggest that VPA enhances pluripotency by direct activating Oct4 transcription and repressing those of differentiation genes. Besides, our results imply that VPA may exert its effect through specific promoter-targeting factors instead through general effects on chromatin structure.
MATERIALS AND METHODS
Plasmids
The promoter region (−1,888 to +52) of Oct4 gene was amplified from the parental construct GOF-18 (a LacZ containing reporter driven by an 18 kb genomic fragment of Oct4 gene) that had been show to mimic Oct4 embryonic expression pattern [Yeom et al., 1996]. This region was then inserted into the KpnI and MluI sites of the pStable-luc vector, a luciferase reporter vector modified from the pGL3-basic vector in which a neomycine-resistant gene expression cassette was inserted into the BamHI site of the basic vector, to create the reporter pStable-Oct4-1.9k-luc that allowed for stable integration of this reporter construct into target cell genomes by G418 selection. The reporter construct GOF18 was a generous gift from Dr. Hans R. Scholer (Max Planck Institute for Molecular Biomedicine, Department for Cell and Developmental Biology, Münster, Germany). The RARα expression vector (pCMX-RARα) was from Dr. Ronald Evans (Department of Molecular and Developmental Biology, Salk Institute; San Diego, USA).
Cell Culture and Stable Cloning of pStable-Oct4-1.9k-luc Into P19 and C2C12 Cells
Proliferating C2C12 cells were kept in DMEM supplemented with 20% fetal calf serum (FCS), and P19 cells were kept in α-MEM with 10% FCS. For transient transfection, aliquots of pStable-Oct4-1.9k-luc DNA were mixed together in 1× Hepes buffer (20 mM Hepes at pH 7.0, 187 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, and 5.5 mM dextrose) in 5 ml test tubes and then liposome (Lipofectamin, Invitrogen) in 1× Hepes buffer were added to the DNA mixture and incubated at room temperature for 10–15 min to allow DNA and liposome complex to form. Aliquots of culture medium were added to each tube and mixed with several inverting. Medium containing the DNA/liposome complex in each tube was transferred to cells grown in 6 mm petridish and the transfection was allowed to proceed overnight before the medium was replaced by fresh medium. G418 (800 µg/ml) was added to the medium 48 h after the transfection and the selection was allowed for 2–3 weeks until monoclonal colonies appeared. For each cell line, 24 colonies were isolated and transferred to 12-well plates. The luciferase activity of each clone was determined after they had grown confluent by injecting aliquots of total lysate (100 µl) into a Bio-Tek Clarity 2 luminometer. The um-isolated colonies on the dish were trypsinized and pooled together as the source of polyclonal stable cells.
Simultaneous Expression of Oct4, Nanog, and Sox2 in C2C12 Myoblasts
The establishment of C2C12 stable clones over-expressing Oct4 and Nanog has been described before [Lang et al., 2009]. Briefly, C2C12 were infected with Oct4 expressing retrovirus first and then selected with G418 for 2–3 weeks. The resulted stable clones were then stably transfected with pPyCAGIP-Nanog and selected with G418 (400 µg/ml) and purimycin (3 µg/ml) at the same time to create Oct4 and Nanog simultaneously over-expressed clones (named as C2C12-OPN). Stable clones over-expressing Oct4, Nanog, and Sox2 simultaneously were created by infecting C2C12-OPN cells with lentivirus carrying Sox2 gene. Cells infected by lentivirus were sorted with their expression of GFP by FACS.
To construct a lentiviral vector conferring Sox2 gene expression under the transcription control of elongation factor alpha promoter with GFP as a selection marker, we first constructed pRRL.PPT.EFS.iresGFPpre where the GFP cDNA fragment in the parental vector pRRL.PPT.EFS.GFPpre [Schambach et al., 2006], a gift kindly provided by Dr. Baum, (Department of Hematology, Hemostaseology, and Oncology, Hannover Medical School), was replaced with the iresGFP fragment isolated from the pMY-IRES-GFP retroviral vector (kindly provided by T. Kitamura, Tokyo, Japan) through BamHI and SalI restriction enzyme digestion. The mouse Sox2 coding region was amplified from an IMAGE cDNA clone (BC057574) by the following primer set: 5′-GGATCCCCATGTATAACATGATGGAGACGG-3′ and 5′-TGTACATCACATGTGCGACAG GGG-3′. The amplified fragment was then cloned into the pGEM®-T vector (Promega Corp) and the BamHI/SalI fragment was excised, purified and cloned into the BamHI/XhoI prepared pRRL.PPT.EFS.iresGFPpre vector fragment.
