High expression of apolipoprotein E impairs lipid storage and promotes cell proliferation in human adipocytes†
Jean-François Carmel and Evelyne Tarnus contributed equally to this work and should be considered as co-first author.
Abstract
Apolipoprotein E (apoE), a key regulator of lipid metabolism, is highly produced by adipose tissue and adipocytes. However, there is little information about its role on adipocyte functions. Because apoE-deficiency in adipocytes was shown to impair adipocyte differentiation, we investigated the consequences of apoE high expression on differentiation and proliferation of a human adipocytic cell line (SW872). SW872 cells were transfected with human apoE to induce a fivefold increase in apoE production and secretion. Adipocyte differentiation and proliferation were assayed by measuring lipid content, adipogenic gene expression, cell number, cell resistance to serum deprivation, and cell division kinetics. Cultured apoE-transfected cells accumulated less triglycerides and less cholesterol than control cells. This decrease in lipid accumulation was associated with a strong downregulation of peroxisome proliferator-activated receptors γ1 and γ2 and stearoyl-CoA desaturase 1. The decrease in lipid accumulation was not dependent on the presence of lipids, lipoproteins, or PPAR-γ agonists in the culture medium, nor was it observed with exogenously added apoE. Moreover, we observed that apoE-transfected cells were more resistant to death induced by serum deprivation, and that these cells underwent more cell divisions than control cells. These results bring new evidence of apoE-involvement in metabolic disorders at the adipocyte level. J. Cell. Biochem. 106: 608–617, 2009. © 2009 Wiley-Liss, Inc.
Abbreviations used:
APO, apolipoprotein; CFDA-SE, carboxyfluorescein diacetate, succinimidyl ester; FBS, fetal bovine serum; HSPG, heparan sulfate proteoglycans; LDLR, low-density lipoprotein receptor; LPDS, lipoprotein-deficient serum; LRP, LDLR-related protein; PPAR-γ, peroxisome proliferator-activated receptor; VLDLR, very low-density lipoprotein receptor.
Global incidence of obesity has increased dramatically during the last decades. The situation has become particularly alarming since obesity epidemic started to spread among children [Rocchini, 2002].
Consequently, the impact of adipose tissue development on health, especially in excess fat accumulation conditions like obesity, has been highly studied. Adipose tissue function is critical for whole body homeostasis; this endocrine organ controls energy metabolism, modulates lipid flux, and secretes factors exhibiting endocrine, paracrine, and autocrine activities [Ahima, 2006; Scherer, 2006].
Apolipoprotein E (apoE), which is a component of lipoproteins, for example, chylomicrons, VLDL, intermediate-density lipoproteins, and HDL, is mainly produced and secreted by the liver [Mahley, 1988]. ApoE is known to regulate both cellular and systemic cholesterol and triglyceride metabolism [Mahley, 1988] and has been shown to exhibit anti-inflammatory, anti-oxidant, and anti-atherogenic properties [Miyata and Smith, 1996; Tamaoka et al., 2000; Davignon, 2005; Atkinson et al., 2008; Tsoi et al., 2007; Jofre-Monseny et al., 2008]. ApoE has been highly studied for its potential role in the etiology of atherosclerosis, diabetes, and obesity, and has been found to be highly expressed in adipose tissue and adipocytes [Zechner et al., 1991; Davignon et al., 1999; Wassef et al., 2004]. However, if apoE expression by adipocytes has been known for many years, only recent studies have focused on adipocyte apoE function and regulation [Wassef et al., 2004; Yue et al., 2004; Huang et al., 2006b, 2007; Gao et al., 2007; Rao et al., 2007; Chiba et al., 2008]. Interestingly, our group has found that apoE protein and mRNA levels increase during maturation of human SW872 adipocytes [Wassef et al., 2004]. This increase in apoE synthesis was closely linked to an increase in cellular content of triglyceride and cholesterol, suggesting a potential role for apoE in adipocyte differentiation and lipid storage regulation. Mazzone et al. reported that adipocyte apoE expression was modulated by nutritional status, by inflammatory messengers, and by peroxisome proliferator-activated receptor (PPAR)-γ agonists [Yue et al., 2004; Huang et al., 2007; Rao et al., 2007]. This group also demonstrated that adipocytes from apoE−/− mice were smaller than those of control mice and accumulated less triglyceride, even under PPAR-γ stimulation [Huang et al., 2006b]. Chiba et al. 2008 reported a decrease of adiposity in leptin deficient apoE−/− mice (ob/ob; apoE−/−), attributable to an impaired adipocyte differentiation. However, Gao et al. 2007 reported that adipocytes from obese Ay mice lacking apoE (apoE−/−; Ay+/−) differentiated normally in vivo.
