Matrilysin (MMP-7) cleaves C-type lectin domain family 3 member A (CLEC3A) on tumor cell surface and modulates its cell adhesion activity
Abstract
Matrilysin (MMP-7) plays important roles in tumor progression. Previous studies have suggested that MMP-7 binds to tumor cell surface and promotes their metastatic potential. In this study, we identified C-type lectin domain family 3 member A (CLEC3A) as a membrane-bound substrate of MMP-7. Although this protein is known to be expressed specifically in cartilage, its message was found in normal breast and breast cancer tissues as well as breast and colon cancer cell lines. Because few studies have been done on CLEC3A, we overexpressed its recombinant protein in human cancer cells. CLEC3A was found in the cell membrane, extracellular matrix (ECM), and culture medium of the CLEC3A-expressing cells. CLEC3A has a basic sequence in the NH2-terminal domain and showed a strong heparin-binding activity. MMP-7 cleaved the 20-kDa CLEC3A protein, dividing it to a 15-kDa COOH-terminal fragment and an NH2-terminal fragment with the basic sequence. The 15-kDa fragment no longer had heparin-binding activity. Treatment of the CLEC3A-expressing cells with MMP-7 released the 15-kDa CLEC3A into the culture supernatant. Furthermore, the 20-kDa CLEC3A promoted cell adhesion to laminin-332 and fibronectin substrates, but this activity was abrogated by the cleavage by MMP-7. These results suggest that CLEC3A binds to heparan sulfate proteoglycans on cell surface, leading to the enhancement of cell adhesion to integrin ligands on ECM. It can be speculated that the cleavage of CLEC3A by MMP-7 weakens the stable adhesion of tumor cells to the matrix and promotes their migration in tumor microenvironments. J. Cell. Biochem. 106: 693–702, 2009. © 2009 Wiley-Liss, Inc.
Abbreviations used:
CLEC3A, C-type lectin domain family 3 member A; CM, conditioned medium; ECM, extracellular matrix; Lm332, laminin-332; MMPs, matrix metalloproteinases.
Matrix metalloproteinases (MMPs) constitute a large family of zinc-dependent proteinases that are involved in the degradation of extracellular matrix (ECM) proteins such as collagens and laminins. They play critical roles in many physiological and pathological processes such as development, tissue repair, inflammation, and invasion and metastasis of cancer cells [Nelson et al., 2000; Seiki and Yana, 2003]. Many past studies have shown that biological functions of various cell surface molecules are enzymatically modulated by MMPs and other proteinases [Werb, 1997; Seiki and Yana, 2003]. These metalloproteinases are likely to regulate cellular functions by processing or degrading cell surface proteins. These actions of MMPs, as well as the degradation of ECM proteins, are thought to contribute to tumor invasion and metastasis.
Matrilysin (MMP-7) seems to be one of the most important MMPs in cancer progression [Miyazaki et al., 1990; Wilson and Matrisian, 1996; Ii et al., 2006]. The expression of MMP-7 in human colon cancers is highly correlated with malignancy and metastatic potential of the cancers, especially in their liver metastasis [Ishikawa et al., 1996; Hasegawa et al., 1998]. Although MMP-7 is a secreted enzyme, it binds some receptors on cell surface [Yu and Woessner, 2000]. Indeed, there are reports indicating that MMP-7 cleaves membrane proteins such as integrin β4 [Bredow et al., 1997], TNF-α [Haro et al., 2000], syndecan-1 [Li et al., 2002], and E-cadherin [McGuire et al., 2003]. Recently, cholesterol sulfate was identified as a cell surface receptor for the active form of MMP-7 [Yamamoto et al., 2006; Higashi et al., 2008]. The binding of active MMP-7 to colon cancer cells induces cell aggregation and enhances the metastatic potential of the cancer cells in a nude mouse model [Kioi et al., 2003]. Therefore, it seems important for understanding the mechanism of the MMP-7-induced cancer metastasis to identify cell surface proteins, which are specifically cleaved by MMP-7.
The C-type lectin-like domain superfamily is a diverse group of proteins, including asialoglycoprotein receptor (ASGR), collectins, and tetranectin [Zelensky and Gready, 2005]. C-type lectin domain family 3 member A (CLEC3A) was initially isolated as a C-type lectin-like protein from shark and bovine cartilage [Neame et al., 1992]. The same group revealed the human gene structure and chromosomal location of CLEC3A as a cartilage-derived C-type lectin (CLECSF1), which was closely related but not identical to tetranectin [Neame et al., 1999]. However, no further studies to characterize CLEC3A have been reported. Tetranectin, or plasminogen kringle 4-binding protein, has been shown to bind to sulfated polysaccharides and interacts with several kringle domain-containing proteins such as hepatocyte growth factor (HGF) and plasminogen [Lorentsen et al., 2000; Westergaard et al., 2003].
In this study, we identified CLEC3A protein as a membrane-associated substrate for MMP-7 and investigated the biological properties of CLEC3A as well as the consequence of its cleavage by MMP-7. Our results demonstrate that CLEC3A has activity to promote tumor cell adhesion to laminin-332 (Lm332) and fibronectin, and this activity is impaired by the activity of MMP-7.
