Secretory products from human adipocytes stimulate proinflammatory cytokine secretion from human endothelial cells†
Grit Sommer and Susan Kralisch contributed equally to this work.
Abstract
Hyperplasia and hypertrophy of fat cells can be found in obesity and increased adiposity is associated with endothelial dysfunction as an early event of atherosclerosis. However, it is unclear whether human adipocytes directly influence endothelial protein secretion. To study the crosstalk between fat and endothelial cells, human umbilical venous endothelial cells (HUVECs) were cultured in infranatants (Adipo) of primary differentiated human adipocytes. Interestingly, significantly increased secretion of 23 cytokines and chemokines from HUVECs was detected in four independent experiments after Adipo stimulation by protein array analysis detecting a total of 174 different proteins. Among those, time-dependent Adipo-induced upregulation of cytokine secretion in HUVECs was confirmed by ELISA for interleukin (IL)-8, monokine induced by gamma interferon, macrophage inflammatory protein (MIP)-1β, MIP-3α, monocyte chemoattractant protein-1, and IL-6. Factors besides adiponectin, leptin, resistin, and tumor necrosis factor α appear to mediate these stimulatory effects. Our findings suggest that endothelial cell secretion is significantly influenced towards a proinflammatory pattern by adipocyte-secreted factors. J. Cell. Biochem. 106: 729–737, 2009. © 2009 Wiley-Liss, Inc.
Cardiovascular diseases are nowadays the most common causes of mortality in industrialized countries [Murray and Lopez, 1997]. The development of atherosclerosis, a key element of this group of diseases, spans several years and first lesions already occur during adolescence [Libby, 2000]. Besides genetic susceptibility, environmental and nutritional parameters influence the progression of atherosclerosis. Here, obesity is a nutritional disorder characterized by the accumulation of adipose tissue with hypertrophy and hyperplasia of fat cells in adipose tissue [Fasshauer and Paschke, 2003], which are closely linked to atherosclerosis [Lusis, 2000]. The connection between obesity and atherosclerosis has been studied in more detail in recent years. Thus, adipocyte-secreted proteins, so-called adipokines, influence endothelial function profoundly [Fasshauer et al., 2004]. Among those, tumor necrosis factor (TNF) α is well known to mediate proatherogenic effects by activating nuclear factor κB (NFκB) that in turn stimulates the expression of proinflammatory genes [Wajant et al., 2003]. Thus, expression of adhesion molecules including intracellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and E-selectin, as well as monocyte adhesion, are induced by TNFα in vivo and in vitro [Kuldo et al., 2005]. Furthermore, TNFα treatment of endothelial cells leads to increased secretion of interleukin (IL)-6, IL-8, and monocyte chemoattractant protein (MCP)-1 [Zachlederova and Jarolim, 2006]. Besides TNFα, leptin, and resistin have been suggested as proatherogenic adipokines. Thus, resistin-induced upregulation of monocyte adhesion and VCAM-1 expression can be found in human endothelial cells in vitro [Verma et al., 2003; Kawanami et al., 2004]. Furthermore, resistin augments NFκB activity in bovine aortic endothelial cells [Kawanami et al., 2004]. Moreover, patients with premature coronary artery disease have higher serum levels of resistin as compared to normal controls [Burnett et al., 2005]. In contrast to TNFα and resistin, leptin does not directly stimulate monocyte adhesion and adhesion molecule expression in human umbilical venous endothelial cells (HUVECs) [Skilton et al., 2005]. However, leptin mediates its proatherogenic effects by upregulating the potent vasoconstrictor endothelin-1 [Quehenberger et al., 2002], increasing oxidative stress [Bouloumie et al., 1999], and stimulating angiogenesis [Park et al., 2001]. Adiponectin has recently been characterized as an antiatherogenic adipokine with potent endothelium-protective effects. Thus, adiponectin reverses the effects of TNFα and resistin on monocyte adhesion by inhibiting endothelial NFκB signaling [Ouchi et al., 1999; Kawanami et al., 2004]. Other beneficial effects of adiponectin include upregulation of NO production [Chen et al., 2003] and suppression of endothelial cell apoptosis [Kobayashi et al., 2004]. Recently, our group has determined for the first time the influence of secretory products from primary human adipocytes on monocyte adhesion in human endothelial cells. We demonstrate that adipocyte infranatant (Adipo) potently stimulates monocyte adhesion via activation of NFκB partly dependent on TNFα [Kralisch et al., 2008].