In view of the above considerations, we investigated whether expression of apoE in human adipocytes could modulate cell differentiation and/or cell proliferation. We show here that high expression of human apoE impairs lipid storage in SW872 adipocytes. We also demonstrate that this impairment in cellular lipid accumulation is associated with a decrease in adipogenic gene expression and an increase in cell proliferation.
MATERIALS AND METHODS
Materials
Human SW872 liposarcoma cells were purchased from American Type Culture Collection (Rockville, MD). Dulbecco's Modified Eagle's Medium (DMEM) high glucose, Ham's F-12 nutrient mixture, and geneticin were obtained from GIBCO (Grand Island, NY). Fetal bovine serum (FBS) was purchased from GIBCO (St. Louis). Bovine lipoprotein-deficient serum (LPDS) was prepared by ultracentrifugation at density 1.25 g/ml using solid KBr and was dialyzed against physiological saline (0.15 M). Goat anti-human apoE and HRP-conjugated goat anti-human apoE antibodies were obtained from Academy Biomedical Compagny (Houston, TX).
SW872 Cell Transfection With pcDNA3 apoE Construct
SW872 cells were transfected (lipofectamine and Plus™ Reagent, Invitrogen, Burlington, Ontario) with the expression vector pcDNA3 containing the cDNA of human apoE3. Human apoE cDNA was prepared as published [Mabile et al., 2003]. The cDNA was sequenced on an Applied Biosystems 373A DNA sequencer using a Thermo Sequenase dye terminator kit (Amersham Pharmacia Biotech, Baie d'Urfé, Quebec), before subcloning into the mammalian expression vector pCDNA3. Stable transfectants were selected with geneticin (G418) and different clones were chosen for further characterization.
SW872 Cell Culture and Treatments
SW872 cells (initially 0.5–1.0 × 106 per well) were plated onto 6-well plates (Falcon) and cultured in DMEM high glucose: Ham's F12 (3:1, v/v) supplemented with NaHCO3 (3.7 g/L), 100 µM non-essential amino acids, 50 U/ml penicillin, 50 µg/ml streptomycin, 10% Fetal bovine serum (FBS), and geneticin (transfected cells; 0.3 mg/ml), in a humidified incubator (5% CO2, 37°C).
LPDS Preparation and Treatment
Lipoprotein-deficient serum (LPDS) was prepared by ultracentrifugation of FBS using previously described methods [Schumaker and Puppione, 1986]. Cells were plated onto 6-well plates and cultured for 15 days with 10% FBS or 10% LPDS. Culture medium was changed every 2 days.
Oleate and Rosiglitazone Treatment
Oleate and rosiglitazone were obtained from Sigma–Aldrich. Cells were grown to 80% confluence in DMEM containing 10% FBS, and then switched to medium containing 500 µM Oleate/BSA (7:1, mol/mol) or 2 µM rosiglitazone for 4 days [Mayerson et al., 2002; Izem and Morton, 2007].
Exogenous apoE Treatment
Wild-type SW872 cells were plated onto 6-well plates and cultured for 15 days in the presence of DMEM/F12 (3:1, v/v) and 10% FBS with control medium (−) (medium from control cells + fresh medium (1:1)) or with E+ medium (+) (medium from E+ cells incubated 2 days + fresh medium (1:1)). Media were changed every 2 days.
Characterization of Stable Cell Lines Expressing High Amount of Human apoE
Confluent cells were washed three times, harvested, resuspended in lysis buffer (25 mM Tris, 2 mM EDTA, 1% triton, protease inhibitor mixture), and spun for 10 min at 10,000g to remove cell debris. Twenty micrograms protein aliquots from cell lysates and cell media were used for SDS–PAGE. ApoE was detected by reaction of blots with a goat polyclonal antibody against human apoE and mouse anti-goat IgG conjugated with peroxidase. Total proteins were stored for 1 day at 4°C before quantification according to Lowry et al. 1951 with albumin as a standard.
