Adenosine triggers the nuclear translocation of protein kinase C epsilon in H9c2 cardiomyoblasts with the loss of phosphorylation at Ser729
Abstract
Adenosine is a major mediator of ischaemic preconditioning (IPC) and cardioprotection. The translocation and activation of protein kinase C epsilon, triggered by adenosine, are essential for these processes. We report here that H9c2 cardiomyoblasts express five PKC isoforms (α, βI, δ, ε and ζ). PKCε is predominantly associated with F-actin fibres in unstimulated H9c2 cells but translocates to the nucleus on stimulation with adenosine. Cytosolic PKCε associated with F-actin fibres is phosphorylated at Ser729 but nuclear PKCε lacks phosphorylation at this site. Adenosine triggers the nuclear translocation after 5 min stimulation. PKCε Ser729Ala and Ser729Glu mutants showed no translocation on adenosine stimulation suggesting both phosphorylation and serine at 729 are critical for this translocation. Among five PKC isoforms (α, βI, δ, ε and ζ) detected, PKCε is the only isoform translocating to the nucleus upon adenosine stimulation. Disruption of microtubules (MTs), but not F-actin-rich fibres, blocked translocation of both endogenous PKCε and overexpressed GFP-PKCε to the nucleus. Ten proteins interacted with cytosolic PKCε; five of which are components of myofibrils. Matrin 3 and vimentin interacted with nuclear PKCε. These findings suggest that adenosine stimulates PKCε translocation to the nucleus in H9c2 cells in a mechanism involving dephosphorylation at Ser729 and MT, which should advance our understanding of the signalling pathways stimulated by adenosine in IPC and cardioprotection. J. Cell. Biochem. 106: 633–642, 2009. © 2009 Wiley-Liss, Inc.
Adenosine is produced by tissues under stress and functions to increase energy supply by vasodilatation and to decrease energy demand [Mubagwa and Flameng, 2001]. Adenosine also protects heart tissue from ischaemic damage and mimics ischaemic preconditioning (IPC) in cardiomyocytes [Bullough et al., 1995; Mubagwa and Flameng, 2001; Donato and Gelpi, 2003]. Adenosine mediates its anti-ischaemic function by interacting with various G-protein-linked receptor subtypes that are coupled to multiple effectors, which include protein kinases, phospholipases, KATP channels, transporters and the cytoskeleton [Liu et al., 1997; Schulz et al., 2001; Baxter, 2002; Martin-Garcia, 2005]. Adenosine is released during preconditioning and its protective action involves the activation of protein kinase C, notably the δ and ε isoforms and their translocation to sarcolemmal and mitochondrial membranes [Takashi et al., 1999; Kloner and Jennings, 2001a,b; Mubagwa and Flameng, 2001; Uchiyama et al., 2003]. PKC translocation leads to increased opening of mitochondrial KATP channels, which contributes to the protective action of preconditioning [Liu et al., 1997; Uchiyama et al., 2003; Martin-Garcia, 2005]. PKCε is implicated in the mechanism of preconditioning and cardioprotection [Mubagwa and Flameng, 2001; Schulz et al., 2001; Baines et al., 2002; Baxter, 2002; Uchiyama et al., 2003; Martin-Garcia, 2005]. IPC causes PKCε translocation to a cell particulate fraction [Wolfrum et al., 2002], which has been more specifically identified as myofibril/nuclear [Yoshida et al., 1996], nuclear [Albert and Ford, 1998], or sarcolemma/mitochondria [Baines et al., 2002]. Adenosine stimulation of cardiomyocytes also induces PKCε translocation to a particulate fraction [Nawas et al., 2000] and to the sarcolemma [Lester and Hofmann, 2000]. In anaesthetic-induced preconditioning isoflurane induces PKCε translocation to the sarcolemma and mitochondria with effects on sarcolemmal KATP channels [Aizawa et al., 2004]. We have shown previously that activation of PKCε causes translocation to the nucleus [Xu and Rumsby, 2004; Xu et al., 2007] so here we have investigated whether adenosine stimulates PKCε translocation to the nucleus in H9c2 cardiomyoblasts and we have asked what proteins associate with PKCε in the cytosol and nuclear compartments.
PKCε requires priming for activation by a series of phosphorylations in the catalytic domain at Thr566, Thr710 and Ser729 [Newton, 2001]. The initial phosphorylation at Thr566 is effected by phosphoinositide-dependent kinase-1 [Newton, 2001]. Phosphorylation of the remaining sites then occurs by autophosphorylation though, in neonatal ventricular cardiomyocytes, Ser729 can be phosphorylated by PKCδ [Rybin et al., 2003]. Phosphorylation at Ser729 increases in cardioprotection induced by moderate ethanol intake [Zhou et al., 2002]. In 3T3 fibroblasts we have found that Ser729 phosphorylation is important for PKCε localisation at the Golgi [England and Rumsby, 2000; England et al., 2001; Xu et al., 2007], loss of the Ser729 phosphate being associated with PKCε translocation to the cell periphery or nucleus [Xu et al., 2007]. It was thus of interest to determine if adenosine stimulation of cardiomyoblasts results in changes in phosphorylation of PKCε at Ser729.
