Resveratrol induces cell-cycle disruption and apoptosis in chemoresistant B16 melanoma
Abstract
Resveratrol, a naturally occurring polyphenol, has been shown to possess chemopreventive activities. In this study, we show that resveratrol (0–500 µM) inhibits the growth of a doxorubicin-resistant B16 melanoma cell subline (B16/DOX) (IC50 = 25 µM after 72 h, P < 0.05). This was accomplished by imposing an artificial checkpoint at the G1–S phase transition, as demonstrated by cell-cycle analysis and down-regulation of cyclin D1/cdk4 and increased of p53 expression level. The G1-phase arrest of cell cycle in resveratrol-treated (10–100 µM) B16/DOX cells was followed by the induction of apoptosis, which was revealed by pyknotic nuclei and fragmented DNA. Resveratrol also potentiated at subtoxic dose (25 µM for 24 h) doxorubicin cytotoxicity in the chemoresistant B16 melanoma (P < 0.01). When administered to mice, resveratrol (12.5 mg/kg) reduced the growth of an established B16/DOX melanoma and prolonged survival (32% compared to untreated mice). All these data support a potential use of resveratrol alone or in combination with other chemotherapeutic agents in the management of chemoresistant tumors. J. Cell. Biochem. 110: 893–902, 2010. © 2010 Wiley-Liss, Inc.
Resistance to chemotherapeutic agents remains a major obstacle in the successful treatment of cancer. Tumor cells exhibit proliferative and invasive potentials and use multiple mechanisms to escape from commonly used anticancer drugs. Chemopreventive phytochemicals which are able to act on signaling molecules, that is, by inhibiting survival proteins or activating pro-apoptotic mediators, represent promising agents.
Recently, many compounds found in the diet and beverages have been identified as potential chemopreventive agents [Li et al., 2007; Thomasset et al., 2007; Khan et al., 2008]. Among them, resveratrol (trans-3,4′,5-trihydroxystilbene; RES) has been shown to exhibit chemopreventive activities against a wide variety of cancers, including breast carcinoma, leukemia, colon carcinoma, prostate adenocarcinoma, and melanoma [Cal et al., 2003; Kundu and Surh, 2008; Goswami and Das, 2009]. This naturally occuring phytoalexin found in grapes and other medicinal plants is synthesized in response to injury, ultraviolet irradiation, or fungal attack. Significant amounts of RES have been found in red wine, and many studies have suggested that it may be in part responsible for the beneficial effect of red wine against coronary heart disease [Kopp, 1998]. Many reports have attributed to RES a large number of cancer preventive properties because of its ability to interfere with tumor initiation, promotion, and progression [Athar et al., 2009]. These effects are related to the inhibition of cyclooxygenase [Kundu et al., 2006] as well as free radical scavenging [Lu et al., 2006] or induction of cell differentiation [Wolter and Stein, 2002].
One of the most attractive approaches to target cancer cells is to disrupt the cell cycle or promote apoptosis. Indeed, loss of cell-cycle checkpoints and resistance to drug-mediated programmed cell death are two features which contribute to an uncontrolled proliferation and to the establishment of malignancies.
It is now well recognized that RES interfers with cell cycle in cancer cells. It has been shown to block cell-cycle progression at G1, S, or G2/M phases depending on the cell type [Ahmad et al., 2001; Kuo et al., 2002; Larrosa et al., 2003; Lee et al., 2004]. These conflicting results of RES-induced cell-cycle arrest might be due to the specific cell type used. Cell-cycle progression is controlled by phase-specific interactions between protein kinases (cdk) and cyclins [Collins et al., 1997]. These complexes are subjected to inhibition via the binding to a class of proteins known as cdk inhibitors, as well as exogenous factors [Sherr and Roberts, 1995]. It is known that the cell-cycle arrest is often associated with programmed cell death, p53 being one of the main regulator of survival and apoptosis balance in malignant cells [Aylon and Oren, 2007]. Since it possesses many biological targets, RES may prevent tumor cell proliferation and trigger apoptosis by interfering with the mitogen-activated protein kinase pathway [Shih et al., 2002], or activating pro-apoptotic signals such as overexpression of bax [Kuo et al., 2002] and down-regulating antiapoptotic proteins expression [Benitez et al., 2007], leading to caspase-dependent or -independent apoptosis.