Helper-free Sox2 lentiviruses were prepared by lipofectamine 2000 (Invitrogen) co-transfection of 90% confluent 293T HEK cells cultured in DMEM supplemented with 10% fetal calf serum. For a 10-cm tissue culture dish, 8 µg each of pRRL.PPT.EFsSox2.iresGFPpre or pRRL.PPT.EFS.iresGFPpre, together with pCMVΔR8.91 and pMD.G plasmids, were co-transfected according to the manufacturer's instruction. Supernatants were collected after 2–4 days and filtered through 0.45 µm filters, aliquoted and stored at −80°C freezer.
To introduce Sox2 or GFP gene into C2C12-Retro-Py (C2C12-RP) and C2C12-Oct4-Py-Nanog (C2C12-OPN) cells, a total of 105 cells were plated in a well of a six-well plate the day before transduction. One ml of thawed viral supernatants were added in the presence of 8 µg/ml polybrene, centrifuged at 2,000 rpm in a cell culture table top centrifuged for 2 h at room temperature. After viral infection, an extra 2 ml of fresh medium was added and the culture medium was replaced with fresh medium the following day. After another 48 h, GFP+ cells were sorted using an unmodified FACS-Vantage SE system (Becton-Dickinson, USA). Post-sort analysis revealed over 97% GFP expressing cells in the FACS-purified C2C12-RPG and C2C12-OPNS cell population. Then, total protein (50 µg/lane) were isolated and examined with Western blot to confirm exogenous expression of Oct4, Nanog, and Sox2. Oct4 antibody was from BD Transduction Laboratory (#611202) and used at 0.25 µg/ml. Antibodies against Nanog, Sox2, and Gapdh were all from ABcam (ab21603, ab15830, and ab9482 respectively) and used at 0.2 µg/ml.
Reverse Transcription and Quantitative Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from the C2C12 and P19 cells using TRIZOLE according to the supplier's instruction. Then, the first strand of cDNA was synthesized using the Superscript III kit for RT-PCR. Briefly, total RNA was denatured at 65°C for 5 min in the presence of 0.5 µg oligo dT and 1 mM dNTP. After chilling on ice for at least 1 min, reverse transcription is allowed to proceed at 25°C for 5 min in the presence of 1× first-strand buffer, 5 mM DTT, and 40 U of RNase inhibitor. Then, reaction was allowed to proceed at 50°C for another 60 min. Reaction was inactivated by heating inactivation at 70°C for 10 min.
Quantitative real-time PCR was performed in a 20 µl reaction mixture containing 5 µM forward/reverse primers, 1× KAPA™ SYBR FAST reaction mix (KAPA Biosystems, Boston, MA), and various amounts (equivalent to about 25, 50, and 100 ng of total RNA) of template. The reaction was performed with preliminary denature for 10 min at 95°C to activate Taq DNA polymerase, followed by 40 cycles of denaturing at 95°C for 15 s, and annealing/extension at 63°C for 1 min. Different amounts of template were used in the same reaction to make sure the linear amplification of PCR products. A tube containing equivalent amount of total RNA was used as reverse transcriptase control and amplified in the same PCR reaction. Only samples shown free of genomic contamination were further analyzed. Gapdh was used as internal control amplified in the same PCR assay. The primer sets used were listed in Table I. All reactions were performed in ABI 7300 sequence detection system.