Translocation of PKC isoforms involves the cytoskeleton. For example, movement of PKCα into the nucleus of 3T3 fibroblasts requires an intact cytoskeleton [Schmalz et al., 1996] while, in isolated perfused hearts, disruption of microtubules (MTs) by colchicine prevents IPC-induced translocation of PKCε [Nakamura et al., 2004]. A RACK 2 may be involved in anchoring PKCε to cardiac myofilaments [Huang and Walker, 2004]. PKCε regulates the cytoskeleton since proteins such as desmin, myosin light chain, troponins I and T and tropomyosin are PKC substrates [Huang et al., 2002; Huang and Walker, 2004]. Overexpression of PKCε alters myofilament properties and composition leading to heart failure [Goldspink et al., 2004] while, on activation, PKCε translocates to cardiac myofilaments to phosphorylate troponin I, troponin T and myosin light chain 2 [Pyle et al., 2002; Metzger and Westfall, 2004; Roman et al., 2004]. Phosphorylation of troponin I may decrease Ca2+-dependent actomyosin ATPase activity [Lester and Hofmann, 2000]. Thus, PKCε can regulate cytoskeleton structure and function in a complex manner.
Here we show that adenosine stimulation of H9c2 line cardiomyoblasts results in translocation of PKCε from F-actin fibres to the nucleus and that this movement requires MTs and dephosphorylation at Ser729. We have also identified 10 proteins, which interact with cytosolic PKCε; 5 of which are components of myofibrils. In the nucleus we find that matrin 3 and vimentin are associated with PKCε.
MATERIALS AND METHODS
Materials
H9c2 cells were from the American Type Culture Collection (Manassas, VA). Cell culture plastic, four-chamber culture slides and coverglasses were from Nunc (Life Technologies, Paisley, UK). Cell culture reagents were from Gibco BRL (Life Technologies). BCA protein quantification reagents were from Pierce (Luton, UK). The polyclonal antibody to PKCε and peroxidase-conjugated secondary antibodies were from Sigma. The anti-phospho-PKCε (Ser-729) antibody was from Upstate (Milton Keynes, UK). The antibodies for other PKC isoforms were from Santa Cruz. All chemicals were from Sigma unless otherwise stated.
Cell Culture and Treatment
H9c2 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal calf serum and antibiotics in a humidified incubator at 5% CO2. For passage cells were rinsed with warm Tris–saline and released with Tris–trypsin. Cells were resuspended in fresh DMEM/10% FBS and plated into four-chamber culture slides or plastic dishes for 24 h before stimulation with different reagents as follows: 50 µM arachidonic acid (AA) for 20 min; 100 µM adenosine for 20 min; bradykinin 1 µM for 30 min; nocodozole 10 µg/ml for 1 h then plus adenosine 100 µM for 20 min; cytochalasin D 2 µM for 20 min then plus adenosine 100 µM for 20 min.
Construction and Mutation of GFP-PKCε Plasmid
The GFP-PKCε plasmid was constructed as described previously [Xu and Rumsby, 2004]. The mutations Ser729Ala and Ser729Glu were introduced by PCR using a Quikchange Site-Directed Mutagenesis Kit from Stratagene (La Jolla, CA). The mutagenic sense primers were 5′-AGAATTCAAAGGCTTCGCCTACTTTGGTGAAGACC-3′ (S729A), 5′-CCAGGAAGAATTCAAAGGCTTCGAATACTTTGGTGAAGACCTGATGC-3′ (S729E). The mutagenic antisense primers were 5′-GGTCTTCACCAAAGTAGGCGAAGCCTTTGAATTCT-3′ (S729A), 5′-GCATCAGGTCTTCACCAAAGTATTCGAAGCCTTTGAATTCTTCCTGG-3′ (S729E). The mutations were verified by sequencing.
Expression of GFP-PKCε and Its Mutants in H9c2 Cells
Plasmids were purified using a Spin Miniprep Kit from Qiagen (Crawley, UK). H9c2 cells at about 60% confluency in four-chamber slides were transfected with plasmids using Polyfect Transfection Reagent (Qiagen), according to the manufacture's protocol. Fluorescence from GFP-PKCε and the mutants was detectable 12 h after transfection. Cells were treated 24 h after transfection.
Immunofluorescence
Control normal and transfected H9c2 cells were treated with 100 µM adenosine or vehicle for 20 min. Cells were rinsed twice with warm (37°C) PBS, fixed with 4% (w/v) paraformadehyde in PBS for 15 min, quenched in 50 mM NH4Cl and permeablised with 0.2% (w/v) Triton X-100 for 4 min. To detect F-actin, cells were stained with 0.2 µg/ml phalloidin-tetramethylrhodamine B isothiocyanate conjugate (Sigma) in PBS for 30 min. After three washes with PBS, slides of transfected cells were mounted in anti-fade mounting medium (Dako, Ely, UK), coverslips were added, and sealed with nail vanish. For normal non-transfected cells, slides were blocked with 2% BSA plus 5% normal goat serum for 1 h and incubated with Pan-PKCε antibody (1:500) or anti-phospho-PKCε (Serine-729) antibody (1:100) in 2% BSA for 1 h and then labelled with a Cy2-conjugated secondary antibody in 2% BSA. After three rinses with PBS, the slides were mounted and sealed as above.