Although many studies have demonstrated the cell cycle-disrupting and apoptosis-inducing activities of RES on a wide variety of cell types, little data exist concerning its antiproliferative and pro-apoptotic effects in drug-resistant malignant cells, especially in melanoma cells.
In this study, we demonstrate that RES induces a growth inhibition in a mouse B16 melanoma cell line resistant to doxorubicin (B16/DOX) via the induction of G1-phase arrest and subsequently apoptosis. These alterations were mediated by up-regulation of p53 expression. We also assessed the in vivo activity of RES on tumor growth and survival in mice transplanted with B16/DOX cells.
MATERIALS AND METHODS
Materials
RES was purchased from Sigma (Saint Quentin-Fallavier, France). For in vitro studies, stock solutions of 0.05 and 0.01 M resveratrol in absolute ethanol were wrapped in aluminium foil for protection against light, and kept at −20°C. Dilutions of the appropriate stock solution were made in fresh culture medium for experiments. For in vivo studies, a stock solution of 50 mg/ml in absolute ethanol was conserved in the same conditions as previously described and diluted to the required working concentration with PBS. Anti-p53, cyclin D1, cdk2, cdk4, and actin antibodies were from Santa Cruz Biotechnology. MTT (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) and trypan blue were purchased from Sigma. MTT was dissolved in PBS (5 mg/ml) and stored at −20°C. Hoechst 33342 and propidium iodide were from Molecular Probes (Eugene, OR).
Cells and Animals
The mouse B16 melanoma cells resistant to doxorubicin (B16/DOX) was obtained from the National Tumor Institute, Milano [Formelli et al., 1986]. This cell line was shown to express a multidrug-resistant phenotype due to the overexpression of the P-glycoprotein mdr1 gene [Capranico et al., 1989]. Cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (Invitrogen, Cergy-Pontoise, France) in a 5% CO2 and saturated humidity at 37°C. B16/DOX cells were exposed to 35 nM DOX every seven passages to maintain resistance. Cell viability was estimated by the trypan blue exclusion. For cell morphology examination, cells were grown on chambered coverglass system (lab-Tek) and observed with an inverted microscope.
For in vivo experiments, 6- to 8-week-old female B6D2F1 mice, purchased from Charles River Laboratories (Iffa Credo, L'arbresle, France), were housed at the animal maintenance facility. All experiments were conducted according to the Institutional Animal Care and Use Committee Guidelines.
Measurement of Cell Proliferation and Viability
Cell proliferation was determined by the MTT assay. Cells were plated at 1 × 104 cells/well and were allowed to attach for 24 h. The medium was then removed and was replaced by 200 µl of complete medium containing the appropriate concentration of RES or vehicle (0.2% ethanol) in 96-well microplates for 24, 48, or 72 h. Each concentration of RES was used in five wells. Cells were then washed twice with RPMI and 200 µl of fresh medium containing MTT (0.3 mg/ml) were added in each well. After 3 h incubation at 37°C, the supernatant was gently removed and formazan crystals were dissolved in 200 µl DMSO. Absorbance was recorded at 550 and 650 nm using a microplate reader. The effect of RES growth was assessed as the percentage of cell growth (vehicle-treated cells were taken as 100% viable). IC50, expressed as the sample concentration that caused a 50% inhibition of cell growth, was calculated from nonlinear regression with the Prism 3.0 software.
Cell viability of RES-treated cells was measured after 24 or 48 h by the trypan blue exclusion. In order to determine the synergistic effect of RES with DOX, cells were pretreated with 10 or 25 µM RES for 24 h before incubation with 0.25–5 µM DOX for an additional 24 h in a RES-free medium. Cell viability was evaluated using the same method.
Assessment of Apoptosis
Cells grown on chamber coverglass system were fixed with 10% formalin in PBS for 10–15 min at room temperature. Cells were washed twice with PBS and stained with 5 µM Hoechst 33342 in PBS for 10–15 min at room temperature in the dark. Slides were then washed three times with PBS and stained nuclei were observed under a fluorescence microscope.
The TUNEL assay was performed using an in situ cell death detection kit (Roche, ON, USA). After treatment, cells were fixed in acetic acid at −20°C for 5 min and then incubated with digoxigenin-conjugated dUTP in a terminal deoxynucleotidyltransferase-catalyzed reaction for 1 h at 37°C. After washing, a peroxidase-conjugated antibody was added for 30 min. DNA fragments were stained with 3,3′-diaminobenzidine (Sigma) as a substrate for peroxidase.