| NCU code | Gene name | Primer sequence | Amplicon size (bp) |
|---|---|---|---|
| 003007 | Nanog | FP: 5′-GTGGGATCCGCATGAGTGTGGGTCTTCCT-3′ | 225 |
| 015047 | RP: 5′-GCTTTTGTTTGGGACTGGT-3′ | ||
| 003023 | Oct4 | FP: 5′-GAAGAGTATGAGGCTACAGG-3′ | 195 |
| 015049 | RP: 5′-GGCTCTAGATCAGTTTGAATGCATGGG-3′ | ||
| 000010 | Sox2 | FP: 5′-TCAGCATGTACCTCCCCGGC-3′ | 130 |
| 000011 | RP: 5′-CATGTGCGACAGGGGCAGT-3′ | ||
| 015034 | Klf4 | FP: 5′-CTCTTCCCCCAGGATTCCAT-3′ | 210 |
| 015035 | RP: 5′-TTGCCACAGCCTGCATAGT-3′ | ||
| 003016 | Pax7 | FP: 5′-TGGCTGAAGGACGGTCACTGC-3′ | 181 |
| 003017 | RP: 5′-CACACCTGAGCCCTCATCCAGAC-3′ | ||
| 003024 | MyoD | FP: 5′-GGCTACGACACCGCCTACTA-3′ | 186 |
| 003025 | RP: 5′-GTTCTGTGTCGCTTAGGGAT-3′ | ||
| 021016 | Lin28 | FP: 5′-CGGTGTCCAACCAGCAGT-3′ | 145 |
| 021017 | RP: 5′-CATGCGCACGTTGAACCACTT-3′ | ||
| 001042 | Gapdh | FP: 5′-CCTCTGGAAAGCTGTGGCGT-3′ | 190 |
| 001043 | RP: 5′-TTGGCAGGTTTCTCCAGGCG-3′ |
- FP, forward primer; RP, reverse primer.
Western Blots
The protocol for Western blot has been described previously [Lang et al., 2009]. Briefly, P19 and C2C12 cells grown on 10-cm Petri dishes were trypsinized and then lysed with 150 µl RIPA buffer (20 mM Tris, 150 mM NaCl, 1% Deoxycholate, 0.1% SDS, 1% Triton X-100, pH 7.8) supplemented with protease inhibitors to extract total protein. Aliquots of total lysate (100 µg) were run on 10% SDS-PAGE gels before blotted onto PVDF membrane (Pall FluoroTrans W membrane, PALL) by a blotter at 160 mA for at least 16 h. The PVDF membranes were extensively washed with 1× PBS containing 0.5% Tween-20 (PBST) before blocked by 5% skimmed milk in PBST. Primary antibodies against Oct4 (#611202, BD Pharmingen) and Nanog (ab21603-100, Abcam, Cambridge, UK), and Sox2 (#15830, Abcam) were diluted 1:1,000 in blocking solution (5% milk in PBST) and incubated with the blot at 4°C for overnight. After several washes with PBST, HRP conjugated secondary antibody (1:1,000 dilution) was added and incubated at room temperature for 1 h. The signals was detected by a chemiluminescence kit (Amersham Pharmacia Biotech) and visualized on X-ray films (Super RX, Fuji Medical X-film, Tokyo, Japan). For detection of Gapdh as internal control, all the blots were stripped, washed, and then incubated with Gapdh antibody (diluted 1:500; Sc-815, Santa Cruz) and HRP conjugated secondary antibody described as above. For detecting acetylated histone proteins, aliquot of total lysate were run on 15% SDS-PAGE gels and processed as above. Antibodies against acetylated histone H3 (#06-599, Millipore) and H4 (#06-598, Millipore) were diluted with blocking solution to 0.05 µg/ml and 0.5 µg/ml respectively.
Immunocytochemistry
After in differentiation medium (DMEM supplemented with 2% horse serum, 50 nM insulin, and 5 mM LiCl) for 96 h, C2C12-RPG cells were washed with cold PBS before fixed in 4% paraformaldehyde for 20 min. Then, they were quenched in 50 mM NH4Cl for 15 min before permeablized in 2% Triton X-100 over night. Cells were incubated in blocking solution (2%BSA and 2% goat serum in PBS) for 20 min followed by incubating with MHC antibody (1:500 dilution; clone MY-32, Sigma) over night. After extensive wash with PBS, HRP-conjugated secondary antibody (Goat anti mouse IgG, Santa Cruze; diluted 1:500) was added and incubated for an hour. The expression of MHC was visualized with AEC substrate kit (Zymed Laboratories) and the cells were counter-stained with hematoxylin and eosin.