Confocal Analysis
Stained slides were examined using a Zeiss confocal laser scanning microscope (LSM 510 META; Carl Zeiss, Jena, Germany). GFP and Cy2 fluorescence was monitored at a 488-nm excitation wavelength with a 505- to 530-nm band pass barrier filter. Phalloidin-TRITC was monitored at a 543-nm excitation wavelength with a 560-nm long pass barrier filter.
Live Cell Fluorescence Imaging
H9c2 cells were seeded on four-chamber coverglasses and transfected with GFP-PKCε or its Ser729 mutants, 24 h after transfection, cells were treated with 100 µM adenosine for the time indicated, the translocation of GFP-PKCε were monitored by Epifluorescent microscope (Nicon) with the excitation wavelength at 490 nm.
Isolation of Nuclei
Nuclei were purified as described previously [Xu and Rumsby, 2004]. Briefly, cells treated with different agents were scraped from dishes in cold PBS and pelleted by centrifugation at 700g for 5 min. Pelleted cells were resuspended in five volumes hypotonic buffer (15 mM Tris–HCl, 15 mM NaCl, 60 mM KCl, 0.5 mM EDTA, pH 7.4, plus protease inhibitor cocktail) and left on ice for 15 min. Cells were then lysed with Triton X-100 (final concentration 0.5%) and passed several times through a 23-gauge needle. Nuclei were pelleted at 1,000g for 10 min. The supernatant was designated the postnuclear fraction. Pelleted nuclei were washed twice by resuspension in cold PBS and centrifuged at 25,000g for 20 min to remove any residual cytoplasmic material. The purity of nuclear faction was monitored by blotting with α-tubulin as described previously [Xu and Rumsby, 2004].
Co-Immunoprecipitation of Proteins Associating With PKCε and MALDI–TOF-MS Analysis
H9c2 cells were scraped from dishes in immunoprecipitation buffer (50 mM Tris–HCl, 300 mM NaCl, 5 mM EDTA, 1% Triton X-100, plus protease inhibitor cocktail, pH 7.4), passed several times through a 23-gauge needle and then incubated on ice for 30 min. The preparation was then centrifuged and the supernatant made 0.1% with SDS and set on ice for another 30 min. For a nuclear lysate, pelleted nuclei were lysed in immunoprecipitation buffer as above with 0.2% SDS and passed several times through a 25-gauge needle. The lysate was centrifuged at 14,000g for 10 min and the supernatant was analysed for protein content (BCA assay) and then precleared with protein G-sepharose (PGS) for 1 h. The precleared lysate was divided into two samples, each containing equal amounts of protein. One sample was incubated with anti-PKCε antibody (8 µg/ml in final concentration) and the other with rabbit IgG for 2 h. Antibody complexes were recovered with PGS beads. After rinsing the beads three times with immunoprecipitation buffer, proteins were solubilised in Laemmli sample buffer and heated. Immunoprecipitates were resolved by SDS–PAGE (8–18% gels), and proteins were located by silver staining. Target bands were excised into 1 mm blocks. After destaining, the blocks were dehydrated with acetonitrile. Samples were digested with trypsin (Promega, Madison) overnight at 37°C. A 1 µl aliquot of digestion supernatant was spotted onto a MALDI–TOF–MS sample plate with 0.6 µl matrix and allowed to air-dry. An Applied Biosystems 4700 Proteomics Analyser, coupled with tandem mass spectrometry (MS/MS), was used to generate peptide mass fingerprints. These were searched against the NCBInr public database. A mass fingerprint was considered a significant match if the score was ≥75.
Western Blotting
H9c2 cells were treated with different agents, followed by cell fractionation as above. Equal amounts of protein (60 µg) from both postnuclear and nuclear fractions were resolved by SDS–PAGE on 7.5% gels, followed by immunoblotting and probing for PKCε using an anti-Pan-PKCε antibody (1:5,000) or an anti-phospho-Ser729-PKCε antibody (1:1,000).
Results
H9c2 Cells Express Five PKC Isoforms
H9c2 line cardiomyoblasts, derived from rat cardiac tissue, express PKC α, βI, δ, ε and ζ, as detected by Western blotting using brain lysate, 3T3, HeLa and muscle cells as positive controls (Fig. 1). We also probed for PKCβII, PKCγ, PKCη, PKCθ and PKCλ but these isoforms were not detected (Fig. 1). H9c2 cells thus express a range of PKC isoforms especially PKCε and PKCδ, which are implicated in IPC.
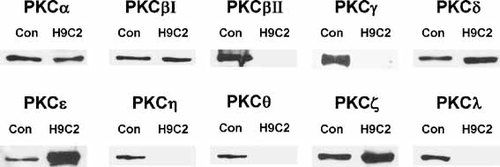
H9c2 cells highly express five PKC isoforms. H9c2 cells were extracted and 60 µg total protein resolved against control cells and tissue as described in the Materials and Methods. Control tissue/cells for PKCα, βj, δ, ε were 3T3 cell lysates; the control tissue/cells for PKCβII, γ. η were rat brain lysates; the control tissue/cells for PKCθ were rat muscle lysate; the control tissue/cells for PKCζ, λ were HeLa cell lyates.