Quantification of Cellular and Extracellular apoE Accumulation by ELISA
Cells were cultured into 24-well plates until confluence. Cells were then lysed in buffer containing 25 mM Tris, 2 mM EDTA, 1% triton, and protease inhibitor mixture, and spun for 10 min at 10,000g to remove cell debris. Hundred microliters of culture medium and 20 µg protein aliquots of cell lysates were used for ELISA quantification as described in [Cohn et al., 1996]. The ELISA is specific for human apoE and does not recognize bovine apoE present in FBS.
Quantification of Cellular Cholesterol and Triglyceride Accumulation
Cells were washed three times with PBS, and lipids were extracted three times (1 h each) with 1 ml hexane: isopropanol (3:2, v/v). Extracts were pooled and centrifuged 10 min at 1,500g. Supernatants were dried under nitrogen. Total cholesterol and triglycerides were quantified by enzymatic reaction using COBAS® reagents obtained from Roche Diagnostics (Indianapolis, IN) and Biomerieux® kit TG PAP 150 (France).
Cellular Lipid Staining
Cultured cells were fixed in a 4% paraformaldehyde solution for 30 min, washed with PBS for 1 min, and incubated in 60% isopropyl alcohol for 2 min. Cells were then incubated for 15 min in 0.3% Oil-Red-O in 60% isopropyl alcohol. After destaining for 1 min in 60% isopropyl alcohol, cells were washed with water for 10 min. Merge images were obtained using inverted microscope and Kodak camera.
Serum Deprivation-Induced Cell Death
Control and apoE-transfected cells were plated onto 6-well plates (0.5 × 106 per well). Twenty-four hours later, medium was removed and fresh medium without FBS was added for 48 h. Living cells were then counted using trypan blue staining. Results are expressed as percentage of living cells.
Cell Division Assay Using Fluorescent-Dye Cell Labeling and Flow Cytometry
CFDA-SE (carboxyfluorescein diacetate, succinimidyl ester) (Vybrant™ CFDA-SE Cell Tracer Kit (V-12883), Molecular Probes, Eugene, OR) was used to verify cell division kinetics. About 8 × 106 cells were centrifuged, resuspended in 0.1% bovine serum albumin phosphate-buffered saline (BSA-PBS) and filtered through a 70 µm nylon filter. A solution containing 0.1% BSA-PBS and 20 µM CFDA-SE (prepared with DMSO provided with the kit) was added to the cells at a final concentration of 10 µM of CFDA-SE. The cells were mixed gently and incubated for 10 min in a 37°C water bath. The reaction was stopped by adding DMEM-F12 (3:1) containing 10% FBS. Cells were washed three times with medium to allow free, unreacted CFDA-SE, to diffuse out into the medium. Cells were plated in 6 wells plate (0.6 × 106 cells/well) and an aliquot was kept to determine parental generation as reference. CFDA-SE-labeled cells were washed two times with PBS and resuspended in 0.1% BSA-PBS before analysis on FACScan (BD Biosciences, Mississauga, Ontario, Canada). The FACS data were collected with Cell Quest Pro software (BD Biosciences) and proliferation analysis done with Mod Fit LT version 3.1.
Quantification of Adipocyte Gene Expression by RT-PCR
RNA was extracted with Trizol© (Invitrogen), according to the manufacturer's instructions and quantified using a spectrophotometer. Reverse transcriptase reaction was performed and cDNA was amplified by real-time PCR on an MX4000 system from Stratagene, using the SYBR green master-mix purchased from Qiagen (Mississauga, Ontario). Analyses were done with the Mx4000 software. PPAR-γ1, PPAR-γ2, SCD1, INSR, CD36, C/EBP-α, and LRP mRNA levels were normalized with ribosomal protein S14. All primers were designed using Primer3 software and purchased from Invitrogen.
Statistical Analysis
Data are expressed as mean ± SD or SEM. Statistical analyses were performed using unpaired Student's t-tests.