For genomic DNA fragmentation analysis, cells were treated with RES or vehicle before genomic DNA isolation, as previously described [Sambrook et al., 1989]. DNA fragments were resolved on a 1.8% agarose gel containing ethidium bromide, following by observation under UV light. A 100–1,000 bp DNA ladder (Eurogentec, Seraing, Belgium) was used as a standard molecular weight.
Cell-Cycle Analysis
Cells were treated with RES (5, 10, 50, and 100 µM) or vehicle. After 24 h, cells were collected by trypsinization, washed with cold PBS, and resuspended in 50 µl cold PBS. After fixation in ice-cold methanol for 1 h at 4°C, the supernatant was discarded by centrifugation (200g, 5 min) and cells were washed twice and finally incubated in PBS containing 50 µg/ml Rnase for 30 min at 37°C. Cells were then chilled on ice for 10 min, stained with 50 µg/ml propidium iodide for 30 min on ice and analyzed by flow cytometry.
Western Blotting
Cells were washed in PBS and lysed in RIPA buffer (10 mM Tris–HCl, pH 7.2, 150 mM NaCl, 0.5% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and 2 mM EDTA) containing protease inhibitors for 15 min on ice. Cell lysates were cleared by centrifugation (20,000g, 15 min). Protein concentration was measured using the Bio-Rad protein assay kit. An equal amount of proteins (30 µg) diluted in loading buffer were boiled for 5 min and then separated on a 4–12% polyacrylamide gel electrophoresis containing SDS. After transfer onto a nitrocellulose membrane for 1 h and blocking nonspecific sites for 30 min in casein-containing blocking solution, the membrane was incubated with the appropriate primary antibody (dilution 1:1,000 in washing buffer) for 1 h and immunodetection was performed using the Western Breeze chemiluminescent immunodetection kit (Invitrogen) according to the manufacturer's instructions. Briefly, the membrane was washed three times and incubated for 30 min with the alkaline phosphatase-conjugated secondary antibody. Revelation was performed by adding the chemiluminescent substrate following by the exposition of the membrane on a Kodak X-MOAT AR film.
Animal Experiments
Mice (n = 5 per group) were subcutaneously (s.c.) challenged with 1 × 106 B16/DOX cells in saline solution in their right flank.
After tumor appearance, mice were s.c. injected with 12.5–50 mg/kg RES or with the same volume of ethanol in the left flank. Tumor size was assessed in a blinded fashion twice a week and recorded as tumor area by measuring the largest perpendicular diameters with callipers. The suppressive rate of tumor growth was calculated as 100 × (mean tumor size in control group − mean tumor size in treated group)/mean tumor size in control group. Experiments were repeated twice. During RES treatment, mice were weighed every 5 days, and their behavior (appetite and mobility) was monitored. The increase in survival time (% IST) was calculated with the following formula: % IST = 100 × (T − C)/C, with T = median value of survival time of treated mice and C = median value of survival time of control mice.
Statistical Analysis
Results were expressed as mean ± SD of the indicated number of experiments. Statistical significance was estimated using a Student's t-test for unpaired observations. A P-value <0.05 was considered to be significant.
RESULTS
Cell Growth Inhibition and Cytotoxicity Induced by RES
We first determined whether RES inhibited cell growth of B16/DOX cells. To this end, the MTT assay was used. As shown in Figure 1A, RES treatment (1–500 µM) resulted in a time- and dose-dependent inhibition of B16/DOX cell proliferation. RES IC50 was dramatically reduced, from more than 200 µM after 24 h to ∼40 and 25 µM (P < 0.05) after 48 and 72 h, respectively. This dose-dependent inhibition of cell proliferation was confirmed by microscopic observations of B16/DOX cells treated with 10–100 µM RES for 48 h (Fig. 1B).
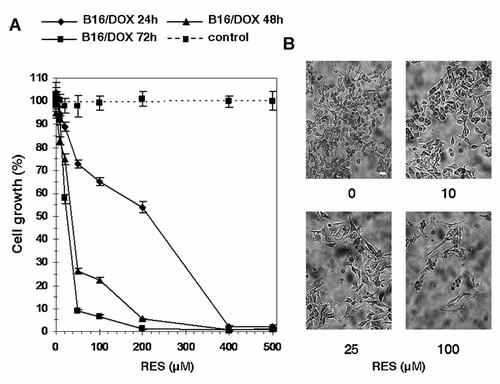
Effect of RES on proliferation of B16/DOX cells. A: Cells were treated with 1–500 µM RES or vehicle for 24, 48, or 72 h, and cell growth was estimated by the MTT assay. Data represent mean ± SD of four independent experiments. The sample concentration that caused a 50% inhibition of cell growth (IC50) was calculated from nonlinear regression. B: Cells growing in the presence of RES were observed under an inverted light microscope. Scale bar: 40 µm.