Adenosine Induces PKCε Translocation to the Nucleus With Dephosphorylation at Ser729
We stimulated H9c2 cells with adenosine, AA and bradykinin and examined PKCε translocation. As shown in a typical result in Figure 2A, stimulation with 100 µM adenosine increased nuclear PKCε (about twofold as judged by densitometry) compared with untreated H9c2 cells, as did stimulation with 1 µM bradykinin (about 1.5-fold), which, like adenosine, is released during IPC. Stimulation of cells with 50 µM AA did not cause significant nuclear translocation of PKCε compared with unstimulated cells. The translocating effect of adenosine was confirmed in a second experiment (Fig. 2B) in which the phosphorylation status at Ser729 of cytosolic and nuclear PKCε was examined. The results again show that adenosine stimulates PKCε translocation to the nucleus. It is also clear that in nuclear PKCε Ser729 is unphosphorylated compared with PKCε in the postnuclear fraction (lower lane), which consists of cytosol, various organelles, plasma membrane and cytoskeletal components. The densitometry bar diagram of Pan-PKCε in postnuclear and nuclear factions (Fig. 2C) showed that adenosine stimulation decreased Pan-PKCε levels in postnuclear fractions (*P < 0.05) and increased Pan-PKCε levels in nuclear fractions (**P < 0.01, Student's t-test). To investigate if other PKC isoforms also translocate to the nucleus upon adenosine treatment, we blotted our postnuclear fractions and nuclear fraction for all four other PKC isoforms, as shown in Figure 2D. Nuclear PKCα was undetectable in H9c2 cells and no significant translocation of PKCβI PKCδ and PKCζ occurred upon adenosine stimulation. The result suggest that PKCε is the only isoform translocating to the nucleus upon adenosine stimulation.
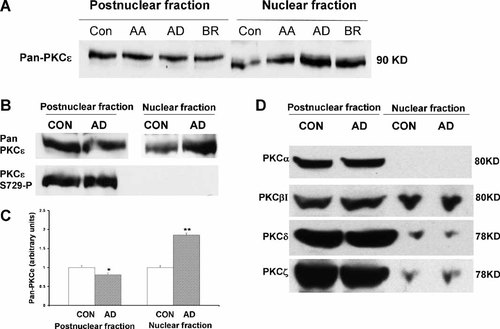
Adenosine induces the nuclear translocation of PKCε accompanied by dephosphorylation at Ser729. A: H9c2 cells were cultured as described in the Materials and Methods and stimulated with adenosine (AD), arachidonic acid (AA) or bradykinin (BR) as described. Cells were harvested and nuclear and postnuclear fractions recovered for resolution of proteins and Western blotting for PKCε. Adenosine and bradykinin increase the nuclear content of PKCε compared with control and AA treated cells. B: Fractions as above were probed again for PKCε and also for PKCε phosphorylated at Ser729. Again adenosine induces PKCε translocation to the nucleus (upper lanes). PKCε in the postnuclear fraction is phosphorylated at Ser729 whereas that in the nucleus is not (lower lanes). C: The bar diagram of Pan-PKCε levels in postnuclear and nuclear fractions upon adenosine stimulation. Results were mean ± SD from three independent experiments. D: The nuclear translocation of all other four PKC isoforms expressing in H9c2 cells were also monitored, nuclear PKCα was undetectable in H9c2 cells and no significant translocation of PKCβI PKCδ and PKCζ occurred upon adenosine stimulation.
PKCε in the Cytosol Is Associated With F-Actin Fibres and Translocates to the Nucleus on Adenosine Stimulation
We transfected H9c2 cells with a GFP-PKCε construct to define the intracellular localisation of the kinase. As shown in Figure 3A, the GFP-PKCε was organised in a linear pattern along the long axis of the cells (Fig. 3A, Con). Staining for F-actin with phalliodin-tetramethylrhodamine B isothiocyanate conjugate revealed a similar pattern of linear fibres. The merged image suggests that GFP-PKCε and F-actin co-localise to these F-actin-rich fibres (merged control). There was little background fluorescence in the cytosol of cells transfected with the GFP-PKCε construct suggesting that most of the kinase was predominately associated with the F-actin-rich fibres. Treatment of H9c2 cells with adenosine triggered the translocation of the GFP-PKCε to the nucleus from the cytosolic fibres. Merged images showed that PKCε and F-actin no longer co-localise. These fluorescence results confirm the Western results in Figure 2 and suggest that the source of the PKCε, which translocates to the nucleus on adenosine stimulation is the F-actin-rich fibres in the cytosol.