RESULTS
VPA Enhances Oct4 Expression in C2C12 Cells
Oct4 is a POU domain transcription factor (also called Oct3 and Pou5f1) expressed exclusively in blastomeres, pluripotent early embryo cells, and the germ cell lineage [Nichols et al., 1998]. Embryos deficient in Oct4 expression can develop to the blastocyst stage, but the inner cell mass (ICM) cells in these embryos are not pluripotent. Instead, they are confined to differentiating along the trophoblast lineage. Therefore, it seems that the production of pluripotent stem cells in the mammalian embryo depends critically on the function of Oct4 [Nichols et al., 1998]. With its importance in the maintaining of pluripotency, it prompted us to examine if VPA enhanced pluripotency of somatic cells through direct activation of Oct4 expression. Using quantitative RT-PCR, we found that VPA treatment significantly enhanced Oct4 expression in vector control C2C12 myoblasts (C2C12-RPG) and those (C2C12-OPNS) over-expressed with Oct4, Nanog, and Sox2 (Fig. 1B). The expression of Nanog was not significantly affected by VPA. In sharp contrast to the up-regulation of Oct4, the expression of MyoD, the master regulator of myogenesis, was significantly reduced and causing the attenuation of myogenesis by VPA treatment (Fig. 1C). These observations suggest that VPA can enhance Oct4 but repress myogenic gene expression in myogenic cells.
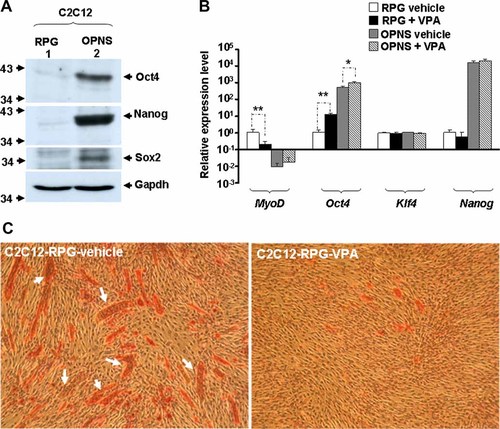
VPA enhances the expression of Oct4 in C2C12 myoblasts. C2C12 were over-expressed with Oct4, Nanog, and Sox2 by three steps as described in the Materials and Methods Section. Firstly, they were infected with Oct4-expressing retrovirus and then stably transfected with pPyCAGIP-Nanog to create Oct4 and Nanog simultaneously over-expressed clones. These clones were later infected with lentivirus carrying either Sox2-IRES-GFP or IRES-GFP gene. A: Expression levels of ectopic Oct4, Nanog, and Sox2 in vector control (C2C12-RPG) and over-expressed (C2C12-OPNS) stable clones were detected by Western blot. B: The expression level of stem cell and myogenic factors in vehicle and VPA treated C2C12 cells were determined by quantitative RT-PCR. For the amplification of each gene, cDNA template equivalent to 25 ng of total RNA was used as template and the expression of Gapdh gene served as the internal control. PCR reactions using equivalent amount of total RNA or no template control (NTC, lane N) were run simultaneously to examine the degree of genomic DNA contamination. Only samples without detectable genomic DNA contamination were further analyzed. *P < 0.05 and **P < 0.01 as compared with that of vehicle only. C: Immunocytochemical staining of myosin heavy chain (MHC) of C2C12-RPG cells treated with vehicle or VPA. Arrow: MHC positive multinucleated myotubes. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To further elucidate whether VPA enhanced Oct4 expression through its activation at transcription level, the promoter (−1,888 to +41) of Oct4 was cloned into luciferase based reporter construct pStable-luc that allowed stable integration of this new reporter construct, pStable-Oct4-1.9k-luc, into target cell genomes. This region of the Oct4 gene has been shown to regulate its cell type-specific expression and response to retinoic acid treatment [Okazawa et al., 1991; Pikarsky et al., 1994] and thus its activity should be a good indicator of Oct4 expression. In polyclonal P19 cells, we found that VPA enhanced Oct4 promoter activity in a dose-dependent manner (Fig. 2A). Addition of retinoic acid (RA) repressed Oct4 promoter activity as reported previously [Sylvester and Scholer, 1994], but this repression could be significantly reversed by VPA treatment at higher dose. This result suggests that VPA can enhance Oct4 expression by directly targeting its promoter and this enhancing effect can overcome the repressive effect caused by RA treatment.