To know whether this decrease in the number in RES-treated living cells was due to an antiproliferative effect or a cytotoxic activity, a trypan blue exclusion assay was carried out on RES-treated B16/DOX cells. Figure 2A shows that cell viability was significantly impaired only when cells were exposed to 50 or 100 µM RES after 24 h treatment. Although the number of living cells decreased in a RES dose-dependent manner for 48 h treatment, cell viability of cells treated with 50 and 100 µM RES was significantly different from untreated cells (Fig. 2B). These results suggest that RES possesses both antiproliferative effects (for concentrations lower than 50 µM) and cytotoxic activity. This was confirmed by the counting of dead cells, which was not greater when cells were exposed to 1–25 µM RES compared to control ones. However, it significantly increased in the presence of 50 µM (P < 0.05) and 100 µM (P < 0.01) RES (Fig. 2C).
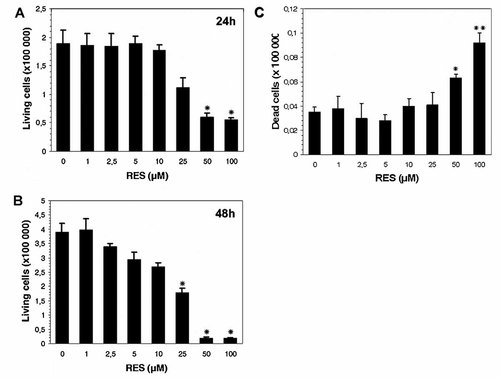
Effect of RES on viability of B16/DOX cells. After treatment with 1–100 µM RES for 24 h (A) and 48 h (B), cell viability was estimated by trypan blue exclusion. C: The number of trypan blue-positive cells were scored after 48 h treatment. Each value corresponds to mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared to untreated cells.
As B16/DOX cells were resistant to doxorubicin, it was also determined whether RES could sensitize B16/DOX cells to DOX. Cells were pretreated with 10 or 25 µM RES for 24 h and subsequently cultured in the presence of 0.25–10 µM DOX for another 24 h. Cell death was evaluated by trypan blue exclusion. As indicated in Figure 3, 10 and 25 µM alone induced low cell death (<3%) whereas pretreatment with RES resulted in an increased cytotoxicity of DOX, in B16/DOX cells.
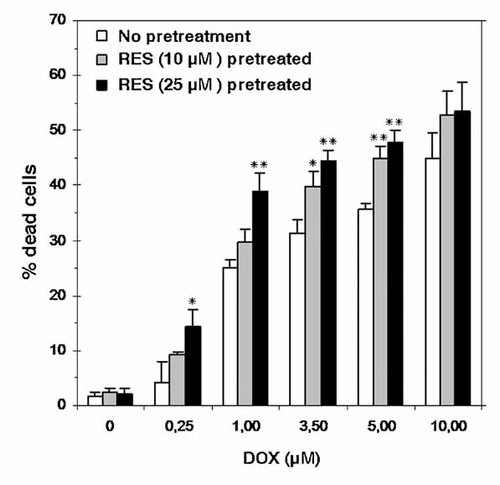
Effect of RES subtoxic doses on DOX cytotoxicity. Cells were exposed to 10 or 25 µM RES for 24 h and then transferred in fresh RES-free medium containing DOX (0.25–10 µM) for 24 h. The percentage of cell death was measured by trypan blue exclusion. Data represent mean ± SD (n = 3). *P < 0.05, **P < 0.01 compared to control cells.
RES Up-Regulates p53 Expression and Induces G1 Phase Arrest
To examine the mechanism of cell proliferation inhibition, the cell-cycle distribution was analyzed by flow cytometry after propidium iodide staining. Treatment of B16/DOX cells with 50 µM (P < 0.05) and 100 µM (P < 0.01) RES induced an accumulation of cells in G1 phase after 24 h (Fig. 4A). This was associated with a concomitant decrease in cell population in G2 and S phases.