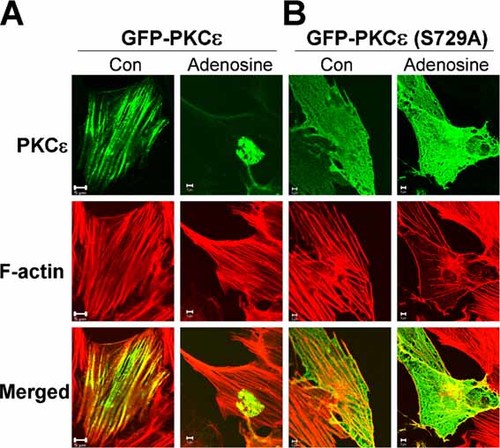
Adenosine triggers the nuclear translocation of GFP-PKCε from actin-rich fibres to the nucleus. A: H9c2 cells were transfected with a GFP-PKCε construct as described in the Materials and Methods and the localisation of the GFP-PKCε construct compared with that of F-actin. In control cells the GFP-PKCε is aligned in linear arrays running the length of the cells. F-actin is associated with linear fibres (stress fibres) running throughout the cytosol. The merged image indicates that PKCε co-localises with F-actin on these stress fibres. Adenosine stimulation has no effect on F-actin organisation but causes PKCε translocation to the nucleus. B: A GFP-PKCε S729A mutant was prepared as described in the Materials and Methods and transfected into H9c2 cells. The distribution of this GFP-PKCε (S729A) construct was different from that of control GFP-PKCε in A and did not co-localise with F-actin stress fibres, no obvious nuclear translocation occurred after adenosine stimulation. Scale bar represents 5 µm.
To examine the role of Ser729 phosphorylation on PKCε subcellular localisation and translocation, we mutated Ser729 to Ala, to prevent phosphorylation at this site. As shown in Figure 3B we observed that the GFP-PKCε S729A mutant was distributed in a lace-like pattern throughout cells compared with the linear distribution of the GFP-PKCε construct. Further the S729A mutant GFP-PKCε showed no tendency to translocate to the nucleus on adenosine stimulation compared with GFP-PKCε (Fig. 3B). F-actin organisation in mutant-transfected cells was the same as in cells transfected with the normal GFP-PKCε construct, that is, in linear fibres. The merged image in Figure 3B shows that mutant S729A PKCε and F-actin did not co-localise suggesting that Ser729 and phosphorylation are important for the interaction of PKCε with the F-actin-rich fibres. GFP alone distributed throughout cells and did not translocate on adenosine stimulation as we described previously [Xu et al., 2007].
Immunofluorescence Confirmed Nuclear PKCε Was Dephosphorylated at Ser729
An anti-phospho PKCε (Ser729) antibody was used to define phosphorylation at Ser729 in PKCε before and after stimulation of H9c2 cells with adenosine. A Pan-PKCε antibody revealed that the kinase in unstimulated cells is localised in the nucleus and throughout the cytoplasm where the pattern of fluorescence suggests that the PKCε is associated with linear fibres running the length of the cell. F-actin was organised as revealed in Figure 3 and the merged image shows some co-localisation of F-actin and PKCε confirming the results also shown in Figure 3 for GFP-PKCε. Adenosine stimulation resulted in some loss of PKCε from the cytosol to the nucleus and there was no longer significant co-localisation of F-actin filaments and PKCε throughout the cell body (Fig. 4A). In cells probed with an anti-phospho-PKCε (Ser729) antibody, we found PKCε phosphorylated at Ser729 was predominantly localised in cytosol (Fig. 4B, Con) and was not detected in the nucleus. In adenosine-treated cells, fluorescence in the cytosol appeared decreased but was not increased in the nucleus in agreement with the Western blot results in Figure 2. In the merged image of unstimulated cells (Fig. 4B) PKCε phosphorylated at Ser729 and F-actin fibres appear to be co-localised though no PKCε phosphorylated at Ser729 was detected in the nucleus.
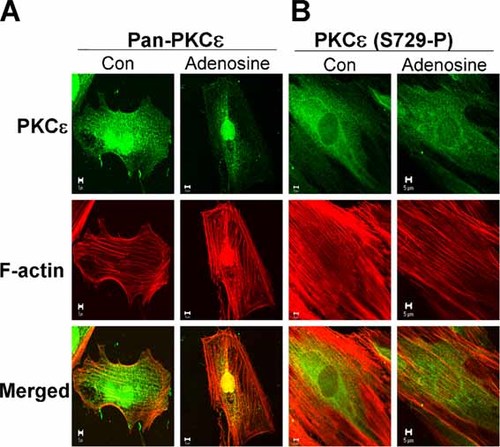
Immunofluorescence confirmed nuclear PKCε was dephosphorylated at Ser729. A: Immunofluorescence detection of PKCε in H9c2 cells was undertaken as described in the Materials and Methods. Using a Pan-PKCε antibody the results reveal that the kinase is distributed in cytoplasm where it appears to be arranged in a linear pattern and in the nucleus (Con). F-actin is distributed as shown in Figure 3. The merged image suggests that PKCε may co-localise with F-actin to stress fibres. Adenosine triggers PKCε translocation from stress fibres to the nucleus (Adenosine). B: An anti-phospho PKCε Ser729 antibody revealed that PKCε phosphorylated at S729 is predominantly localised in the cell body in a punctuate distribution and is not detected in the nucleus. The merged image shows some co-localisation with F-actin on stress fibres. No PKCε phosphorylated at Ser729 was detected in the nucleus after adenosine stimulation. Scale bar represents 5 µm.