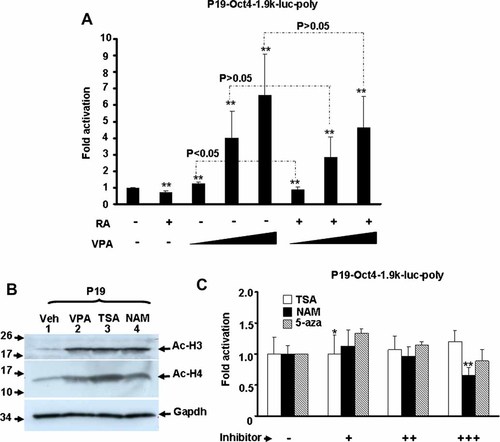
VPA enhances the activity of Oct4 promoter stably integrated into chromatin. A: P19 stable clones containing Oct4 promoter (−1,888 to +41) driven luciferase reporter construct was treated with RA (100 nM) and increasing amount (0.2, 2, and 4 mM) of VPA for 24 h. Then, their luciferase activity was determined. Each treatment was done in triplicate and results shown were means of five independent assays. **P < 0.01 as compared with that of vehicle only. The P-value of the paired comparison between VPA treatment and treatment with VPA and RA was shown on the top. The same cells were also treated with HDAC inhibitors trichostatin A (TSA; 1 nM) and nicotinamide (NAM; 2 mM), and DNA demethylation agent 5-azacytidine (5-aza; 2 µM) and with their acetylated histone H3 and H4 (Ac-H3 and -H4) detected with Western blot (B). The effect of increasing amount of TSA (1, 2, and 4 nM), NAM (1, 2, and 4 mM), and 5-aza (1, 2, and 4 µM) on Oct4 promoter activity was also examined and shown in (C).
VPA was recognized as an inhibitor of histone deacetylase (HDAC) and thus its enhancing effect on chromatin integrated Oct4 promoter could possibly due to general chromatin-remodeling effect. To address this issue, we examined the effect of other widely used HDAC inhibitors, including trichostatin A (TSA) and nicotinamide (NAM), and DNA demethylation agent 5-azacytidine (5-aza) on chromatin-integrated Oct4 promoter activity. Although these inhibitors significantly enhanced the acetylation of histones H3 and H4 (Fig. 2B), only TSA marginally increased Oct4 promoter activity at low concentration while NAM showed toxicity at high concentration (Fig. 2C). These observations imply that VPA enhanced Oct4 expression is not due to general effect on chromatin acetylation.
Independent monoclonal P19 cells carrying pStable-Oct4-1.9k-luc was also isolated and treated with VPA. The luciferase activity of clone #7 was significantly higher than that of clone #8 (Fig. 3A). Their difference in basal promoter activity might possibly reflect the local chromatin structure of their integration site; higher activity implies loose chromatin structure and lower activity means compact chromatin structure. If the effect of VPA is exerted through inhibition of HDAC, differential activation should be observed between these two monoclonal cells. To our surprise, VPA treatment enhanced the promoter activity of both clones (Fig. 3B,C). Their repression by RA treatment was also rescued. Similar to what observed in polyclonal cells, treatment with TSA, NAM, and 5-aza failed enhanced Oct4 activity in cone #7 cells (Fig. 3D). These results further confirm that VPA may specifically enhance the activity of factors acting/binding on Oct4 promoter, instead of acting as a general HDAC inhibitor.