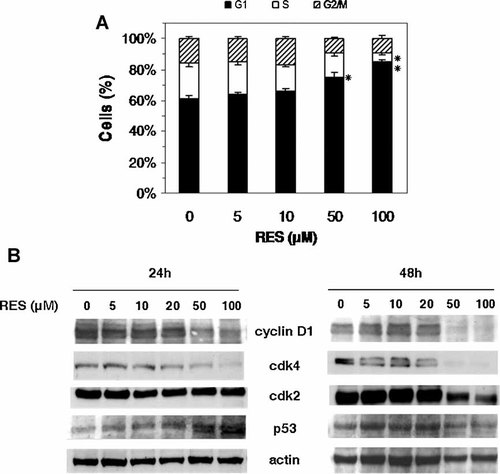
Effect of RES on cell-cycle progression. A: Cell-cycle distribution in B16/DOX cells following RES treatment for 24 h. Cells were fixed and stained with propidium staining before flow cytometry analysis. *P < 0.05, **P < 0.01 compared to untreated cells. B: Immunoblot analysis of cyclin D, cdk2, cdk4, and p53 in RES-treated cells for 24 or 48 h (one representative of three independent experiments). Actin: loading control.
The RES-mediated G1 phase cell-cycle arrest was confirmed by the analysis of cyclin D and cdk2 and cdk4 expression. These proteins play in fact a major role in G1 phase progression and G1–S transition. A dose-dependent decrease of cyclin D expression was observed after 24 h RES treatment, and this was more pronounced after 48 h (Fig. 4B). Cdk4 expression was also down-regulated in RES-treated cells. After 48 h, cdk4 was nondetectable when cells were treated with 50 and 100 µM RES. The decrease in cdk2 expression was less pronounced than that of cdk4. After 24 h, cdk2 level remained virtually unchanged, and a slight decrease was observed for 50 µM RES treatment after 48 h.
Since the observed cell-cycle changes could be related to the up-regulation of p53, a Western blot analysis was performed to analyze p53 expression. Data showed that p53 expression was up-regulated upon RES treatment for 24 h, but did not increase after 48 h (Fig. 4B). Taken together, these results show that RES arrests cell cycle in G1 phase via a p53-dependent inhibition of cell cycle-associated proteins.
RES Induces Apoptosis in B16/DOX Cells
As cell-cycle arrest often precedes apoptosis, we determined whether RES treatment may lead to programmed cell death in B16/DOX cells. First, cells treated with RES were stained with Hoechst 33342 and visualized by fluorescence microscopy. Figure 5A shows the morphological changes induced by RES, including chromatin condensation and formation of apoptotic bodies, whereas such features could not be seen in the untreated cells (P < 0.05 with 25 µM RES, P < 0.01 with 100 µM RES; Fig. 5D).
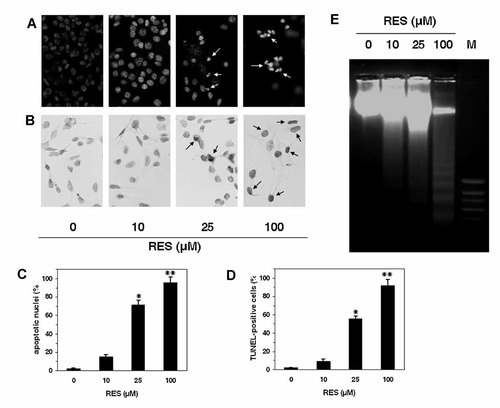
RES-induced apoptosis in B16/DOX cells. A: Morphological changes observed after Hoechst 33342 staining under a fluorescence microscope. B: Apoptosis was assessed by a TUNEL assay. Cells were observed by light microscopy. Apoptotic nuclei (C) and TUNEL-positive cells (D) were recorded in three independent experiments. Data represent mean ± SD. *P < 0.05, **P < 0.01 compared to control cells. E: After RES treatment, DNA was extracted and separated on 1.8% agarose gel containing ethidium bromide. DNA fragments were visualized under UV light. M, standard molecular weight.
Internucleosomal DNA fragmentation, which occurs during late apoptosis, was also assessed using a TUNEL assay and DNA gel electrophoresis. Whereas no TUNEL-positive cells were found in untreated B16/DOX cells, a positive staining was observed in cells treated with RES, indicating that the cells underwent apoptosis when they were treated with RES after 48 h (Fig. 5B,D, P < 0.05 with 25 µM RES, P < 0.01 with 100 µM RES). DNA electrophoresis of RES-treated cells revealed a typical DNA ladder pattern, characteristic of apoptotic cells (Fig. 5E). The extent of internucleosomal cleavage was RES dose-dependent, whereas the DNA of untreated cells remained intact.