MATERIALS AND METHODS
Materials
The sources of reagents used are as follows: human tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) from Calbiochem (San Diego, CA); plasma kallikrain from Protagen AG (Läutelfingen, Switzerland); human plasmin, bovine thrombin and factor Xa, human fibronectin, mouse anti-FLAG monoclonal antibody, and Hoechst 33432 from Sigma (St. Louis, MO); human recombinant MMP-9 (gelatinase-B), human recombinant interstitial collagenase (MMP-1), and human stromelysin (MMP-3) from Chemicon (Temecula, CA); human recombinant MMP-7 and heparin from Wako Pure Chemical Industries (Osaka, Japan); human normal and tumor tissues cDNAs from BioChain (San Leandro, CA); the synthetic MMP inhibitor TAPI-1 (N-(R)-(2-(hydroxyaminocarbonyl)methyl)-4-methylpentanoyl-L-naphthylalanyl-L-alanine-2-aminoethyl amide) from Peptides Institute (Osaka, Japan). The anti-human-MMP-7 mouse monoclonal antibody 11B4G was a kind gift from Dr. T. Tanaka (Nagahama Institute of Oriental Yeast Co., Shiga, Japan). Human gelatinase A (MMP-2) [Kioi et al., 2003] and human recombinant Lm332 (laminin-5) [Kariya et al., 2002] were prepared in our laboratory as reported. All other chemicals used for the experiments were of analytical grade or the highest quality commercially available.
Cell Lines and Culture Conditions
Human cell lines used and their sources are as follows: colon carcinoma cell lines (Colo201, Colo320DM, DLD-1, WiDr, HT29, and CaR-1), breast carcinoma cell lines (MDA-MB, BT20), lung carcinoma cell lines (Lu 134, Lu 65, Lu 99, and PC-3), glioblastoma cell line T98G, gastric carcinoma cell line AZ521, bladder carcinoma cell line EJ-1, fibrosarcoma cell line HT1080, leukemia cell line K562, and epidermoid carcinoma cell line A431 from Japanese Cancer Resources Bank (Osaka, Japan); and mammary gland cell line MCF10A, breast cancer cell line MCF-7, and embryonic kidney cell line HEK293 from American Type Culture Collection (ATCC; Manassas, VA). These cell lines were maintained in a mixture of Dulbecco's-modified Eagle's medium and Ham's F-12 medium (DME/F12) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum.
Plasmid Construction and Transfection
To overexpress recombinant CLEC3A in HT1080 and DLD-1 cells, total RNA was prepared from WiDr cells using TRIZOL (Invitrogen) according to the manufacturer's protocol. A human CLEC3A cDNA was prepared using the WiDr-derived RNA as a template by reverse transcription-polymerase chain reaction (RT-PCR) using the following primer set: ATGGCAAAGAATGGACTTGTAATTTGCATCCTGGTG (forward primer) and CTATTGAGGGATGGTGAACTCGCATATGTATCTC (reverse primer). The synthesized CLEC3A cDNA was extended with a FLAG sequence using a specific primer by polymerase chain reaction and inserted into the pBos-CITE-neo mammalian expression vector. HT1080 and DLD-1 cells were separately transfected with the CLEC3A expression vector and with the control vector using the Lipofectamine reagent (Invitrogen) and OPTI-MEM medium (Invitrogen) according to the manufacturer's protocol. To establish stable transfectants, the transfected cells were cultured in the growth medium supplemented with 200 µg/ml Geneticin (G-418; Sigma–Aldrich).
Preparation of Conditioned Media and ECM Fraction
HT1080 or DLD-1 cells expressing recombinant CLEC3A (CLEC3A-HT1080 on CLEC3A-DLD-1 cells) were grown to confluence in 90 or 60 mm culture dishes with the growth medium. Confluent cultures were washed twice with Ca2+-, Mg2+-free phosphate-buffered saline (PBS) and further incubated in serum-free DMEM/F-12 for 2 days. The resultant serum-free conditioned media were collected and concentrated by lyophilization or precipitation with 10% trichloroacetic acid. After collecting the serum-free conditioned media, the cells in the dishes were lysed with 50 mM Tris–HCl buffer (pH 7.5) containing 150 mM NaCl and 0.5% Triton X-100, and the remaining ECM components on the dishes were washed with the buffer supplemented with 5 mM CaCl2 and 0.01% Brij 35 and then solubilized in the SDS sample buffer.
SDS–PAGE and Immunoblotting Analyses
SDS–PAGE was performed on polyacrylamide gels by the standard Laemmli's method. Separated proteins were detected by staining with Coomassie Brilliant Blue R250. For immunoblotting, proteins separated on gels were transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA) and visualized by the alkaline phosphatase method or the enhanced chemiluminescence (ECL) method (GE Healthcare, Buckinghamshire, UK) using specific antibodies.