GFP-PKCε, But Not Its S729A Nor Its S729E Mutants, Translocates to the Nucleus Upon Adenosine Stimulation
To further investigate the mechanism of PKCε translocation and the exact time point of PKCε translocation. We mutated Ser729 to glutamic acid, expecting the negative charge of glutamic acid can mimic phosphorylation on Ser729, and monitored the real-time translocation of GFP-PKCε by live cell fluorescence imaging. As shown in Figure 5, wild-type GFP-PKCε was located in the cytosol, and the nuclei were clearly distinguishable with some fibres passing over them (red arrow), GFP-PKCε began to translocate the nuclei around 5 min with a dramatic increase of fluorescence in the nuclei and a slight decrease of fluorescence in the cytosol. The nuclei were hardly distinguishable from the cytosol at 10–20 min, suggesting the further translocation of GFP-PKCε. While GFP-PKCε Ser729Ala was located in the whole cell with no distinguishable nuclei, adenosine treatment failed to induce further translocation. The distribution of GFP-PKCε Ser729Glu was similar to GFP-PKCε Ser729Ala with slightly distinguishable nuclei, and no translocation after adenosine treatment.
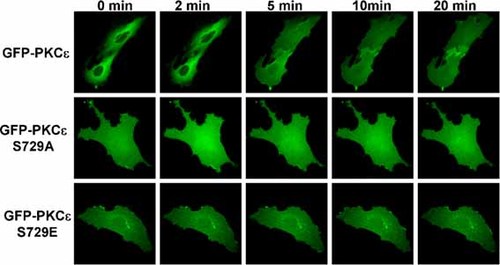
GFP-PKCε, but not its S729A nor its S729E mutants, translocates to the nucleus on adenosine stimulation. H9c2 cells transfected with GFP-PKCε or its mutants in chambered coverglasses were treated with 100 µM adenosine, the fluorescent images were taken at 0, 2, 5, 10 and 20 min. Wild-type GFP-PKCε was located in the cytosol, and the nuclei were clearly distinguishable with some fibres passing over them (red arrow), GFP-PKCε began to translocate the nuclei around 5 min with a dramatic increase of fluorescence in the nuclei and a slight decrease of fluorescence in the cytosol. The nuclei were hardly distinguishable from the cytosol at 10–20 min, suggesting the further translocation of GFP-PKCε. While GFP-PKCε Ser729Ala was located in the whole cell with no distinguishable nuclei, adenosine treatment failed to induce further translocation. The distribution of GFP-PKCε Ser729Glu was similar to GFP-PKCε Ser729Ala with slightly distinguishable nuclei, and no translocation after adenosine treatment.
The live cell fluorescence images of Figure 5 were slightly different to the fixed cell confocal images of Figure 3, which F-actin was stained with phalloidin-tetramethylrhodamine B isothiocyanate conjugate. In Figure 3, GFP-PKCε was localized more clearly on F-actin rich fibres and the translocation of GFP-PKCε looked more dramatic. This difference may be due to the better resolution and better focusing of confocal microscopy.
Microtubules Are Involved in PKCε Nuclear Translocation
To address whether MTs and/or the actin-rich fibres are involved in adenosine-induced nuclear translocation of PKCε, cells were treated with nocodozole or cytochalasin D and then stimulated with adenosine. Nuclear and postnuclear fractions were then analysed for PKCε by Western blotting. As shown in Figure 6A, adenosine or nocodozole treatment did not alter cell morphology, while cytochalasin D induced a dramatic change in shape. Cells were reduced in size and had extended several long fine processes. Western blot results in Figure 6B (upper panel) indicate that, as reported above, adenosine triggered translocation of PKCε to the nucleus but that this movement was reduced by initial treatment of cells with nocodozole, PKCε remaining in the postnuclear fraction. Disruption of F-actin-rich fibres with cytochalasin D did not have such a marked effect on PKCε translocation induced by adenosine even though it caused a dramatic morphology change. To further investigate the involvement of microtububles in PKCε nuclear translocation, we transfected H9c2 cells with GFP-PKCε and tested if nocodozole can also block GFP-PKCε translocation, as shown in Figure 6B (lower panel), the general translocation pattern of GFP-PKCε is similar to that of endogenous PKCε, adenosine triggers the nuclear translocation of PKCε, which is blocked by nocodozole but not cytochalasin D, the nuclear translocation of GFP-PKCε is even more dramatic to that of endogenous PKCε which is consistent with and Figure 5 (upper panel) showing almost no fluorescence in nuclei before adenosine treatment.
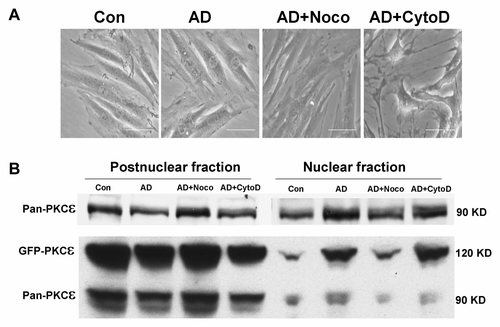
Microtubules are involved in the nuclear translocation of PKCε. H9c2 cells were cultured and treated with nocodozole or cytochalasin D before stimulation with adenosine as described in the Materials and Methods. A: Effects on cell morphology. Adenosine or nocodozole had no effect on cell morphology while cytochalasin D caused cells to shrink and extend long processes. Scale bar represents 5 µm. B (upper panel): Nuclear and postnuclear fractions were recovered from treated cells and analysed for PKCε by Western blotting. Adenosine triggers the nuclear translocation of PKCε (nuclear AD), while nocodozole reduced this translocation (nuclear AD + Noco). Cytochalasin D does not inhibit the nuclear translocation of PKCε through it causes dramatic changes in cell shape. B (lower panel): the general translocation pattern of GFP-PKCε is similar to the of endogenous PKCε, adenosine triggers the nuclear translocation of both endogenous PKCε and GFP-PKCε, which is blocked by nocodozole but not cytochalasin D, the nuclear translocation of GFP-PKCε is even more dramatic than that of endogenous PKCε. Result is a typical of three repeats.