In Vivo Effect of RES on the Growth of B16/DOX Cells
Considering the above in vitro results showing that RES initiated apoptosis in B16/DOX cells, we investigated whether the compound may have in vivo inhibitory effects on the growth of B16/DOX cells. Mice were challenged with 1 × 106 B16/DOX cells and after tumor appearance, they were treated every 2 days with 12.5, 25, or 50 mg/kg RES or vehicle for 30 days. Treatment with RES did not induce loss of weight or changes in behavior. Figure 6A shows that each RES treatment resulted in an impaired tumor growth, as revealed by the size of tumors in each group during the 30 days of treatment. However, the tumor growth inhibition was not related to the dose of RES administered, as expected. After 30 days of treatment, the tumor growth in the 12.5 mg/kg-treated group was 2.5 and 1.9 times lower compared to the 25 and 50 mg/kg-treated groups, respectively. The highest inhibitory effect was obtained 17 days after tumor appearance regardless of the RES dose used (Fig. 6B). At this time, the inhibition of tumor growth was 62% compared to control when mice were treated with 12.5 mg/kg RES and remained ∼50% after 30 days. When 25 and 50 mg/kg were used, the inhibition of melanoma growth reached 43% and 35%, respectively, followed by a rapid decrease, in particular in the 50 mg/kg-treated group, suggesting that growth of B16/DOX cells became uncontrollable in spite of RES administration.
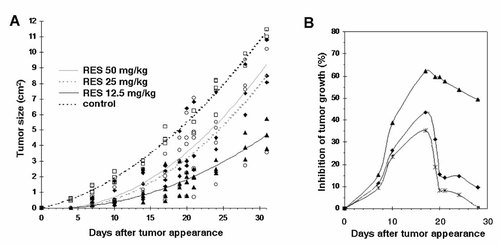
In vivo effect of RES on the growth of B16/DOX cells. After a s.c. challenge of 1 × 106 B16/DOX cells, B6D2F1 mice received RES injections (12.5, 25, or 50 mg/kg) after tumor appearance every 2 days for 30 days. A: Tumor sizes were assessed twice a week. Data are recorded as tumor area for each mouse (n = 5 per group). Determination coefficients were comprised between 0.85 and 0.98. B: Inhibition of tumor growth (% compared to untreated mice) was calculated as expressed in the Materials and Methods Section. ♦, Treatment with 12.5 mg/kg, ▴, 25 mg/kg, 50 mg/kg.
The IST in mice treated with RES was correlated with tumor growth. Administration of 12.5, 25, or 50 mg/kg RES prolonged the survival of melanoma-bearing mice (Fig. 7). IST was 32% when 12.5 mg/kg were used whereas treatment with 25 and 50 mg/kg gave 15% and 20%, respectively. These results show that RES inhibits the in vivo growth of B16/DOX cells, but the efficacy of treatment is not correlated with the dose of RES administered.
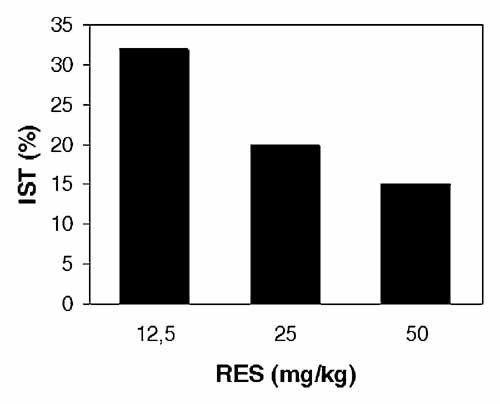
Effect of RES on the median survival time of mice. Mice bearing B16/DOX melanoma were treated as described above. The increase in survival time (IST, % compared to control) was calculated as described in the Materials and Methods Section.