Purification of Recombinant CLEC3A
CLEC3A-HT1080 cells were grown to confluence and incubated in the serum-free DMEM/F12 medium, and the resulting serum-free conditioned medium (CM) was harvested every 2 days. The collected CM was concentrated by protein precipitation with 80%-saturated ammonium sulfate and dissolved in 50 mM Tris–HCl buffer (pH 7.5) containing 150 mM NaCl, 5 mM CaCl2, and 0.1% Triton X-100, and then applied to affinity chromatography on an anti-FLAG M2 affinity gel column (Sigma). Bound proteins were eluted from the affinity column with 100 mM acetate buffer (pH 3.5) and immediately neutralized with a small volume of 1 M Tris–HCl (pH 8.0). To exclude an NH2-terminally deleted CLEC3A, fractions containing CLEC3A were applied to a heparin–Sepharose-6B column (GE Healthcare). Full-length CLEC3A was bound to the column and eluted at 0.6–1.2 M NaCl. The final CLEC3A preparation showed a purity of 80–90% as judged by SDS–PAGE.
Cleavage of CLEC3A by Proteinases and Determination of NH2-Terminal Amino Acid Sequence
The proforms of MMPs were activated by incubation with 1 mM p-aminophenyl mercuric acetate (APMA). Purified CLEC3A (2 µg protein) was incubated with proteinases at indicated concentrations in 100 µl of a reaction buffer consisting of 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 5 mM CaCl2, and 0.01% Brij 35 at 37°C for indicated lengths of time. The reaction was stopped by mixing with the SDS sample buffer, and the reaction mixture was analyzed by SDS–PAGE and immunoblotting. For the determination of NH2-terminal sequences, the digested proteins were separated by SDS–PAGE, transferred onto PVDF membranes, and stained with Coomassie Brilliant Blue R-250. Stained protein bands were cut off from the membranes and analyzed by a Procise 49X cLC protein sequencer (Applied Biosystems, Foster City, CA).
Cell Adhesion Assay
Cell adhesion assay was carried out as described previously [Hirosaki et al., 2002]. Briefly, each well of 96-well enzyme-linked immunosorbent assay plates (Costar, Cambridge, MA) was coated with FLAG peptide (Sigma) or purified CLEC3A in the presence or absence of purified Lm332 (or laminin-5) or fibronectin at 4°C overnight and blocked with 1.2% (w/v) bovine serum albumin (BSA). Cells were suspended in serum-free medium at a density of 2–5 × 105 cells/ml, and a 100-µl aliquot of the cell suspension was inoculated into each well of the plates and incubated at 37°C for 1.5 h. After non-adherent cells were removed, adherent cells were fixed and stained with Hoechst 33432. The fluorescence intensity of each well of the plates was measured using the spectrofluorometer Plate Chameleon (Hidex; Turku, Finland).
RESULTS
Identification of CLEC3A as a Substrate of Matrilysin
To identify substrate proteins on tumor cell surface for MMP-7, we analyzed membrane proteins, which were specifically released from WiDr human colon carcinoma cells after treatment with active MMP-7. When the carcinoma cells were incubated with MMP-7, a small protein of approximately 5 kDa and some other proteins were increasingly released into the culture medium as the concentration of MMP-7 was increased (Fig. 1A). The NH2-terminal amino acid sequence of the 5-kDa protein was determined to be “LNWDRAQPNGGK” by the automated protein sequencing, and this sequence was identical to a partial amino acid sequence of CLEC3A (amino acid no. 152–163) (Fig. 1B).
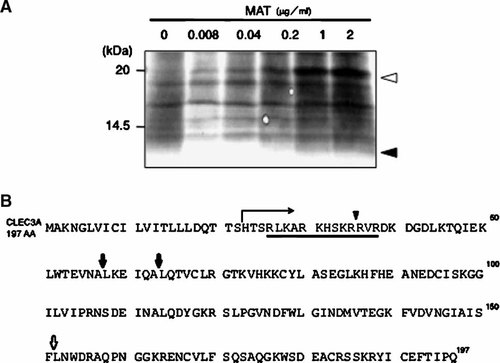
Identification of CLEC3A as a substrate of matrilysin. A: WiDr cells were inoculated at a density of 5 × 106 cells per 60-mm culture dish in the growth medium and incubated for 2 days. The cultures were washed twice with the serum-free DME/F12 medium and incubated at 37°C for 3 h with or without matrilysin at the indicated concentrations. Proteins released into the culture medium were separated by SDS–PAGE on a 5–20% gradient gel, and some protein bands were subjected to the analysis of NH2-terminal amino acid sequence as described in Materials and Methods Section. Closed arrowhead, a 5-kDa fragment of CLEC3A; open arrowhead, matrilysin. B: Amino acid sequence of CLEC3A and its proteolytic cleavage sites. Proteolytic cleavage sites determined for CLEC3A fragments are shown as follows: open arrow, the 5-kDa fragment obtained in (A); closed arrows, the 15-kDa CLEC3A (see Fig. 4A); and closed arrowhead, the 17-kDa CLEC3A (see Fig. 4A). The horizontal arrow indicates the NH2-terminal amino acid sequence of the 20-kDa CLEC3A (see Fig. 4A), and the underline indicates a sequence rich in basic amino acids.