Identification of Proteins Associating With PKCε in the Cytosol and Nucleus
To get a better understanding of the role of PKCε in cardiomycytes, we immunoprecipitated the kinase and characterised major proteins associated with the kinase in the postnuclear and nuclear fractions. Ten proteins co-immunoprecipitated with PKCε from the postnuclear fraction. These were identified by MALDI–TOF–MS (Table I) as myosin heavy chain, drebrin 1, gelsolin, caldesmon 1, Grp75, vimentin, β-actin, tropomyosin 1, myosin regulatory light chain and myosin alkali light chain. Full details of the accession numbers of these proteins, their molecular size and their protein score in MALDI–TOF–MS as well as a possible role in H9c2 cells are described in Table I. Nuclei were isolated from adenosine-stimulated cells and PKCε immunoprecipitated. Two proteins co-immunoprecipitated with the PKCε. These were identified as matrin 3′ and vimentin as shown in Table II where accession numbers and MALDI–TOF–MS scores are given.
| Protein | Accession number | Molecular weight (kDa) | Protein score in MALDI–TOF–MS | Possible role in H9c2 |
|---|---|---|---|---|
| Myosin heavy chain | gi|639999 | 200 | 737 | Myofibril component |
| Drebrin 1 | gi|13591936 | 95 | 419 | Inhibits tropomyosin-actin binding |
| Gelsolin | gi|34853856 | 90 | 206 | Actin-depolymerising factor |
| Caldesmon 1 | gi|6978589 | 60 | 233 | Stimulates polymerisation of actin |
| Grp75 | gi|1000439 | 75 | 247 | Ischaemia protection |
| Vimentin | gi|14389299 | 55 | 815 | Cytoskeleton component |
| Actin beta | gi|55575 | 42 | 330 | Myofibril component |
| Tropomyosin 1 | gi|71601 | 35 | 859 | Myofibril component |
| Myosin regulatory light chain | gi|8393781 | 19 | 293 | Myofibril component |
| Myosin alkali light chain | gi|13487933 | 16 | 403 | Myofibril component |
| Protein | Accession number | Molecular weight (kDa) | Protein score in MALDI–TOF–MS | Possible role in nucleus |
|---|---|---|---|---|
| Matrin 3 | gi|9506881 | 125 | 181 | RNA splicing |
| Vimentin | gi|14389299 | 55 | 115 | Chromatin organisation |
Discussion
H9c2 line cardiomyoblasts, derived originally from rat cardiac tissue, have been frequently used as a model heart cell system to investigate signalling pathways in IPC [Sakamoto et al., 1998; Sueur et al., 2005]. Their morphological, biochemical and electrophysiological characteristics have been defined [Hescheler et al., 1991]; it was concluded that these cells are suitable for investigating aspects of signal transduction in heart muscle cells even though their morphology is more similar to embryonic cardiomyoblasts and they lack myofibrils with obvious sarcomeres. Our results reveal that H9c2 cells express a range of PKC isoforms including PKCδ and PKCε, which are linked to the signal transduction pathways regulating IPC [Mubagwa and Flameng, 2001; Schulz et al., 2001; Baxter, 2002; Aizawa et al., 2004; Martin-Garcia, 2005]. H9c2 cells are therefore a reasonable model system for investigating the translocation of PKCε induced by adenosine.
Our results show that in unstimulated H9c2 cells PKCε in the nucleus is not phosphorylated at Ser729 while in the cytosol the PKCε, which is largely associated with F-actin-rich structures defined as stress fibres [Hescheler et al., 1991], is phosphorylated at Ser729. Such observations suggest that removal of the phosphate at Ser729 is important for nuclear translocation. We found a similar pattern in fibroblasts [Xu et al., 2007]. In addition, we have also shown [Xu et al., 2007] that PKCε localisation to the Golgi in fibroblasts requires phosphorylation at Ser729 while translocation from this site to the cell membrane involves the loss of the Ser729 phosphate. Our results here and in our other studies suggest that phosphorylation at Ser729 is important for localising PKCε to organelles and stress fibres in the cell body and that dephosphorylation at the Ser729 site is needed for translocation [England and Rumsby, 2000; England et al., 2001]. It is of interest that PKCδ is expressed by H9c2 cardiomyoblasts since in neonatal ventricular cardiomyocytes Ser729 in PKCε can be phosphorylated by PKCδ [Rybin et al., 2003]. However, autophosphorylation and/or another kinase are also possibilities for phosphorylation at Ser729. A PP2A catalytic subunit may be responsible for dephosphorylation at Ser729 in vitro [Cenni et al., 2002; Mumby, 2007].