Endothelial cells are also known to have endocrine functions by secreting various factors including cytokines and adhesion molecules [Libby, 2000]. Since the development of cardiovascular diseases is associated with a change in the secretion pattern of endothelial cells [Libby, 2000], we studied the influence of Adipo on the secretion of cytokines by HUVECs, a well-characterized model for human endothelial cells, in vitro. For this purpose, 174 cytokines were analyzed by protein arrays. A subset of targets relevant for the pathogenesis of atherosclerosis and upregulated by Adipo was further characterized by ELISAs.
MATERIALS AND METHODS
Materials
Cell culture reagents were purchased from Life Technologies, Inc. (Grand Island, NY), Biochrom KG (Berlin, Germany), and PAA (Garching, Austria). RayBio® Human Cytokine Antibody Array G Series 2000 and ELISA kits were obtained from RayBiotech, Inc. (Norcross, GA). Recombinant adiponectin (Axxora, Lörrach, Germany), leptin (Biomol, Hamburg, Germany), resistin (Biocat, Heidelberg, Germany), and TNFα (Sigma, Deisenhofen, Germany) were purchased as indicated. Unless specified otherwise, all other reagents were purchased from Sigma.
Isolation and Culture of Human Adipocytes
Adipocytes were isolated from adipose tissue obtained from healthy women undergoing surgical mammary reduction as described recently [Kralisch et al., 2008]. All women were free of metabolic or endocrine diseases. Informed consent was obtained from all donors before the surgical procedure. The study was approved by the ethical committee of the University of Leipzig. In brief, after collagenase digestion and several washing steps, isolated floating adipocytes were cultured with adipocyte medium consisting of DMEM/F12, 10% FCS, 15 mM glucose, 13.5 mM HEPES, 2 mM L-glutamine, 1.2 g/L NaHCO3, 16.5 µM biotin, 8.5 µM pantothenate, 100 U/ml penicillin, 100 µg/ml streptomycin, and 200 µg/ml kanamycin. Adipocytes were cultured at 37°C in a humidified atmosphere for 16 h. After that period, the conditioned medium (Adipo) was collected and sterile-filtered through a 0.22 µm filter. Adipo was stored at −20°C until use. Before stimulation of HUVECs with Adipo and control medium, 0.4% endothelial cell growth supplement (ECGS), 5 U/ml heparin, 0.1 ng/ml epidermal growth factor (EGF), and 1 ng/ml fibroblast growth factor (FGF) were added.
Since adipose tissue was completely dissected by collagenase digestion and only floating adipocytes were used to generate Adipo after several washing and centrifugation steps, it is unlikely that other cell types such as macrophages contributed to Adipo. An endotoxin contamination could not be detected in Adipo (data not shown).
Isolation and Culture of HUVECs
Isolation of HUVECs by collagenase digestion of human umbilical veins was performed as described by Jaffe et al. 1973. HUVECs from passages 1 and 2 were used for all experiments. 1 × 104 cells/well were seeded in 96-well plates. After culturing for 3 days in EC-20 medium consisting of medium 199 supplemented with 20% FCS, 17 µg/ml ECGS, 100 units/ml penicillin, and 100 µg/ml streptomycin, HUVECs were cultured overnight in EC-2 medium consisting of MCDB131 medium supplemented with 2% FCS, 5 U/ml heparin, 0.1 ng/ml EGF, 1 ng/ml FGF, 1 µg/ml hydrocortisone, and 0.4% ECGS. Adipocyte medium or Adipo supplemented with ECGS, heparin, EGF, and FGF as detailed above were used for stimulation experiments.
Culture of THP-1 Cells and Human Mesenchymal Stem Cells (hMSC)
THP-1 cells (ATCC, London, UK) and hMSC (Lonza, Verviers, Belgium) were cultured with standard protocols. The cells were cultured in adipocyte medium for 16 h to obtain cell culture supernatants. These supernatants were supplemented with ECGS, heparin, EGF, and FGF as detailed above before stimulation experiments.