CLEC3A was initially isolated as a mammalian C-type lectin like protein (CLECSF1) from shark and bovine cartilage [Neame et al., 1992], and its message appears to be expressed specifically in the cartilage [Neame et al., 1999]. Little is known about its physiological functions. To investigate biological roles of CLEC3A, we first analyzed the expression of CLEC3A message in various human cancer cell lines by RT-PCR. As shown in Figure 2, the CLEC3A PCR product of approximately 0.6 kb was clearly detected in two (WiDr and HT29) of six human colon cancer cell lines and all human breast cancer cell lines (MDA-MB, BT20, and MCF-7) tested. The message was not detected in 14 other cancer cell lines and a transformed cell line (HEK-293). When the expression of CLEC3A message was analyzed for a human tissue cDNA library, it was detected only in normal breast and breast cancer tissues (data not shown). Any other normal tissues (brain, esophagus, skin, kidney, prostate, uterus, colon, and lung) and cancer tissues (colon, lung, breast, and skin) tested did not show the signal for the transcript (data not shown).
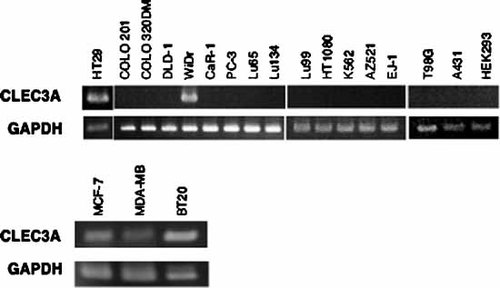
RT-PCR analysis for CLEC3A message expressed by various types of cancer and transformed cell lines. Total RNA was isolated from each cell line using the TRIZOL reagent. One microgram of total RNA was used for the reverse transcription. The cDNAs were amplified using the following specific primer set: 5′-ATGGCAAAGAATGGACTTGTAATTTGCATCCTGGTG-3′ (forward primer) and 5′-CTATTGAGGGATGGTGAACTCGCATATGTATCTC-3′ (reverse primer). The reaction was carried out for 30 cycles of 30 s at 60.5°C, 30 s at 95°C, and 1 min at 72°C. Aliquots of PCR products were run on 1% agarose gels in Tris–borate–EDTA buffer, and stained with ethidium bromide. GAPDH, glyceraldehyde-phosphate dehydrogenase.
Expression of CLEC3A Protein and Its Extracellular Localization
To characterize CLEC3A protein, we cloned a CLEC3A cDNA from WiDr cells, constructed an expression vector, and introduced it into HT1080 human fibrosarcoma cells and DLD-1 human colon cancer cells, both of which did not express intrinsic CLEC3A, to express CLEC3A as a COOH-terminally FLAG-tagged recombinant protein.
To examine the localization of the recombinant CLEC3A protein, three cellular fractions were prepared from the culture of the CLEC3A-transfected HT1080 (CLEC3A-HT1080) cells: CM, cell lysate, and ECM deposited by the HT1080 cells. As shown in Figure 3A, immunoblotting analysis with an anti-FLAG antibody detected the CLEC3A protein in all the fractions. Interestingly, the CM showed two bands of 20 and 17 kDa, only the former of which was detected in the cell lysate and ECM. The NH2-terminal amino acid sequence of the intact 20-kDa CLEC3A was determined to be “HTSRLKARKHSKR” (amino acid no. 23–35), while that of the 17-kDa protein was “RVRDKDGDLKTQI” (amino acid no. 36–48) (Fig. 1B). It was assumed that the 17-kDa CLEC3A fragment might be produced by an endogenous proteinase.
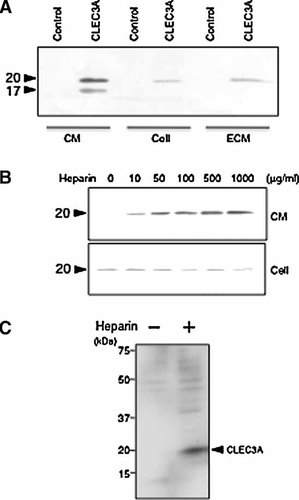
Analysis of recombinant CLEC3A protein produced by CLEC3A-HT1080 cells. A: Analysis of CLEC3A secreted by transfected cells. Three cellular fractions, conditioned medium (CM), whole cells (Cell), and extracellular matrix (ECM), were obtained from serum-free confluent cultures of the CLEC3A-HT1080 (CLEC3A) and control HT1080 (Control) cell lines and subjected to immunoblotting with the anti-FLAG monoclonal antibody. Arrowheads indicate CLEC3A protein bands of 20 or 17 kDa. Other experimental conditions are described in Materials and Methods Section. B: Heparin-dependent release of CLEC3A from monolayer cultures. Confluent monolayer cultures of CLEC3A-HT1080 cells in 35 mm dishes were incubated in the serum-free medium without or with the indicated concentrations of heparin at 37°C for 3 h. CLEC3A protein released into the culture medium was analyzed as above. C: Heparin-dependent release of CLEC3A from CLEC3A-HT1080 cells in suspension. The suspension cultures of CLEC3A-HT1080 cells were incubated at 37°C for 3 h with or without 500 µg/ml heparin, and CLEC3A released into the culture supernatant was analyzed as above.