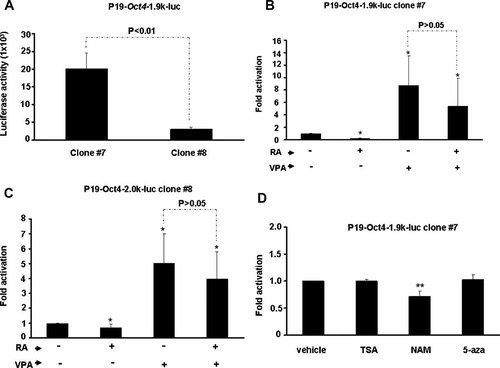
VPA enhances the activity of Oct4 promoter in p19 monoclonal cells. A: Luciferase activity of two P19 monoclones containing Oct4 promoter (−1,888 to +41) driven luciferase reporter. They were treated with RA (100 nM) and VPA (4 mM) for 24 h before assayed for their luciferase activity. Their relative fold activations by these two treatments were shown in (B) and (C) respectively. Each treatment was done in triplicate and results shown were means of three independent assays. **P < 0.01 as compared with that of vehicle only. The P value of the paired comparison between VPA treatment and treatment with VPA and RA was shown on the top. D: Effect of HDAC inhibitors and 5-aza on Oct4 promoter activity in P19-Oct4-1.9k-luc clone #7 cells was examined as in Figure 2.
Although VPA could enhance Oct4 promoter activity in P19 EC cells, it is not known whether the same aspect can be observed in somatic cells, such as myoblasts. To answer this question, C2C12 myoblasts stable clones carrying pStable-Oct4-1.9k-luc were established. As in P19 EC cells, polyclonal C2C12 stable clone cells responded well to VPA treatment (Fig. 4D). Monoclones with differential basal activity were also isolated (Fig. 4A) and subjected to VPA and RA treatments. Similar to what observed in P19 cells, VPA treatment enhanced Oct4 promoter activity and rescued its repression by RA treatment (Fig. 4B,C). No significant difference in the degree of VPA enhanced activity between monoclones was observed. However, the degree of promoter activity enhanced by VPA in C2C12 was significantly lower than that in P19 cells, implying that some factors targeted by VPA might be lacking in C2C12 cells. Treatments with TSA, NAM and 5-aza also failed to enhance Oct4 promoter in C2C12 cells (Fig. 4D). In sharp contrast to what observed with Oct4 gene, the promoter activity and expression level of N-cadherin gene was significantly reduced by VPA (Fig. 4E). These observations further suggest that VPA increased Oct4 expression is a gene-specific response, not due to general effects on the chromatin structure.
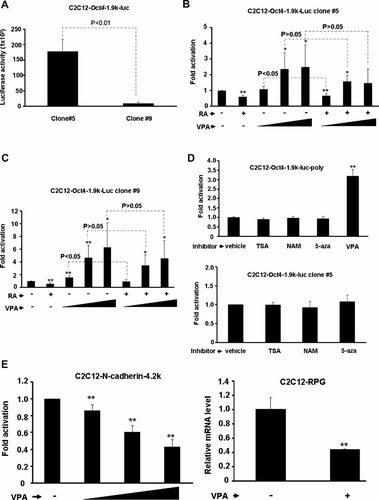
VPA enhances the activity of Oct4 promoter in C2C12 monoclonal cells. A: Basal luciferase activity of two C2C12 monoclones containing Oct4 promoter (−1,888 to +41) driven luciferase reporter. They were treated with RA (100 nM) and increasing amount (0.2, 2, and 4 mM) of VPA for 24 h before assayed for their luciferase activity. Their relative fold activations by these two treatments were shown in (B) and (C). Each treatment was done in triplicate and results shown were means of three independent assays. *P < 0.05 and **P < 0.01 as compared with that of vehicle only. The P value of the paired comparison between VPA treatment and treatment with VPA and RA was shown on the top. D: Effect of HDAC inhibitors and 5-aza on Oct4 promoter activity in C2C12-Oct4-1.9k-luc-poly and clone #5 cells were examined as in Figure 2. The promoter of N-cadherin (−3,785 to +1,032) was also stably transfected into C2C12 and treated with VPA. Their luciferase activity was shown in the left panel of (E). The expression levels of N-cadherin gene in response to VPA was also determined by qPCR as described above and the results were shown on the right panel of (E).