Analysis of Cytokine Secretion by Protein Chip
After treatment of HUVECs for 24 h with Adipo or control medium, cells were washed once with serum-free MCDB131 medium and maintained in freshly added serum-free MCDB131 medium for 1 h. The HUVEC supernatants during that 1 h period were used for all subsequent analyses. The secretion of cytokines was analyzed by using RayBio Human Cytokine Antibody Array G Series 2000 according to the manufacturer's instructions. Briefly, after adjusting the protein glass chip into the incubation chamber, the chip was blocked by adding 100 µl 1× blocking buffer to each incubation well for 30 min at room temperature. Then, the blocking buffer was discarded and 100 µl of HUVEC supernatants were added to the wells of the protein chip for 2 h at room temperature. After that period, supernatants were discarded and the chips were washed five times for 2 min with 150 µl wash buffer I and twice for 2 min with 150 µl wash buffer II. Seventy microliters of biotin-conjugated antibody solution was added to each well of the protein chip and incubated for 2 h at room temperature. Again, chips were extensively washed with wash buffers I and II. Subsequently, 70 µl of 1,500-fold diluted Alexa Fluor 555-conjugated streptavidin solution was added to each well and incubated overnight at 4°C in the dark. After additional washing steps with wash buffers I and II, glass chips were removed from the incubation frame and rinsed in 30 ml washing buffer I and II. Glass chips were dried and fluorescence was quantified at 532 nm using an Axon GenePix2000 laser scanner (Molecular Devices, Sunnyvale, CA). In case of our protein array data, none of the cytokine spots was under detectable fluorescence level and, therefore, we were able to calculate significance for all cytokines.
Analysis of Cytokine Secretion by ELISA
Cytokine secretion into HUVEC supernatants was quantified with commercially available ELISAs from RayBiotech Inc. according to the manufacturer's instructions. HUVECs were cultured for 16 h in EC-2 medium and after treatment of HUVECs with Adipo, hMSC supernatants, THP-1 supernatants, recombinant adipokines, or control medium, cells were washed once with serum-free MCDB131 medium before the same medium was added for another 1 h. Cytokine secretion into the serum-free MCDB131 medium during this 1 h period was quantified by ELISA.
Statistical Analysis
Results are shown as mean ± SE. If not indicated otherwise, differences between various treatments were analyzed by Mann–Whitney U-test with P values <0.01 considered highly significant and <0.05 considered significant.
RESULTS
Protein Array Analysis of Cytokines Released by HUVECs Treated With ADIPO
After treatment of HUVECs with Adipo for 24 h, cells were washed and medium was changed to serum-free MCDB131 medium for 1 h. Using protein arrays, 174 different cytokines were analyzed concerning a potential Adipo-mediated regulation in HUVECs during this 1 h period (Fig. 1A,B). Significant upregulation of 23 cytokines in Adipo-treated HUVECs was observed as compared to controls in four independent experiments (Table I).
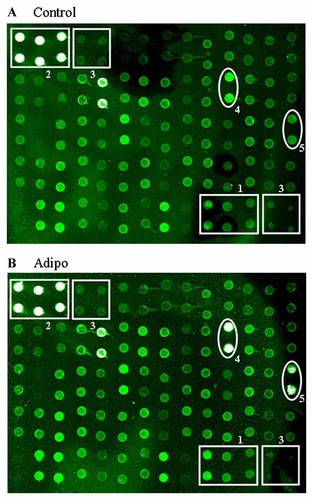
Laser scans of protein arrays. HUVECs were cultured for 16 h in EC-2 medium before (A) adipocyte medium and (B) Adipo was added for 24 h. After this period, cells were washed with serum-free MCDB131 medium and maintained for another 1 h in MCDB131 medium. Subsequently, HUVEC supernatants obtained during this 1 h period were collected and analyzed by protein array. An internal control (1) was used for normalization. Positive (2) and negative controls (3) were included. As shown in the representative array laser scans, the proinflammatory cytokines GRO (4) and IL-8 (5) were induced in Adipo-treated HUVECs (B) as compared to controls (A). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