RESULTS
Characterization of Stable ApoE-Transfected Adipocytes
SW872 adipocytes were transfected with empty pcDNA3 (control cells) or with pcDNA3 containing human apoE cDNA (E+ cells). After 3 weeks of selection in geneticin, several clones were cultured until confluence. Levels of cellular (bound to cell surface and intracellular) and secreted apoE were assessed by Western blot and quantified by ELISA in cell lysates and media from wild-type cells, from empty-vector transfected cells (control), and from apoE-transfected cells (E+ cells). Wild-type cells and empty-vector transfected cells secreted the same amount of apoE in the medium (80 ng of apoE/mg of cell protein in 48 h) and accumulated the same amount of cellular apoE in cell lysates (20 ng of apoE/mg of cell protein in 48 h). In E+ cells, the amount of cellular and secreted apoE was three- to fivefold higher than in control cells (Fig. 1). One can note the amount of apoE secreted by the E+ cells appears higher when evaluated by Western blotting than by ELISA (Fig. 1). ELISA technique is quantitative whereas Western blotting is semi-quantitative. For this reason, ELISA results for the precise level of apoE secretion by the E+ cells should prevail.
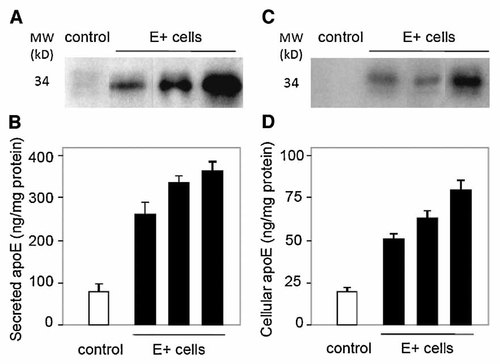
Characterization of stable cell lines with high expression of human apoE. Medium apoE (A,B) and cellular apoE (C,D) were analyzed by Western Blot (A,C) and quantified by ELISA (B,D) after cells were incubated with DMEM/F12 (3:1, v/v) and 10% fetal bovine serum for four days. Results are shown for vector-only transfected cells (control) and for apoE-transfected cells (E+ cells). Results represent mean ± SD of triplicates.
High Expression of apoE Inhibited Lipid Accumulation in Human Adipocytes
To evaluate the effects of apoE high expression on cellular lipid accumulation, triglycerides and cholesterol contents were measured in control and apoE-transfected cells (E+). Cells were grown in 10% FBS for 15 days. After 2 days (day 2), control and E+ cells accumulated the same amount of cholesterol and triglycerides (Fig. 2A,B). In contrast, after 15 days, E+ cells accumulated less triglycerides (∼75%, P < 0.001) and less cholesterol (∼45%, P < 0.001) than control cells (Fig. 2A,B). The marked decrease in lipid content in E+ cells compared to control, was confirmed by Oil-Red-O staining as shown in Figure 2E.
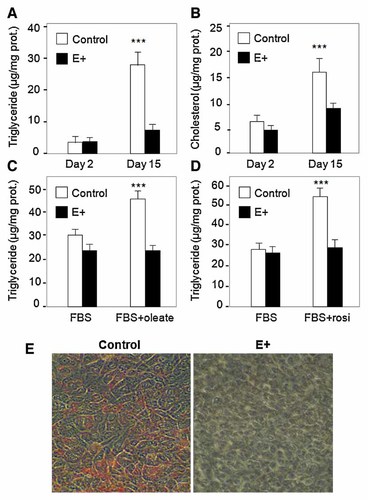
High expression of apoE inhibits lipid accumulation in human adipocytes. A,B: Cells were cultured for fifteen days in the presence of DMEM/F12 (3:1, v/v) and 10% FBS. Cellular triglyceride (A), and cellular cholesterol (B) were determined in vector-only transfected cells (control) and apoE-transfected cells (E+). C,D: Cells were grown to 80% confluence days in the presence of DMEM/F12 (3:1, v/v) and 10% FBS, and then switched to medium containing 500 µM Oleate/BSA (C) or 2 µM rosiglitazone (D) for 4 days. Cellular triglyceride content was determined in vector-only transfected cells (control) and apoE-transfected cells (E+). Results represent the average ± SD of five wells. ***P < 0.001, significantly different from control cells by Student's t-test. E: Vector-only transfected cells (Control) and apoE-transfected cells (E+) were cultured for 15 days in the presence of DMEM/F12 (3:1, v/v) and 10% FBS. Lipids were then stained using O-Red-Oil staining protocol (see Research Design and Protocols).