The Proximal Region of Oct4 Promoter Mediates VPA Effect
From our observations in P19 and C2C12 cells, it suggests that VPA exerts its effect independent of local chromatin structure. Therefore, it is of interest to know whether VPA can enhance the activity of Oct4 promoter not stably integrated into chromatin. In transient transfection assays, we found that VPA also enhanced the activity of pStable-Oct4-1.9k-luc (Fig. 5A), thus confirming its chromatin-independent effect. Since RA and VPA antagonized each other on the Oct4 promoter, it led us to examine if over-expression of retinoic acid receptor (RAR) could affect the activation effect of VPA. As expected, over-expression of RARα blocked VPA mediated activation of Oct4 promoter (Fig. 5B). This result suggests that specific transcription factors are recruited to Oct4 promoter by VPA treatment and this recruitment was prevented when RAR is binding to Oct4 promoter.
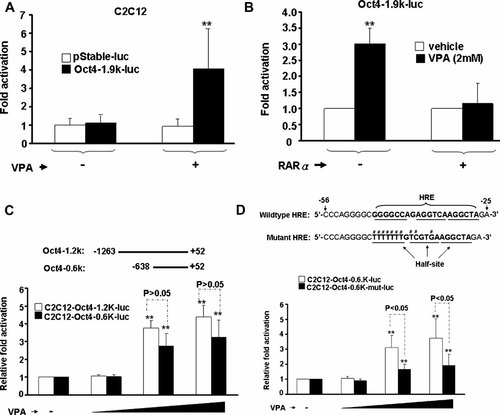
The proximal hormone response element (HRE) mediates VPA effect on Oct4 promoter. A: The reporter construct pStable-Oct4-1.9k-luc (0.67 µg/well) was transiently transfected into C2C12 cells and its luciferase activity in the presence or absence of VPA was determined 24 h after the treatment. B: Similar to (A), but with RARα expression vector (0.33 µg/well) co-transfected with the pStable-Oct4-1.9k-luc and treated with VPA or not. C,D: Deletion and HRE mutants of Oct4 promoter were stably transfected into C2C12 cells to create polyclonal stable lines. Their luciferase activity in the presence and absence of VPA was determined as described in Figure 4. Each treatment was done in triplicate and results shown were means of at least three independent assays. D: The sequence (−56 to −25) of wild-type and mutant HRE are shown on the top and bases mutated are denoted with # on their top. The three half-sites in the HRE are underlined. **P < 0.01 as compared with that of vehicle only.
To further define the regions in Oct4 promoter that were targeted by VPA, a series of 5′-end deletion mutants of this promoter were made and tested for their activation by VPA. Deletion of the −1,888 to −1,264 region had no effect on the VPA mediated activation (Fig. 5C). Further deletion to −638 gave similar results (Fig. 5C). These results suggested that the binding sites of factors mediating VPA effect may locate within the region close to transcription initiation site, especially the region between −638 and +52. A hormone response element (HRE, −46 to −27) located within this region was shown previously to be targeted by many nuclear hormone receptors, including TR2, SF1, RAR, COUP-TFII, and GCNF [Pikarsky et al., 1994; Schoorlemmer et al., 1994; Fuhrmann et al., 2001; Yang et al., 2007; Gupta et al., 2008]. Binding of RAR, COUP-TFII, and GCNF to this HRE strongly repressed the expression of Oct4 in ES and EC cells. Conversely, TR2 and SF1 tethered to this HRE enhance the expression of Oct4. As over-expression of RAR blocks the activation of Oct4 promoter by VPA treatment (Fig. 5), it implies that this HRE plays critical role in the VPA activation effect. This speculation was further confirmed when mutation of the HRE significantly reduced VPA activation effect on the region between −638 and +52 (Fig. 5D). It will be interesting to know whether VPA treatment affects the binding of these nuclear hormone receptors to this HRE that leads to the activation of Oct4 promoter.
DISCUSSION
Before its pluripotency-promoting effect was identified, VPA had been prescribed by doctors worldwide for the treatment of epilepsy and bipolar disease for more than 35 years [Perucca, 2002]. Its pharmacological effects involve various mechanisms that affect the transmission of nerve signals in the patients. Several intracellular pathways, including the Ras-ERK pathway [Jung et al., 2008], were affected by VPA, however, its major targets in the cells were found to be histone deacetylases (HDACs) [Gottlicher et al., 2001; Gurvich et al., 2004] and glycogen synthase kinase 3β (GSK3β) [Bug et al., 2005]. In addition to neuronal diseases, VPA is also effective for treating acute myeloid leukemia (AML) by inducing differentiation or apoptosis of leukemic blasts and stimulating the proliferation and self-renewal of normal hematopoietic stem cells [Bug et al., 2005]. The therapeutic effect of VPA on AML is mainly mediated by its inhibition of GSK3β.