DISCUSSION
DOX is a chemotherapeutic agent to which clinical melanomas have been found refractory. However, the mechanisms of clinical melanoma DOX resistance were not clear. In addition to new regimens in melanoma treatment associating paclitaxel and bevacizumab [González-Cao et al., 2008], another important part of chemoresistance in malignant melanoma toward agents currently used in clinical for this malignancy, such as dacarbazine and temozolamide [Middleton et al., 2000; Danson et al., 2003], is mediated by molecular mechanisms, such as increased cellular sulfhydryls, altered signal transduction, and increased DNA repair [Bradbury and Middleton, 2004]. Therefore, the mechanism of melanoma chemoresistance to other agents, such as DOX, required further investigation. Frank et al. 2005 have reported the role of ABCB5 protein in resistance to DOX in G3361 melanoma cells. Recently, ABCB8 was shown to be involved in DOX resistance in melanoma cell lines [Elliott and Al-Hajj, 2009] and another model proposed that ABC transporters involved in melanogenesis could mediate multidrug resistance in melanoma [Chen et al., 2009]. Therefore, there is a need to identify compounds that overcome chemoresistance.
RES is one such compound and has been extensively studied because of its multifunctional inhibitory effects on multistage carcinogenesis [Ulrich et al., 2005]. In vitro as well as in vivo studies have clearly demonstrated that RES possesses cancer inhibitory effects, in particular against melanoma [Larrosa et al., 2003; Hsieh et al., 2005; Belleri et al., 2008; van Ginkel et al., 2008], but only few reports used chemoresistant cell lines as a model. Among these studies, RES mas found to inhibit cell growth and induce apoptosis in HT29 cells resistant to etoposide [Hwang et al., 2007], and affected the cell-cycle progression in two MCF7 cell sublines resistant to arabinosylcytosine and 2′-desoxy-5-fluorouridine [Bader et al., 2008]. As melanoma is refractory to chemotherapy, we used the chemoresistant B16/DOX cell line which has been established in the presence of DOX and expresses ABCB1/P-glycoprotein [Capranico et al., 1989].
In our study, we found that RES inhibited the growth of B16/DOX cells in a time- and concentration-dependent manner. RES IC50 was 40 µM after 48 h treatment. In human SK-mel28 and A375 melanoma cell lines, IC50 were, respectively, 60 and 15 µM [Niles et al., 2003], indicating that RES-mediated inhibition of proliferation varies according to the cell type.
DNA cell-cycle analysis demonstrated that RES-mediated inhibition of cell growth was attributable to a G1-phase arrest of the cell cycle. This accumulation of cells in G1-phase was previously observed in different cell lines, such as LNCaP prostate [Benitez et al., 2007], epidermoid carcinoma A431 [Ahmad et al., 2001], liver Hep G2 [Kuo et al., 2002], or leukemia cell lines [Lee et al., 2008]. In other cell lines, RES-induced cell-cycle arrest in S-phase [Larrosa et al., 2003; Niles et al., 2003], showing that RES may interfere with the cell cycle via different pathways depending on the cell type. Down-regulation of cdk4 and cyclin D1 was in agreement with the G1-phase cell-cycle blockade [Tashiro et al., 2007]. The cyclin D1/cdk4 complex is responsible for the cell-cycle progression during the early G1-phase and phosphorylates the tumor suppressor pRb, leading to its inactivation [Seville et al., 2005]. pRb in its hypophosphorylated state sequesters the transcription factor E2F in the cytosol which suppresses protein expression required for S-phase, thus causing a blockade in the G1-phase.
The tumor suppressor gene p53 is considered as a key element in controlling the balance between cell growth and death [Aylon and Oren, 2007]. In response to DNA damages, p53 triggers cell-cycle regulatory events to limit the proliferation of abnormal cells. Here, we demonstrated that p53 expression was increased in B16/DOX cells after RES treatment. Although we did not measure the level of p21WAF1 expression, it is likely that p53-mediated cell-cycle arrest occurs through the induction of p21WAF1, which negatively regulates the kinase activity of cdks [Choisy-Rossi et al., 1998] leading to the blockade of G1–S transition.
In our study, the p53-mediated cell-cycle arrest was followed by apoptosis induction in RES-treated B16/DOX cells. In this work, Hoechst staining, TUNEL assay, and DNA electrophoresis clearly demonstrated that RES triggered a dose-dependent apoptosis in B16/DOX cells. Although apoptosis induction by RES was triggered through several ways, it is possible that in our study, apoptosis may be elicited through the mitochondrial pathway as it has previously been described [Kuo et al., 2002; Benitez et al., 2007], since pro-apoptotic proteins such as Bax are p53-targeted genes [Mitry et al., 1997].