Because lipid content in adipocytes is known to be rapidly stimulated by oleate [Izem and Morton, 2001, 2007] and rosiglitazone [Mayerson et al., 2002], control and E+ cells were incubated with oleate or rosiglitazone for 4 days. As shown in Figure 2C,D, control cells incubated with oleate or rosiglitazone (rosi) accumulated more triglycerides than untreated cells (∼50%, P < 0.001). However, in E+ cells, lipid content was affected by neither oleate nor rosiglitazone.
We previously showed that exogenous lipoproteins from the serum can modulate cellular lipid content in SW872 cells [Wassef et al., 2004]. To avoid any effect from exogenous lipoproteins on lipid accumulation, control and E+ cells were incubated in medium containing lipoprotein-depleted serum (LPDS). As shown in Figure 3A,B, cellular cholesterol and triglyceride contents were increased in control cells incubated with LPDS (3.5- and 2-fold, respectively, P < 0.001). However, in E+ cells incubated with LPDS, cholesterol and triglyceride cellular contents remained lower (∼70% and ∼45%, respectively, P < 0.001) than in control cells.
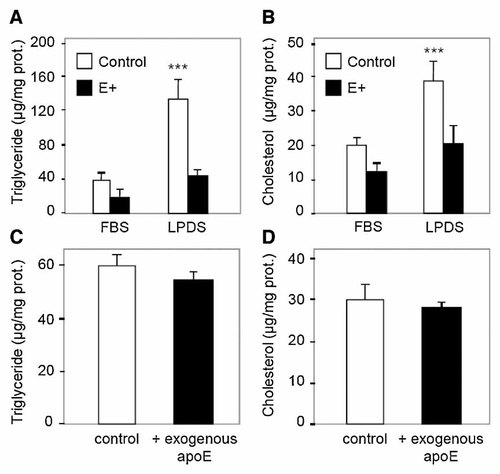
Exogenous lipoproteins and exogenous apoE have no effect on cellular lipid accumulation in human adipocytes. A,B: Cells were cultured for 15 days in the presence of DMEM/F12 (3:1, v/v) and 10% FBS or lipoprotein-depleted serum. Cellular triglyceride (A), and cellular cholesterol (B) were determined in vector-only transfected cells (control) and apoE-transfected cells (E+). C,D: Wild-type SW872 cells were plated onto 6-well plates and cultured for 15 days in the presence of DMEM/F12 (3:1, v/v) and 10% FBS with control medium (−) (medium from control cells + fresh medium (1:1)) or with E+ medium (+) (medium from E+ cells incubated 2 days + fresh medium (1:1)). Medium was changed every 2 days. Cellular triglyceride (C), and cellular cholesterol (D) were determined. Results represent the average ± SD of five wells. ***P < 0.001, significantly different from control cells by Student's t-test.
As apoE is a secreted protein involved in lipid efflux [Davignon et al., 1999], we next wanted to determine whether exogenous apoE, added to the medium, will have the same effects on lipid accumulation. Wild-type SW872 cells were incubated with media from control (−) or E+ cells (+). Medium from control cells contained 30 ng apoE/ml; medium from E+ cells contained 140 ng apoE/ml. After 15 days in culture, cellular lipid content was measured. As shown in Figure 3C,D, exogenous apoE had no effect on triglyceride or on cholesterol accumulation in SW872 adipocytes, suggesting that endogenously produced apoE is responsible for the inhibition of lipid accumulation.