Because CLEC3A contains a region rich in basic amino acids in the NH2-terminal sequence (residues 26–38), it may interact with heparan sulfate proteoglycans on cell membrane and ECM. When CLEC3A-HT1080 cells were treated with increasing concentrations of heparin for 3 h, the amount of CLEC3A released into the culture medium increased dose-dependently whereas that found in the cell lysate slightly decreased (Fig. 3B). The amount of CLEC3A in the culture medium clearly increased even when CLEC3A-HT1080 cells were treated with heparin for 3 h in suspension culture (Fig. 3C). When CLEC3A-HT1080 cells were subjected to the cell-based ELISA analysis with the anti-FLAG antibody, the antigen signal was clearly detected (data not shown). All these results indicated that CLEC3A exists on the tumor cell surface, probably binding to heparan sulfate proteoglycans such as syndecans and glypican. DLD-1 cells transfected with the CLEC3A cDNA similarly produced the recombinant CLEC3A protein (data not shown).
Cleavage of CLEC3A by Matrilysin and Other Proteinases
Recombinant CLEC3A secreted from CLEC3A-HT1080 cells was purified by affinity chromatographies on an anti-FLAG M2 antibody column and then on a heparin affinity column. When the purified CLEC3A protein of 20 kDa was incubated with active MMP-7, the 20-kDa CLEC3A band was partially converted to a clear band of 15 kDa and a faint band of 10 kDa (Fig. 4A). Protein sequencing analysis of the 15-kDa, cleaved CLEC3A protein showed the NH2-terminal amino acid sequences of two protein fragments, “LKEIQALQTVCLRG” (amino acid no. 58–71) and “LQTVCLRGTKVHKK” (amino acid no. 64–77) (Fig. 1B). In this experiment, we could not detect the 5-kDa fragment of CLEC3A shown in Figure 1A, probably due to its excess degradation. These results suggest that MMP-7 cleaves at least four peptide bonds of CLEC3A, that is, Ala57–Leu58, Ala63–Leu64, Phe151–Leu152, and an unidentified bond for the 10-kDa fragment (Fig. 1B).
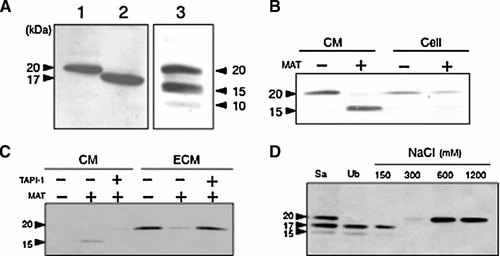
Cleavage of CLEC3A by matrilysin. A: Purified CLEC3A and its cleavage by matrilysin. Two forms of recombinant CLEC3A, the 20-kDa form (lane 1) and the 17-kDa form (lane 2), were isolated from the serum-free culture of CLEC3A-HT1080 cells. The 20-kDa CLEC3A (20 µg/ml) was digested with 100 nM matrilysin at 37°C for 3 h (lane 3). The protein samples were separated by SDS–PAGE, transferred onto a PVDF membrane, and visualized by the staining with Coomassie Brilliant Blue R250. Arrowheads indicate the individual forms of CLEC3A. The 20 and 17-kDa CLEC3A and a 15-kDa CLEC3A obtained by the matrilysin digestion (lane 3) were analyzed for the NH2-terminal amino acid sequences. The sequences determined are shown in Figure 1B. B: Cleavage of CLEC3A by matrilysin in culture. Confluent monolayer cultures of CLEC3A-HT1080 cells in 35 mm dishes were incubated in 2 ml of the serum-free medium without or with 50 nM matrilysin (MAT) at 37°C for 3 h. CLEC3A proteins released into the culture medium (CM) or remaining on the cells (Cell) were analyzed by immunoblotting. Arrowheads, 20 or 15-kDa band of CLEC3A. C: Cleavage of CLEC3A in ECM fraction by matrilysin. The ECM fraction was obtained from the cultures of CLEC3A-HT1080 cells in 35 mm dishes. The ECM fractions were treated with matrilysin (MAT) in the presence or absence of the MMP inhibitor TAPI-1 as shown above, and CLEC3A proteins released into the culture medium (CM) or remaining on ECM were analyzed. D: Affinity of CLEC3A and its proteolytic products for heparin. The serum-free conditioned medium (CM) (10 ml) obtained from confluent culture of HT1080-CLEC3A cells was concentrated 10-fold by lyophilization and incubated at 37°C for 3 h with 50 nM matrilysin (MAT) in 1 ml of 50 mM Tris–HCl buffer (pH 7.5) containing 50 mM NaCl and 5 mM CaCl2 and 0.01% Brij 35, and then the reaction was stopped by mixing with the MMP inhibitor TAPI (4 µM). The digest was applied to a heparin–Sepharose column (2 ml). Proteins bound to the column were eluted with the buffer supplemented with the indicated concentrations of NaCl. CLEC3A in these protein fractions were analyzed by immunoblotting. Sa, the digested sample applied to the heparin column; Ub, the unbound protein fraction from the column. Other experimental conditions are described in Materials and Methods Section.