It was found that inhibitors of both DNA methyltransferase and HDACs can improve the reprogramming efficiency of somatic cells [Huangfu et al., 2008a]. However, in our study, we found that only VPA treatment is effective; treatments with TSA and 5-aza-cytidine had no significant effect on the activity of Oct4 promoter (Figs. 2C and 4D). The Oct4-GFP reporter used in the previous study [Huangfu et al., 2008a] was driven by an 18 kb Oct4 genomic fragment in which the proximal enhancer (PE) was deleted (identical to GOF18ΔPE but with the LacZ replaced by EGFP). The failure of TSA and 5-aza-cytidine to activate Oct4 promoter in our study might due to the shorter promoter region used. An alternative explanation is that, instead of activating Oct4 promoter directly, both TSA and 5-aza-cytidine may induce the expression of Oct4 via indirect pathways. Nevertheless; our results indicate that VPA specifically target the proximal promoter region of Oct4 to activate its expression, instead of through non-specific inhibition of HDACs. It is also of interest to know whether VPA activates Oct4 promoter by inhibition of GSK3β. Treatment with LiCl and other GSK3β inhibitors had no apparent effects on the Oct4 promoter activity (data not shown), suggesting that VPA exerts its effect through factors that yet to be defined. The identification of factors mediating the effects of VPA on Oct4 expression is now underway in our laboratory.
A few factors targeting the proximal hormone response element (HRE) in Oct4 promoter have been identified, including TR2, SF1, RAR, COUP-TFII, and GCNF [Pikarsky et al., 1994; Schoorlemmer et al., 1994; Fuhrmann et al., 2001; Yang et al., 2007; Gupta et al., 2008]. Their binding to Oct4 promoter in ES and EC cells can either activate or repress the promoter activity. The importance of this HRE in the VPA activation effect has been demonstrated by the fact that over-expression of RARα blocks the activation of Oct4 promoter by VPA (Fig. 5B) and mutation of HRE significantly reduces this activation (Fig. 5D). It will be interesting to know whether VPA treatment affects the binding of these nuclear hormone receptors to this HRE and/or the interactions among these nuclear hormone receptors.
Recent progresses in this field have identified several other small compounds that can also promote the efficiency of iPS cell reprogramming. BIX-01294, the inhibitor of the G9a histone methyltransferase, can improve the reprogramming efficiency in neural progenitor cells (NPCs) transduced with Oct4 and Klf4 [Shi et al., 2008a]. Addition of PD0325901, an inhibitor of MEK, at later stage of reprogramming helps to select colonies that have been successfully reprogrammed [Shi et al., 2008a]. Small compounds, such as Bayk (an L-calcium channel agonist) and RG108 (a DNA methyltransferase inhibitor) that can act synergistically with BIX-01294 are also identified [Shi et al., 2008b]. It will be interesting to know whether these small compounds can act synergistically with VPA in the activation of Oct4 promoter and subsequent reprogramming of somatic cells. If the answer is positive, and then the development of a reprogramming cocktail consists of these small compounds will be achievable in the near future.
Acknowledgements
The authors like to thank Dr. Hans R. Scholer (Max Planck Institute for Molecular Biomedicine), Dr. Christopher Baum (Hannover Medical School), and Dr. Ronald M. Evans (Salk Institute; San Diego) for providing plasmids; Dr. Uwe Dressel (The University of Queensland) for careful reading of this manuscript. This study is supported by funding from the National Science Council of Taiwan, ROC to Shen Liang Chen (NSC-96-2311-B-008-006-MY3) and from Armed Forces Taoyuan General Hospital to Yu-Liang, Kuo (AFTYGH-9507 and AFTYGH-9803) and to Ta Wei Chu (AFTYGH-9407).