| Cytokine | Fold increase | SE | P-value |
|---|---|---|---|
| BMP-6 | 1.325 | 0.129 | <0.05 |
| b-NGF | 1.755 | 0.626 | >0.05 |
| EGF | 1.930 | 0.237 | <0.01 |
| Eotaxin-2 | 1.293 | 0.169 | >0.05 |
| FGF-7 | 1.340 | 0.110 | <0.05 |
| Fractalkine | 1.133 | 0.055 | >0.05 |
| GCP-2 | 1.973 | 0.221 | <0.01 |
| GM-CSF | 1.325 | 0.084 | <0.01 |
| GRO | 1.780 | 0.272 | <0.05 |
| GROα | 1.503 | 0.037 | <0.0001 |
| HGF | 1.470 | 0.147 | <0.05 |
| ICAM-1 | 1.150 | 0.018 | <0.01 |
| IGFBP-2 | 1.758 | 0.330 | >0.05 |
| IGFBP-3 | 1.188 | 0.148 | >0.05 |
| IL-1 R4/ST2 | 1.063 | 0.014 | <0.01 |
| IL-10 | 1.708 | 0.239 | <0.05 |
| IL-12 p40 | 1.255 | 0.139 | >0.05 |
| IL-15 | 1.460 | 0.038 | <0.0001 |
| IL-16 | 1.433 | 0.165 | <0.05 |
| IL-17 | 1.180 | 0.085 | >0.05 |
| IL-1α | 1.675 | 0.276 | >0.05 |
| IL-1ra | 1.285 | 0.118 | >0.05 |
| IL-2 | 1.645 | 0.309 | >0.05 |
| IL-2 Rα | 1.130 | 0.059 | >0.05 |
| IL-5 | 1.630 | 0.260 | >0.05 |
| IL-7 | 1.888 | 0.294 | <0.05 |
| IL-8 | 4.025 | 0.507 | <0.01 |
| Lymphotactin | 1.158 | 0.114 | >0.05 |
| MDC | 1.513 | 0.115 | <0.01 |
| MIF | 1.205 | 0.104 | >0.05 |
| MIG | 1.895 | 0.230 | <0.01 |
| MIP-1α | 1.700 | 0.315 | >0.05 |
| MIP-1β | 2.093 | 0.130 | <0.01 |
| MIP-3α | 7.520 | 1.626 | <0.01 |
| NAP-2 | 1.743 | 0.342 | >0.05 |
| NT-3 | 1.520 | 0.295 | >0.05 |
| PARC | 1.693 | 0.293 | >0.05 |
| RANTES | 1.398 | 0.112 | <0.05 |
| SDF-1 | 1.468 | 0.127 | <0.05 |
| TARC | 1.565 | 0.146 | <0.01 |
| TGFβ-1 | 1.973 | 0.242 | <0.01 |
| TIMP-1 | 1.148 | 0.080 | >0.05 |
| TNFα | 1.510 | 0.275 | >0.05 |
| TNFβ | 1.815 | 0.350 | >0.05 |
| VEGF R3 | 1.075 | 0.032 | >0.05 |
- Differences were assessed by unpaired Student´s t-test. Cytokines in bold letters were further analyzed by ELISA.
Time-Dependent Upregulation of Cytokine Secretion From HUVECs by Adipo
Next, we tested whether Adipo-induced upregulation of various cytokines detected in the protein chip experiments could be confirmed by commercial ELISAs of the respective antigens. Additionally, we analyzed MCP-1 and IL-6 by ELISA since these two cytokines in the supernatants of control endothelial cells already showed an antibody saturation of the protein array. Furthermore, the time-dependence of these effects was determined by treating HUVECs with Adipo for 1, 6, and 24 h. Again, the medium was changed to MCDB131 medium for 1 h after a washing step and secretion into the MCDB131 medium during this 1 h period was determined.
As shown in Figure 2A, Adipo significantly stimulated MCP-1 secretion into supernatants after 1–6 h treatment of HUVECs. Maximal 2.8-fold upregulation was found after 1 h (P < 0.01) as compared to untreated cells (Fig. 2A). In contrast, MCP-1 secretion returned to control levels after 24 h stimulation (Fig. 2A).
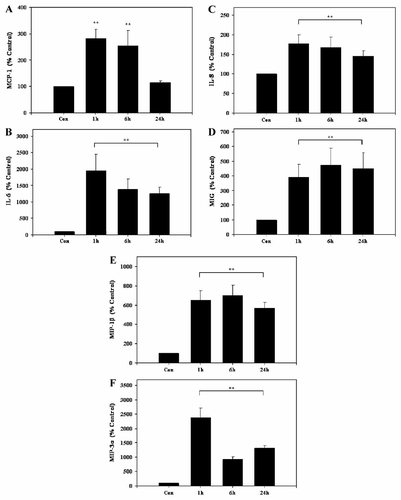
Adipo-stimulated cytokine secretion in HUVECs. HUVECs were cultured for 16 h in EC-2 medium and incubated with Adipo for 1, 6, and 24 h. After this period, cells were washed with serum-free MCDB131 medium and incubated for 1 h in serum-free MCDB131 medium. Cell supernatants obtained during this 1 h period were collected and analyzed by ELISA. Concentrations of (A) MCP-1, (B) IL-6, (C) IL-8, (D) MIG, (E) MIP-1β, and (F) MIP-3α were determined as described in Materials and Methods Section. Results are the means ± SE of four independent experiments relative to controls (100%). **P < 0.01 Adipo-treated versus untreated HUVECs.
Adipo significantly induced IL-6 secretion by HUVECs. As compared to untreated HUVECs, 19.5-fold stimulation after 1 h, 13.8-fold induction after 6 h, and 12.5-fold upregulation after 24 h, respectively, was observed (P < 0.01) (Fig. 2B).