High Expression of apoE Decreased Adipogenic Gene Expression in Human Adipocytes
PPAR-γ and CEBP-α are two adipogenic transcription factors, which play a major role in adipocyte terminal differentiation. PPAR-γ1, PPAR-γ2, and CEBP-α gene expression levels were determined by real-time PCR, in control and transfected cells (primer sequences are specified in Table I). As shown in Figure 4A,B, mRNA levels of PPAR-γ1 and PPAR-γ2 γ are elevated in confluent immature cells (day 2) and do not increase with time of differentiation, as already observed in previous studies by our group [Wassef et al., 2004]. In comparison to control cells, PPAR-γ1 and PPAR-γ2 gene expression in E+ cells significantly decreased at day 8 (∼35% and ∼55% vs. control cells, respectively) and at day 15 (Fig. 4A,B). In contrast, no changes in CEBP-α level were observed (Fig. 4C).
| Assessed mRNA | Forward primer 5′–3′ | Forward primer 5′–3′ |
|---|---|---|
| S14 | CCACAGGAGGAAATAGGACAAAGA | AATCTTGTTCACAGACGGCGAC |
| INSR | CAACGAGGAGTGTGGAGACA | GCAGCCGTGTGACTTACAGA |
| LRP | CACCTTAACGGGAGCAATGT | GTCACCCCAGTCTGTCCAGT |
| CD36 | TGGTCAAGCCATCAGAAAAA | CAGCACCACACCAACACTGA |
| C/EBP-α | AGTCGGTGGACAAGAACAGC | TTGTCACTGGTCAGCTCCAG |
| SCD1 | CCCCTACGGCTCTTTCTGAT | GCGTACTCCCCTTCTCTTTG |
| PPAR-γ1 | CCTCGAGGACACCGGAG | TGATCCCAAAGTTGGTGGGC |
| PPAR-γ2 | GCAAGGGTTTCTTCCGGA | AGGCATTTCTGAAACCGACA |
- S14, ribosomal protein; INSR, insulin receptor; LRP, LDL receptor-related protein; CD36, scavenger receptor; C/EBP-α, CCAAT/enhancer binding protein; SCD1, stearoyl-CoA desaturase 1; PPAR-γ1 and 2, peroxisome proliferator-activated receptor gamma 1 and 2.
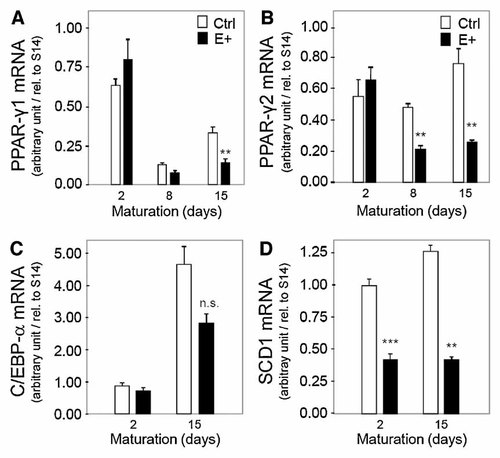
High expression of apoE decreases PPAR-γ1, PPAR-γ2 and SCD1 gene expression in human adipocytes. Cells were cultured 2, 8, and 15 days in the presence of DMEM/F12 (3:1, v/v) and 10% FBS. Results are shown for vector-only transfected cells (control), and for apoE-transfected cells (E+, expressing five times more cellular apoE than control cells). PPAR-γ1 mRNA (panel A), PPAR-γ2 mRNA (panel B), C/EBP-α mRNA (panel C), and stearoyl CoA desaturase 1 mRNA (panel D) were quantified by real-time RT-PCR and were expressed relative to S14 mRNA. Results represent mean ± SEM and are representative of at least three independent experiments. **P < 0.01; ***P < 0.001, significantly different from control cells by Student's t-test.
We next measured mRNA expression of several genes involved in lipid and glucose metabolism in control and E+ cells cultured for 15 days. Expression of stearoyl-CoA desaturase 1 (SCD1), which is an enzyme involved in the biosynthesis of monounsaturated fatty acids necessary for triglycerides formation, was significantly decreased at day 2 (−57%, P < 0.001) and day 15 (−63%, P < 0.001) as seen in Figure 4D. In contrast, no changes in gene expression were observed for insulin-receptor (INS-R), LDL receptor-related protein (LRP), and the scavenger receptor CD36, as shown in Table II.
| Gene | Day 2 (maturation) | Day 15 (maturation) | ||
|---|---|---|---|---|
| Control | E+ | Control | E+ | |
| INSR | 0.046 ± 0.005 | 0.050 ± 0.007 | 0.088 ± 0.013 | 0.068 ± 0.003 |
| LRP | 0.272 ± 0.011 | 0.198 ± 0.022 | 0.497 ± 0.079 | 0.586 ± 0.045 |
| CD36 | 0.00054 ± 0.00011 | 0.00036 ± 0.00003 | 0.00055 ± 0.00008 | 0.00026 ± 0.00011 |
- Cell lines were incubated for 15 days with FBS. INSR (insulin receptor), LRP (LDL receptor-related protein) and CD36 (scavenger receptor) mRNA levels are relative to S14. Data represent mean ± SEM of four wells from a minimum of three independent experiments.