We have shown that in both 3T3 fibroblasts and H9c2 cardiomyoblasts removal of a Ser729 phosphate accompanies translocation while PKCε associated with actin-rich fibres in the cytosol or the Golgi is phosphorylated at Ser729 [England et al., 2001; Xu et al., 2007]. Other cell types need to be analysed to determine if this is a common feature of PKCε translocation. In rat heart tissue IPC induces PKCε translocation to the nucleus [Uecker et al., 2003] indicating that the same translocation that we observe in H9c2 line cardiomyoblasts occurs in heart tissue cells in situ. Thus, PKCε translocation to the nucleus may be of importance in the mechanisms controlling IPC. Uecker et al. 2003 noted that overall phosphorylation at Ser729 in PKCε did not change during IPC though the phosphorylation status of nuclear PKCε was not specifically examined in this study.
We note that the largely cytosolic distribution of GFP-PKCε in control cells (Fig. 3A, upper left panel) seems different to that of Pan-PKCε (Fig. 4A, upper left panel), which is distributed both in cytosol and nucleus. One possibility for this is that newly expressed GFP-PKCε is autophosphorylated at Ser729, so it is predominantly localised in cytosol, adenosine can trigger the loss of phosphorylation at Ser729, so the dephosphorylated PKCε moves to nucleus.
Our finding that MTs are somehow associated with PKCε translocation to the nucleus agrees with results for PKCα in smooth muscle cells [Battistella-Patterson et al., 2000; Dykes et al., 2003]. In intact heart tissue MTs are essential for the translocation of PKC in IPC [Nakamura et al., 2004]. Disruption of stress fibres with cytochalasin D caused severe changes in H9c2 cell morphology but did not block the nuclear translocation of PKCε as effectively as disruption of MTs. However, in fibroblasts cytochalasin B treatment inhibited nuclear translocation of PKCα [Schmalz et al., 1996]. Our findings are in keeping with the recognised nuclear transport function of MTs [Lam et al., 2002; Ogawa-Goto et al., 2003] and that MTs associate with the nuclear membrane and penetrate into the nucleus [Georgatos et al., 1997].
The mechanisms underlying PKC-mediated preconditioning, especially the delayed form of preconditioning are not fully clear. Notably, PKCε has an actin binding site so, not unexpectedly, co-localises with F-actin stress fibres in H9c2 cells as well as with myofilaments in cardiac myocytes [Huang and Walker, 2004] where a RACK 2 or similar protein may anchor the kinase. Our finding that 5 of the 10 proteins associated with PKCε in the postnuclear fraction are myofibril components while another four are involved in the regulation of myofibrils indicates that PKCε plays a key role in regulating myofibril function in heart muscle cells as reported for PKCε by Mansour et al. 2004. Ping et al. 2001 also identified several cytoskeletal and contractile proteins associated with PKCε in mouse heart tissue. AA causes PKCε translocation to myofilaments [Huang et al., 1997] where it could phosphorylate troponin 1 and myosin light chain 2 [Damron et al., 1995].
We identified matrin 3 and vimentin as PKCε-associating proteins in the nucleus as in fibroblasts [Xu and Rumsby, 2004] though these proteins were not detected by Ping et al. 2001 in a proteomic screen of PKCε complexes from mouse heart. Matrin 3, a component of the fibrogranular nucleoskeleton, influences transcription and RNA splicing [Nakayasu and Berezney, 1991; Matsushima et al., 1998]. Vimentin, an intermediate filament protein involved in chromatin organisation and nuclear matrix core filament construction [Shoeman et al., 2001], is a phosphorylation target for PKCε [Ivaska et al., 2005]. So the nuclear translocation of PKCε may mediate preconditioning through changing phosphorylation status of its binding partners or anchors on its binding partners and changes the activity of other kinases such as p38-MAPK [Sato et al., 2000].
Further investigation of the role of PKCε in cardiomyblasts is warranted to advance our understanding of the signalling mechanism involved in ischaemic preconditioning and cardioprotection.
In conclusion, we find H9c2 cardiomyoblasts express five PKC isoforms (α, βI, δ, ε and ζ). We demonstrate that adenosine stimulates PKCε translocation to the nucleus in H9c2 cells via a mechanism involving dephosphorylation at Ser729. Both phosphorylation and serine at 729 are critical for the subcellular localization of PKCε. Among five PKC isoforms (α, βI, δ, ε and ζ) detected, PKCε is the only isoform translocating to the nucleus upon adenosine stimulation. We show that MTs are involved in the nuclear translocation of both endogenous PKCε and overexpressed GFP-PKCε. Our results also indicate 10 proteins interact with cytosolic PKCε; 5 of which are components of myofibrils and matrin 3 and vimentin interact with nuclear PKCε, which strongly suggested the involvement of this kinase in the function of cardiomyoblasts.
Acknowledgements
This research was funded by BBSRC Grant 87/C14680 to M.G.R. We thank Dr. Peter O'Toole and Ms. Meg Stark for help with confocal microscopy.