To examine whether MMP-7 cleaves CLEC3A bound to cell surface and ECM, CLEC3A-HT1080 cells and a cell-free ECM fraction were individually incubated with purified MMP-7 under culture conditions without serum. These MMP-7 treatments significantly decreased the amounts of the 20-kDa CLEC3A in the cell lysate and ECM, while they increased the amounts of the 15-kDa CLEC3A fragment released into both culture media (Fig. 4B,C). The cleavage of CLEC3A by MMP-7 was inhibited by an MMP inhibitor (TAPI-1).
Next, we compared heparin-binding activities of the intact CLEC3A and its proteolytic fragments. The CM containing the 20-, 17-, and 15-kDa CLEC3A proteins, which were obtained from the MMP-7-treated culture of CLEC3A-HT1080 cells, was applied to a heparin-conjugated column. The MMP-7-cleaved, 15-kDa CLEC3A did not bind to the column (Fig. 4D). Although the 17-kDa proteolytic fragment mostly flew through the column without adsorption, it appeared to have weak affinity to the heparin column. In contrast, the 20-kDa protein strongly bound to the column and was eluted from the column at over 0.6 M NaCl. These results indicate that the heparin-binding activity of CLEC3A is localized in the NH2-terminal basic amino acid region (amino acid no. 26–40), and this region is removed from CLEC3A by the MMP-7 digestion.
We also examined whether proteinases other than MMP-7 could cleave purified CLEC3A. As shown in Figure 5, MMP-2 cleaved the 20-kDa recombinant CLEC3A to an 18-kDa form. Two other MMPs, MMP-3 and MMP-9, scarcely degraded CLEC3A, while MMP-1 appeared to degrade it weakly. As for serine proteinases, kallikrain, plasmin, thrombin, factor Xa limitedly cleaved the recombinant CLEC3A. Especially, plasmin produced a similar size of fragment to the 15-kDa fragment produced by MMP-7. Trypsin and chymotrypsin unlimitedly degraded CLEC3A (data not shown). These results suggest that CLEC3A has relatively high susceptibility to various proteinases.
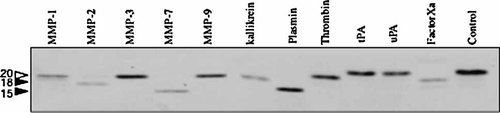
Cleavage of purified CLEC3A by various proteinases. The recombinant CLEC3A (100 nM) was incubated in 100 µl of a reaction mixture without (control) or with 2 nM each of MMP-1, MMP-2, MMP-3, MMP-9, and matrilysin (MMP-7), or 0.4 mU kallikrein, 2.2 nM plasmin, 2 U thrombin, 2.7 nM tissue-type plasminogen activator (tPA), 3.8 nM urokinase-type plasminogen activator (uPA), or 2.1 nM factor Xa at 37°C for 16 h. The digested samples were subjected to non-reducing SDS–PAGE followed by immunoblotting for CLEC3A. Arrowheads indicate the 20-, 18-, and 15-kDa forms of CLEC3A. Other experimental conditions are described in Materials and Methods Section.
Cell Adhesion Activity of Recombinant CLEC3A
Because heparin-binding proteins often modulate cell adhesion, we assayed cell adhesion activity of recombinant CLEC3A using the breast cancer cell line MCF-7. The basement membrane protein Lm332, formerly laminin-5, is know to have a very high cell adhesion activity by binding its cell surface receptors, that is, integrins α3β1, α6β4, and α6β1 [Miyazaki, 2006]. When CLEC3A and Lm332 were separately coated at various concentrations on culture plates, Lm332 strongly promoted the attachment of MCF-7 cells to the plate in serum-free medium but CLEC3A did only slightly at higher concentrations (Fig. 6A). Effective concentration of Lm332 for the half-maximal adhesion (ED50) was determined to be approximately 0.5 µg/ml for MCF-7 cells. However, when CLEC3A was co-coated on plastic plates with a low concentration (0.15 µg/ml) of Lm332, which by itself did not support the cell attachment, CLEC3A dose-dependently promoted the cell attachment (Fig. 6B). The maximal cell adhesion effect of CLEC3A was attained at 0.25 µg/ml. Morphological observation showed that CLEC3A enhanced cell spreading in the presence of a low concentration of Lm332 (Fig. 6C). Similar synergistic adhesion activity of CLEC3A was obtained with fibronectin (Fig. 6D).