Few studies reported the sensitizing effect of RES to chemotherapy-induced apoptosis. As we used a DOX-resistant melanoma cell line, we tested whether RES could sensitize these cells to DOX. Indeed, RES-enhanced DOX cytotoxicity, resulting in an increased cell death. RES has been found to enhance TRAIL- or bortezomib and thalidomide-induced apoptosis, mainly through the down-regulation of STAT-3 and NF-κB [Bhardwaj et al., 2007; Ivanov et al., 2008]. Since the B16/DOX cells express the ABCB1/MDR1 gene which codes for the ABC transporter P-glycoprotein involved in the limited uptake of antineoplastic drugs [Perrin et al., 2007], it is not excluded that RES may interfer with survival pathways as well as with P-glycoprotein-mediated multidrug resistance, as it has been described [Quan et al., 2008]. Another data to explain this enhanced DOX cytotoxicity is that RES can act as a pro-oxidant agent, causing oxidative breakage of cellular DNA. This may result in synergistic antitumour activities when RES is combined with conventional chemotherapeutic agents or cytotoxic compounds [Cal et al., 2003; Fulda and Debatin, 2006; de la Lastra and Villegas, 2007].
Using B16/DOX cells, we also assessed the in vivo anticancer activity of RES. Although some studies showed that RES failed to inhibit melanoma growth because of its rapid metabolization [Niles et al., 2006], we and others have demonstrated that the stilbene reduced tumor growth, angiogenesis, and metastasis formation [Asensi et al., 2002; Belleri et al., 2008; van Ginkel et al., 2008].
In studies reporting the in vivo antitumor activity of RES, the doses used were very variable. Zhou et al. 2005 administered 500–1,500 mg/kg in mice beside tumor body whereas other studies used 40–80 mg/kg (200–400 µM) [Gao et al., 2002; Chen et al., 2004]. It is obvious that such concentrations cannot be used with classical anticancer agents. But resveratrol and other plant compounds displayed less adverse side effects than conventional anticancer agents, and showed protective effects in normal cells [Garg et al., 2005]. Nevertheless, the doses of RES used in our study were lower than the concentrations used in the studies cited above (12.5–50 mg/kg). At the dose of 20 mg/kg, which is close to ours, a rapid plasmatic peak which reached 2.6 µM was obtained when resveratrol was administered by intragastric route [Asensi et al., 2002]. The authors estimated that ∼1.5% RES reached the plasma compartment, and which was explained by a low bioavailability and a rapid metabolism in liver (mainly as glucuronide conjugate). To establish a comparison, it was shown that 70% RES given orally was absorbed, and found essentially under a metabolized form.
In our study, an increase in survival and a reduction of tumor size were observed in RES-treated mice. Surprisingly, the beneficial effects of RES were inversely proportionnal to the dose administered. These results are in disagreement with several studies showing the in vivo effects RES in xenograft models [Zhou et al., 2005; Garvin et al., 2006]. However, most of these studies were performed using nude mice, which do not have a functional immune system. Besides, it is known that RES exhibit immunomodulatory properties, such as suppression of lymphocyte proliferation, cytokine production, and development of cell-mediated cytotoxicity [Falchetti et al., 2001; Gao et al., 2001]. Therefore, to explain our in vivo data, we postulate, that a dose of 12.5 mg/kg RES may not be more efficient in itself to inhibit the tumor growth than 25 and 50 mg/kg. Indeed, our in vitro data show that RES had a dose-dependent effect on B16/DOX cells. But at the highest doses, RES may exert both tumor growth inhibitory and immunosuppressant effects, and it is now well recognized that the immune system plays a key role in the surveillance of tumor growth, at least in the first steps. Moreover, Feng et al. 2002 demonstrated that low doses of RES-enhanced immune response in mice. Other studies performed using mice having a functional immune system reported beneficial effects of RES at low/moderate doses (1–10 mg/kg) [Bråkenhielm et al., 2001; Kimura and Okuda, 2001]. Therefore, the dose of RES to be administered may be important, and moderate doses of RES may be more efficient in vivo. Further investigations linking the antitumor effect of RES and the state of the immune system activation are needed to support the in vivo antitumor activity of RES.
In summary, we showed that RES imposes an artificial checkpoint at G1–S phase transition, causing a G1-phase arrest of cell cyle and leading to apoptosis in DOX-resistant B16 melanoma. This work, together with other studies, supports that RES, alone or in combination with other compounds, may be a promising compound in cancer chemoprevention and therapy, in particular against tumors resistant to chemotherapy.