Similarly, Adipo significantly induced IL-8 secretion into supernatants of HUVECs at all time points tested (Fig. 2C). Thus, IL-8 levels were upregulated 1.8-, 1.7-, and 1.5-fold after 1, 6, and 24 h, respectively (P < 0.01) (Fig. 2C).
Monokine induced by gamma interferon (MIG) secretion by HUVECs treated with Adipo was also stimulated (Fig. 2D). Here, MIG levels were significantly increased between 3.9- and 4.7-fold (P < 0.01) with maximal effects seen after 6 h of stimulation (Fig. 2D).
Adipo-induced macrophage inflammatory protein (MIP)-1β release from HUVECs is shown in Figure 2E. Secretion of this cytokine from HUVECs was upregulated 6.5-fold after 1 h, 7.0-fold at 6 h, and 5.7-fold after 24 h stimulation as compared to untreated controls (P < 0.01) (Fig. 2E).
HUVECS stimulated with Adipo showed significantly higher MIP-3α secretion as compared to controls. Thus, after stimulation with Adipo for 1 h, levels of the cytokine were induced 23.9-fold as compared to untreated HUVECs (P < 0.01) (Fig. 2F). After 6 and 24 h treatment with Adipo, 9.2- and 13.2-fold elevated MIP-3α levels, respectively, persisted (P < 0.01) (Fig. 2F).
In contrast, secretion of the cytokines EGF, neutrophil activating protein (NAP)-2, regulated upon activation, normally T-expressed, and presumably secreted (RANTES), and stromal derived factor (SDF)-1α was below the detection limit of the respective ELISAs in control and Adipo-stimulated HUVECs (data not shown).
Upregulation of Cytokine Secretion From HUVECs by Independent Adipo Isolates
The effect of Adipo derived from four different women on secretion of IL-6, IL-8, MCP-1, MIG, MIP-1β, and MIP-3α was determined. To this end, HUVECs were stimulated for 1 h with four independent Adipo isolates (1–4) before cells were washed once with serum-free MCDB131 medium and the same medium was added for another 1 h. Cytokine secretion into serum-free MCDB131 medium during this 1 h period was quantified by ELISA. The four independent Adipo isolates significantly induced IL-6, IL-8, MCP-1, MIP-1β, and MIP-3α (Table II). In the case of MIG, Adipo 1-induced cytokine secretion missed statistical significance while Adipo 2, 3, and 4 significantly increased MIG secretion relative to untreated HUVECs (Table II).
| Adipo 1 | Adipo 2 | Adipo 3 | Adipo 4 | |
|---|---|---|---|---|
| IL-6 | 11,336 ± 1,826* | 17,267 ± 3,739* | 21,292 ± 4,803* | 11,873 ± 2,455* |
| IL-8 | 732 ± 331* | 1,385 ± 610* | 1,731 ± 837* | 536 ± 67* |
| MCP-1 | 432 ± 46* | 463 ± 32* | 549 ± 62* | 308 ± 68* |
| MIG | 206 ± 80 | 959 ± 423* | 8,914 ± 4,063* | 374 ± 352* |
| MIP-1β | 2,633 ± 1,791* | 2,537 ± 1,646* | 4,457 ± 3,017* | 3,638 ± 2,504* |
| MIP-3α | 2,028 ± 562* | 4,517 ± 1,350* | 5,222 ± 1,593* | 5,100 ± 1,167* |
- HUVECs were cultured for 16 h in EC-2 medium and incubated with Adipo for 1 h. After this period, cells were washed with serum-free MCDB131 medium and incubated for 1 h in serum-free MCDB131 medium. Cell supernatants obtained during this 1 h period were collected and analyzed by ELISA. Results are means ± SE of three independent experiments and data are shown relative to controls (100%).
- * Indicates P < 0.05 Adipo-treated versus untreated HUVECs.