High Expression of apoE Promoted Cell Proliferation in Human Adipocytes
As adipocyte differentiation is in balance with cell proliferation, we next studied the effects of apoE high expression on cell proliferation. Control and E+ cells were cultured for 15 days in the presence of FBS, and cell growth was assayed by cell count and total cell protein measurements. In E+ cells, a twofold increase was observed in both cell number and total cell protein, as shown in Figure 5A,B. A time-course study showed that the effect of apoE on cell growth was already apparent after 8 days in culture (data not shown).
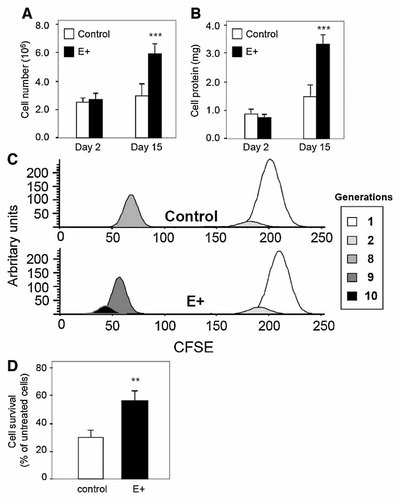
High expression of apoE promote cell proliferation in human adipocytes. A,B: Cells were cultured for 15 days in the presence of DMEM/F12 (3:1, v/v) and 10% FBS. Cell number (A), and total cell protein (B) were determined in vector-only transfected cells (control) and apoE-transfected cells (E+). C: Cells were cultured for 9 days in the presence of DMEM/F12 (3:1, v/v), 10% FBS, and 10 µM of carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE). Results are shown for vector-only transfected cells (control), and for apoE-transfected cells (E+, expressing five times more cellular apoE than control cells). Measurement of cell division was done using CFDA-SE and Flow Cytometry. During maturation of cells, the peaks initially at day 0 (parents) will be shifted to the left after each cell division (generation). Results are representative of two independent experiments of three wells. D: Control and apoE-transfected cells were plated onto 6-well plates (0.5 × 106 per well). Twenty-four hours later, medium was removed and fresh medium without FBS was added for 48 h. Cells were trypsinized and living cells were counted using trypan blue staining. Results were expressed as percentage of living cells. Results represent the average ± SD of three independent experiments. **P < 0.01, significantly different from control cells by Student's t-test.
We next wanted to determine if the increase in cell proliferation of E+ cells was due to an increase in cell division kinetics.
An assay using CFDA-SE was performed with control and E+ cells. CFDA-SE is a fluorescent compound, which diffuses passively into the cells and is retained inside the cells when its acetate groups are conjugated with intracellular amines. Each round of mitotic cell division distributes CFDA-SE equally between the daughter cells and results in a twofold decrease of the fluorescence intensity. Control and E+ cells were stained with CFDA-SE for 10 min and cultured for 9 days in presence of 10% FBS. Figure 5C shows a peak corresponding to generation 1 at day 0 (control 92% and E+ 90%). At day 9, 92% of control cells were in generation 8, whereas 77% of E+ cells were already in generation 9 and 17% had reached generation 10, showing that high expression of apoE accelerated cell division in SW872 adipocytes.
To determine if the increase in cell number of E+ cells was also related to a resistance to cell death, control and E+ cells were incubated for 48 h in serum-deprived (SD) medium, which promotes cell death through the caspase pathway. Surviving cells were counted using the trypan blue method. As shown in Figure 5D, E+ cells were more resistant to serum deprivation than control cells (+57%, P < 0.01).