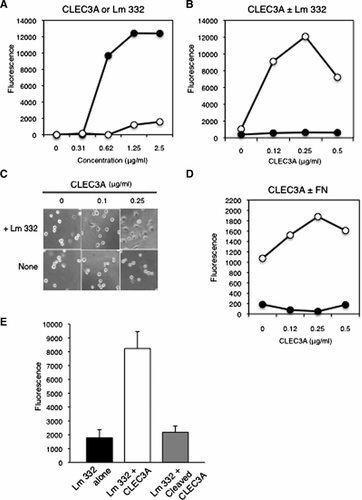
Promotion of cell adhesion by CLEC3A. A: Cell attachment activity of laminin-332 (Lm332) and CLEC3A toward MCF-7 cells. Ninety-six-well plates were coated with the indicated concentrations of Lm332 (closed circles) or CLEC3A (open circles) and incubated with MCF-7 cells in the serum-free medium at 37°C for 1 h. After the incubation, relative numbers of cells attached to the substrates were determined by measuring fluorescent intensity. Each point represents the mean ± SD for triplicate determinations. Other experimental conditions are described in Materials and Methods Section. B: Synergistic cell adhesion activity of CLEC3A with Lm332. Assay plates were coated with varied concentrations of CLEC3A with (open circles) or without (closed circles) a fixed concentration of Lm332 (0.15 µg/ml). The attachment of MCF-7 cells to these plates were analyzed as described above. C: Morphology of MCF-7 cells incubated on CLEC3A ± Lm332 substrates. Assay plates were coated with the indicated concentrations of CLEC3A with or without (none) a fixed concentration of Lm332 (0.15 µg/ml). MCF-7 cells were incubated on these substrates for 1 h and observed under a phase-contrast microscope. Original magnification is 300×. D: Synergistic cell adhesion activity of CLEC3A with fibronectin. The cell attachment assay was carried out on the plates pre-coated with the indicated concentrations of CLEC3A and with (open circles) or without (closed circles) a fixed concentration (1 µg/ml) of fibronectin, as described in (B). E: Effect of matrilysin treatment of CLEC3A on its cell adhesion activity. CLEC3A (0.25 µg/ml) was digested with 50 nM matrilysin at 37°C overnight. Assay plates were coated with 0.15 µg/ml Lm332 and without or with 0.25 µg/ml of the intact or matrilysin-cleaved CLEC3A. The attachment of MCF-7 cells to these substrates were assayed as described above.
Next, we investigated whether the CLEC3A cleavage by MMP-7 affects the synergistic cell adhesion activity of CLEC3A with Lm332. When the MMP-7-digested and undigested CLEC3A samples were co-coated with Lm332 and used as cell adhesion substrates, the synergistic cell adhesion activity with Lm332 was not evident in the digested CLEC3A compared to the intact one, indicating that the cleavage of CLEC3A by MMP-7 to the 15-kDa form extinguished the activity (Fig. 6E).
Finally, we compared cell adhesion efficiency to Lm332 and fibronectin between control HT1080 (mock-HT1080) and CLEC3A-expressing HT1080 (CLEC3A-HT1080) cell lines. CLEC3A-HT1080 cells adhered at significantly higher efficiency to both substrates than mock-HT1080 cells (Fig. 7A,B). This also showed that CLEC3A had cell adhesion activity.
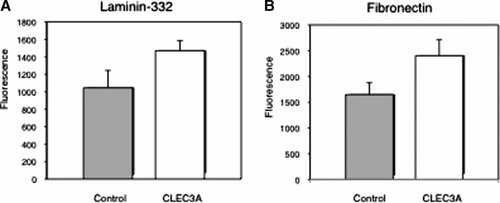
Cell adhesion activity of control and CLEC3A-expressing HT1080 cells toward Lm332 (A) and fibronectin (B). Ninety-six-well plates were coated with 0.125 µg/ml Lm332 (A) or 1 µg/ml fibronectin. (B) Control HT1080 cells (control) transfected with the empty vector and CLEC3A-HT1080 cells (CLEC3A) were incubated on the plates in the serum-free medium at 37°C for 1 h. After the incubation, relative numbers of adherent cells were determined. Each column represents the mean ± SD for triplicate determinations.
DISCUSSION
The present study identified CLEC3A, a poorly characterized member of the C-type lectin superfamily, as a novel membrane-bound and matrix-deposited substrate for the MMP member MMP-7. CLEC3A was originally identified as a cartilage protein from shark and bovine [Neame et al., 1992]. Later, the structures of human CLEC3A gene and cDNA were determined [Neame et al., 1999]. To our knowledge, no other studies have been reported regarding this protein. Although the past study showed the specific localization of CLEC3A in cartilage [Neame et al., 1999], we could detect its message in human tissues of normal breast and breast cancer as well as human cancer cell lines of the breast and colon. Therefore, it is expected that CLEC3A may play some specific roles in not only the cartilage but also in the breast and cancers.
CLEC3A is closely related to tetranectin in structure. There are considerable numbers of studies on tetranectin [Neame et al., 1999]. Tetranectin, a homotrimeric C-type lectin, was originally isolated from plasma as a protein having affinity for the kringle-4 domain of plasminogen [Clemmensen et al., 1986; Holtet et al., 1997]. It is also distributed in various tissues such as the liver, pancreas, pituitary, and thyroid [Christensen and Clemmensen, 1989]. An NH2-terminal, hydrophobic sequence of tetranectin is involved in the trimerization of this molecule [Nielsen et al., 1997], while a COOH-terminal carbohydrate recognition domain is responsible for the binding to various kringle-domain-containing proteins including plasminogen, apolipoprotein, HGF, and tPA [Kluft et al., 1989; Westergaard et al., 2003]. The NH2-terminal domain of tetranectin also contains a heparin-binding sequence [Lorentsen et al., 2000]. These structural features of tetranectin suggest a possibility that it may play in the matrix organization. In addition, its binding capacity to HGF and proteinases is expected to contribute to tissue remodeling under various pathological conditions.