Modulation of Cytokine Secretion From HUVECs by Selected Adipokines
We determined the influence of recombinant adiponectin (5 µg/ml), leptin (1, 0.1, and 0.01 µg/ml), resistin (3, 0.3, and 0.03 ng/ml), or TNFα (25, 2.5, and 0.25 ng/ml) on IL-6, IL-8, MCP-1, MIG, MIP-1β, and MIP-3α secretion from HUVECs. To this end, confluent HUVECs were treated with the different adipokines for 1 h before cells were washed once with serum-free MCDB131 medium and the same medium was added for another 1 h. Cytokine secretion into the serum-free MCDB131 medium during this 1 h period was quantified by ELISA. Adiponectin at 5 µg/ml significantly induced IL-6 protein secretion fourfold as compared to control conditions (Table III). In contrast, the secretion of IL-8, MCP-1, MIG, MIP-1β, and MIP-3α was not significantly influenced by adiponectin (Table III). Leptin at a concentration of 1 µg/ml significantly decreased IL-8 and MCP-1 protein secretion (Table III). In contrast, lower concentrations of the adipokine (0.1 and 0.01 µg/ml) significantly induced MIP-3α secretion while the other cytokines were not influenced by leptin in our setting of experiments (Table III). Resistin significantly induced IL-6 cytokine secretion 1.8-fold at a concentration of 0.03 ng/ml (Table III). Similarly, IL-8 secretion into HUVEC supernatants was significantly upregulated by 0.3 and 3 ng/ml resistin (Table III). TNFα dose-dependently induced MCP-1 protein secretion with maximal 6.6-fold induction after treatment with 25 ng/ml effector (Table III). Furthermore, IL-8 was significantly induced 20-fold in HUVECs treated with 25 ng/ml TNFα (Table III).
| Adiponectin (5 µg/ml) | Leptin (1 µg/ml) | Leptin (0.1 µg/ml) | Leptin (0.01 µg/ml) | Resistin (3 ng/ml) | Resistin (0.3 ng/ml) | Resistin (0.03 ng/ml) | TNFα (25 ng/ml) | TNFα (2.5 ng/ml) | TNFα (0.25 ng/ml) | |
|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | 444 ± 62* | 154 ± 33 | 445 ± 230 | 186 ± 66 | 78 ± 27 | 209 ± 58 | 184 ± 40* | 103 ± 63 | 114 ± 93 | 215 ± 168 |
| IL-8 | 237 ± 69 | 15 ± 9* | 53 ± 32 | 90 ± 25 | 198 ± 78* | 145 ± 51* | 126 ± 77 | 2,045 ± 739* | 310 ± 203 | 208 ± 85 |
| MCP-1 | 120 ± 50 | 25 ± 10* | 28 ± 19* | 78 ± 44 | 142 ± 65 | 123 ± 55 | 112 ± 77 | 659 ± 85* | 446 ± 114* | 184 ± 58* |
| MIG | 89 ± 50 | 101 ± 21 | 196 ± 83 | 171 ± 62 | 81 ± 18 | 71 ± 13* | 72 ± 17 | 134 ± 38 | 107 ± 27 | 97 ± 15 |
| MIP-1β | 178 ± 69 | 116 ± 71 | 299 ± 196 | 283 ± 163 | 90 ± 50 | 82 ± 46 | 120 ± 43 | 93 ± 34 | 102 ± 28 | 88 ± 26 |
| MIP-3α | 72 ± 30 | 144 ± 32 | 278 ± 106* | 168 ± 27* | 170 ± 82 | 157 ± 59 | 163 ± 32 | 103 ± 17 | 104 ± 17 | 95 ± 25 |
- After this period, cells were washed with serum-free MCDB131 medium and incubated for 1 h in serum-free MCDB131 medium. Cell supernatants obtained during this 1 h period were collected and analyzed by ELISA. Results are the means ± SE of three independent experiments and data are shown relative to controls (100%).
- * Indicates P < 0.05 adipokine-treated versus untreated HUVECs.
Effects of Non-Adipose hMSC and Monocytic THP-1 Cell Supernatants on Cytokine Secretion From HUVECs
The influence of conditioned medium from non-adipose hMSC and monocytic THP-1 cells on cytokine secretion from HUVECs was tested. To this end, HUVECs were stimulated with hMSC and THP-1 cell culture supernatants for 1 h before cells were washed once with serum-free MCDB131 medium and the same medium was added for another 1 h. IL-6, IL-8, MCP-1, MIG, MIP-1β, and MIP-3α secretion into the serum-free MCDB131 medium during this 1 h period was quantified by ELISA. Human MSC culture supernatants significantly induced MCP-1 and reduced MIP-3α secretion whereas the other cytokines were not significantly influenced by hMSC supernatants (Table IV). Human monocytic THP-1 cell supernatants downregulated IL-6 but did not significantly influence secretion of the other five cytokines (Table IV).
| hMSC | THP-1 | |
|---|---|---|
| IL-6 | 175 ± 68 | 58 ± 5* |
| IL-8 | 168 ± 50 | 112 ± 31 |
| MCP-1 | 335 ± 27* | 937 ± 677 |
| MIG | 94 ± 10 | 91 ± 31 |
| MIP-1β | 113 ± 37 | 689 ± 370 |
| MIP-3α | 61 ± 3* | 86 ± 24 |
- After this period, cells were washed with serum-free MCDB131 medium and incubated for 1 h in serum-free MCDB131 medium. Cell supernatants obtained during this 1 h period were collected and analyzed by ELISA. Results are means ± SE of three independent experiments and shown relative to controls (100%).
- * Indicates P < 0.05 supernatant-treated versus untreated HUVECs.
DISCUSSION
In the current study, we show that Adipo significantly influences the secretion pattern of proinflammatory cytokines in HUVECs. Out of 174 proteins tested, we find significant upregulation of 23 cytokines in Adipo-treated HUVECs as compared to control cells using protein array (Table I). These results suggest that Adipo but not non-adipose hMSC and monocytic THP-1 cell culture supernatants contain bioactive proinflammatory mediators which affect endothelial secretory function profoundly.
In agreement with this notion, we have recently shown that Adipo stimulates monocyte adhesion via induction of VCAM-1, ICAM-1, and E-selectin protein expression in HUVECs [Kralisch et al., 2008]. Interestingly, this effect is reversed by heat inactivation, suggesting that one or more adipocyte-secreted proteins are involved [Kralisch et al., 2008]. Furthermore, by performing experiments with TNFα-neutralizing antibodies, we define TNFα as one cytokine partially mediating the effects of Adipo on monocyte adhesion [Kralisch et al., 2008].
In the current study, we have focused on cytokines that might be involved in the development of obesity-associated cardiovascular diseases. Therefore, we have selected EGF, IL-8, MIG, MIP-1β, MIP-3α, NAP-2, RANTES, and SDF-1α for further examination by ELISA. Additionally, we have analyzed MCP-1 and IL-6 by ELISA since these two cytokines in the supernatants of control endothelial cells already show an antibody saturation of the protein array.
We show a time-dependent Adipo-induced upregulation of the proinflammatory CC-chemokine MCP-1 in HUVECs. Circulating MCP-1 is increased in obesity in mice [Sartipy and Loskutoff, 2003; Xu et al., 2003; Kamei et al., 2006]. The cytokine is well known as a major mediator of monocyte recruitment into atherosclerotic lesions [Baggiolini, 1998]. In agreement with this notion, high concentrations of MCP-1 are found in atherosclerotic plaques with a high number of macrophages [Nelken et al., 1991]. In apolipoprotein (Apo) E-deficient mice, a well-characterized model for atherogenesis, MCP-1 overexpression accelerates the development of atherosclerosis, whereas MCP-1-deficient animals show severely reduced aortic lipid deposition [Aiello et al., 1999; Shin et al., 2002]. In ApoE/MCP-1 knockout mice, migration of macrophages into blood vessel walls is reduced as compared to control animals [Gu et al., 1998]. Significantly elevated MCP-1 serum concentrations are found in patients with coronary artery disease [Martinovic et al., 2005]. Similarly, serum levels of MCP-1 are significantly elevated in patients with ischemic stroke and myocardial infarction as compared to healthy subjects [Arakelyan et al., 2005]. This association is also found in subjects with peripheral arterial disease [Petrkova et al., 2004]. Furthermore, circulating MCP-1 levels are significantly and positively associated with markers of obesity such as body mass index (BMI) and waist circumference [Kim et al., 2006]. Taking these findings into consideration, induction of MCP-1 by fat-secreted proteins might contribute to obesity-associated endothelial dysfunction and atherosclerosis.
Adipo time-dependently induces IL-6 secretion from HUVECs. The proinflammatory cytokine IL-6 is secreted by activated leukocytes, adipocytes, endothelial cells, and various other cell types. The role of IL-6 in the pathogenesis of endothelial dysfunction and atherosclerosis has been better elucidated in recent years. Thus, IL-6 stimulates MCP-1 and cell adhesion molecules including ICAM-1, VCAM-1, and E-selectin in endothelial cells [Lau et al., 2005]. Clinical studies demonstrate that IL-6 serum levels at baseline predict future cardiovascular disease. Thus, elevated IL-6 levels are related to an increased risk of myocardial infarction as seen in a study group of apparently healthy men [Ridker et al., 2000]. Moreover, elevated plasma IL-6 concentrations are associated with an increased common carotid artery lumen diameter in subjects with manifest or suspected coronary artery disease [Larsson et al., 2005]. Interestingly, a study population of 80 obese men shows higher plasma concentrations of the proinflammatory cytokine [Giugliano et al., 2004]. These and our findings implicate that Adipo-induced IL-6 expression in endothelial cells might be another important factor promoting atherogenesis in obesity.