In the present study, recombinant CLEC3A was found in the culture medium, ECM, and cell membrane of human cancer cell lines, which had been introduced with the CLEC3A cDNA. As expected, CLEC3A had a strong heparin-binding activity and this activity was abrogated after the cleavage by MMP-7. When CLEC3A-transfected HT1080 cells in suspension were treated with heparin, CLEC3A was increasingly released into the culture medium. This suggests that CLEC3A binds to tumor cell surface via its heparin-binding activity. Many heparin-binding proteins also bind heparan sulfate proteoglycans like syndecans on cell surface and modulate integrin function and hence cell adhesion activity of cells. We have seen such effects in two heparin-binding domains of Lm332 [Hirosaki et al., 2002; Ogawa et al., 2007] and angiomodulin (IGFBP-rP1/TAF/mac25) [Sato et al., 1999]. Purified CLEC3A protein by itself scarcely promoted cell adhesion, but it markedly promoted tumor cell adhesion and spreading onto an Lm332 or fibronectin substrate. Although it is unknown whether CLEC3A, like tetranectin, forms a homotrimer complex, we found that it produced a homodimer as analyzed by SDS–PAGE under non-reducing conditions (data not shown). Therefore, it is likely that CLEC3A links a cell surface receptor with ECM, thus enhancing cell adhesion. The synergistic effect of CLEC3A with Lm332 or fibronectin seems to result from its indirect activation of integrins through interaction with heparan sulfate proteoglycans on tumor cell surface. Indeed, we obtained data indicating that the synergistic cell adhesion activity of CLEC3A with Lm332 was completely blocked by the addition of 500 µg/ml heparin into the culture (data not shown). Thus, our results strongly suggest the possibility that CLEC3A acts as a cell adhesion modulator.
MMP-7 efficiently cleaved human recombinant CLEC3A, producing a 15-kDa COOH-terminal fragment in a test tube, which lacked its NH2-terminal, heparin-binding domain, into the culture medium. This 15-kDa form of CLEC3A was also produced in the suspension culture of CLEC3A-HT1080 cells after a short time treatment with MMP-7, indicating that MMP-7 hydrolyzed CLEC3A and released its COOH-terminal fragment. As expected, the 15-kDa CLEC3A showed neither heparin binding nor cell adhesion activity. MMP-7 is highly expressed in various types of human cancers including the colon and breast cancers [Wilson and Matrisian, 1996; Nagashima et al., 1997]. It is tempting to speculate that MMP-7 may cleave CLEC3A, leading to the release of tumor cells from the stable adhesion to surrounding ECM and the resultant enhancement in the cell migration. Many other proteinases also cleaved purified CLEC3A protein. Especially, MMP-2, plasmin, and factor Xa produced COOH-terminal CLEC3A fragments, which apparently lost the heparin-binding domain. It is also noted that both CLEC3A-HT1080 and CLEC3A-DLD-1 cells secreted a 17-kDa CLEC3A fragment into the culture medium, which had been cleaved by an endogenous proteinase within the heparin-binding sequence, besides the 20-kDa intact form. The 17-kDa CLEC3A showed little affinity for heparin. Thus, all these enzymes are capable of modulating the functions of CLEC3A in vivo. Initially, we detected a 5-kDa fragment of CLEC3A when WiDr human colon cancer cells were treated with MMP-7. However, we could not detect this fragment in the cultures of CLEC3A-HT1080 cells after treatment with MMP-7. This might be due to further degradation of the 5-kDa fragment in WiDr cells. Alternatively, other proteinases, which were activated by MMP-7, might be responsible for the production of this fragment.
MMP-7 hydrolyzes a broad range of matrix and non-matrix substrates [Miyazaki et al., 1990; Fosang et al., 1992; Sires et al., 1993; Powell et al., 1999; Sawey et al., 2007]. The active form of MMP-7 efficiently binds to cholesterol sulfate on tumor cell membrane, having its enzyme activity [Higashi et al., 2008]. We recently found that MMP-7 cleaves annexin II bound onto tumor cell membrane, leading to a concomitant enhancement in the binding of tPA to the tumor cell surface [Tsunezumi et al., 2008]. In this respect, tetranectin is known to be a plasminogen-binding protein. Preliminary, we examined whether CLEC3A could bind plasminogen. Immunoprecipitation using the anti-FLAG antibody showed the association of both intact and MMP-7-cleaved CLEC3A proteins with plasminogen (data not shown). However, these interactions were found only in the absence of calcium ion. Therefore, it remains to be clarified whether CLEC3A is able to modulate the plasminogen/plasminogen-activator system in physiological conditions.
In conclusion, we first identified CLEC3A as a membrane-bound substrate for MMP-7 and showed that CLEC3A is a heparin-binding, cell adhesion modulator. It can be speculated that the cleavage of CLEC3A by MMP-7 in tumor microenvironments may affect tumor cell invasion and metastasis by modulating tumor cell adhesion and the plasminogen/plasminogen-activator system.
Acknowledgements
We thank Dr. Yuichiro Sato and Kayano Moriyama for technical support of plasmid construction and protein sequencing. We are grateful to Dr. Kazuhiro Yamamoto and Dr. Takashi Ogawa for helpful discussion. This work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan.