IL-8, which is also significantly induced by Adipo in HUVECs, is a proinflammatory and proatherogenic chemokine that is involved in several processes contributing to the development of atherosclerosis. Thus, it recruits neutrophils and T-lymphocytes into the intima of blood vessels and mediates the adhesion of monocytes to endothelial cells by triggering the expression of adhesion molecules on endothelial cells [Gerszten et al., 1999]. Furthermore, IL-8 promotes proliferation and migration of endothelial cells and smooth muscle cells, leading to enlargement of atherosclerotic plaques [Yue et al., 1994]. High concentrations of IL-8 are found in human foam cells [Wang et al., 1996]. Furthermore, convincing evidence has been presented that plaque rupture is enhanced via activation of matrix-degrading metalloproteinases (MMPs) through IL-8-mediated inhibition of tissue inhibitor of metalloproteinase (TIMP)-1 production [Moreau et al., 1999]. In unstable coronary heart disease patients, elevated levels of circulating IL-8 concentrations are found as compared to subjects with stable coronary heart disease, as well as controls [Romuk et al., 2002]. Furthermore, in patients with carotid artery atherosclerosis, increased circulating IL-8 levels are associated with common carotid artery lumen diameter and cross-sectional intima-media thickness [Larsson et al., 2005]. In addition, circulating levels of IL-8 are significantly and positively associated with visceral obesity-related parameters including BMI and waist circumference [Kim et al., 2006]. In agreement with these findings, IL-8 is likely to be another Adipo-induced candidate molecule secreted by endothelial cells playing a major role in obesity-associated atherogenesis.
We demonstrate that the three chemokines MIG, MIP-1β, and MIP-3α are significantly induced by Adipo in HUVECs. These proteins have all been suggested to contribute to the pathogenesis of atherosclerosis. Thus, MIG is a chemokine recruiting activated T-lymphocytes into the intima of atherosclerotic lesions by CXC-receptor 3 in situ [Mach et al., 1999]. Furthermore, Anger et al. 2007 report increased MIG gene expression in patients with end-stage calcified stenotic aortic valves, which is partially reversed by statin treatment. MIP-1β has chemoattractant properties for monocytes and T-lymphocytes [Taub et al., 1993] and recruits macrophages into atherosclerotic plaques leading to accumulation of macrophages and foam cells [Wilcox et al., 1994]. MIP-3α is another chemotactic protein that plays an important role in psoriasis, recruiting T-lymphocytes in cutaneous lesions [Homey et al., 2000]. Taking these data into consideration, MIG, MIP-1β, and MIP-3α are potential candidates in the interaction between adipocytes and endothelial cells leading to endothelial dysfunction and atherosclerosis.
We show that four independent Adipo isolates cause upregulation of IL-6, IL-8, MCP-1, MIG, MIP-1β, and MIP-3α secretion from HUVECs. However, the extent of upregulation is different between independent isolates, which is most probably due to both individuals´ background and the components of Adipo.
The adipokines adiponectin, leptin, TNFα, and resistin do not induce IL-6, IL-8, MCP-1, MIG, MIP-1β, and MIP-3α secretion from HUVECs to an extent similar to Adipo. These results suggest that factors besides or in addition to these adipokines induce secretion of IL-6, IL-8, MCP-1, MIG, MIP-1β, and MIP-3α. These factors have to be isolated and characterized in future experiments. Furthermore, the biochemical properties of proinflammatory and chemotactic cytokines induced by Adipo will be investigated by performing gene expression and western blotting analyses.
It needs to be pointed out that we have not been able to detect significant amounts of EGF, NAP-2, RANTES, and SDF-1α in HUVEC supernatants from both, control and Adipo-stimulated cells despite an Adipo-mediated upregulation seen in the protein array experiments. These results indicate that all regulations detected by protein arrays must be validated by ELISA before safe conclusions can be drawn.
Taken together, we demonstrate for the first time that adipocyte-secreted factors induce secretion of various proinflammatory and chemotactic cytokines from endothelial cells. Further studies are needed to better elucidate the mechanisms by which human adipocyte infranatants modulate the proinflammatory secretion pattern in endothelial cells.
Acknowledgements
We thank Jurgen Janke and Stefan Engeli (Charité, Berlin) for their support in establishing human adipocyte culture. We are grateful to Mario Lorenz (Charité, Berlin) for his help with endothelial cell culture. This study was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG), KFO 152: “Atherobesity”, project FA476/4-1 (TP 4), the IZKF Leipzig (Project B25) to M.F., and by a grant from the FORMEL1 program of the University of Leipzig to S.K.