DISCUSSION
In this study, we showed that high expression of apoE in human adipocytes impaired lipid storage and promoted cell proliferation. Indeed, high expression of apoE in adipocytes induced a decrease in cellular triglyceride and cellular cholesterol accumulation, and downregulated the expression of adipogenic genes such as PPAR-γ1, PPAR-γ2, and stearoyl-CoA desaturase 1, a PPAR-γ-induced enzyme, which is involved in triglyceride synthesis. The decrease in lipid accumulation was not dependent on the presence of lipid or lipoproteins in the medium, nor was it observed with exogenously added apoE. We also showed that high expression of apoE increased cell resistance to serum deprivation. ApoE over-expression also accelerated cell division leading to an increase in cell proliferation. Our results clearly show that the observed apoE-induced inhibition of differentiation is caused by both a large increase in cell proliferation and a decrease in adipogenic gene expression.
As of today the role of apoE in adipocyte metabolism has not been clearly established. Some results suggest that the absence of apoE expression in adipocytes inhibits lipid storage, whereas other results show that adipocytes from apoE-deficient mice exhibit a normal expression of adipogenic genes. Our group has already shown that during human adipocyte differentiation, increased apoE secretion was closely linked to lipid accumulation and adipogenic gene expression [Wassef et al., 2004]. Mazzone et al. reported that apoE knockout (apoE−/−) mice on a chow-diet had less adipose tissue and smaller adipocytes than wild-type controls; they also showed that adipocytes isolated from apoE−/− mice failed to accumulate lipids, proposing that lower adipocyte apoE expression limited further expansion of adipocyte lipid content and size [Huang et al., 2006b]. Chiba et al. 2003 reported that apoE-deficient obese (ob/ob;apoE−/−) mice, when fed on a high-fat diet, did not show an increase in their body fat mass compared to ob/ob mice. However, when mice are fed a chow diet, ob/ob; apoE−/− mice gained more body fat mass than did apoE−/− mice in spite of apoE deficiency. Conversely, Gao demonstrated that adipocyte differentiated normally in apoE-deficient obese Ay (apoE−/−: Ay/−) mice; indeed, no changes were observed in adipogenic gene expression of CEBP-α or PPAR-γ in these mice.
In the present study though, we showed that high expression of apoE in adipocytes also impairs lipid accumulation. We hypothesize that, in our cellular model, endogenous over-expression of apoE inhibits lipid uptake from the medium. This is similar to a study by Ho et al. 2000 showing that lipid uptake was reduced in macrophages transfected with apoE, compared to macrophages that did not express apoE. The effect of apoE on lipid accumulation by adipocytes appears to be dependent on an optimal concentration. The mechanisms for lipid uptake inhibition in excess apoE are most probably different from what happens in absence of apoE. We surmise that the interaction between the high amount of apoE and the extracellular lipids is responsible for the inhibition of lipid uptake and therefore for the inhibition of cellular lipid accumulation. In several cellular models, secreted apoE was shown to form nascent lipoproteins, which could reduce lipid uptake [Basu et al., 1982; Herscovitz et al., 1992]. The question why endogenously secreted apoE can inhibit lipid uptake but not exogenous apoE remain to be answered.
Consistent with our finding, Mazzone demonstrated that endogenous apoE was required for adipocyte triglyceride accumulation, which was not compensated by exogenous apoE from lipoproteins added to the medium [Huang et al., 2006b]. Similarly, our results showed that exogenous apoE could not substitute for the effect of endogenous apoE. Thus, we propose that intracellular apoE has a predominant role on adipocyte differentiation. Within the cell, apoE is known to modulate cellular sterol flux and metabolism [Huang and Mazzone, 2002] and could therefore influence plasma membrane structure, including the function and number of caveolae [Huang et al., 2006b]. Changes in adipocyte cholesterol flux mediated by apoE could lead to downstream changes in caveolin expression, which was shown to be modulated by cellular free cholesterol [Covey et al., 2007]. Moreover, caveolin expression have been shown to have important implications in cell proliferation, since over-expression of caveolin-1 inhibits cell proliferation by arresting cell cycle at the G0/G1 phase [Galbiati et al., 2001].
Other mechanisms by which endogenous apoE could influence adipocyte proliferation must also be considered. An increased apoE expression was associated with several types of cancer, including breast, colon, ovarian and liver carcinoma [Zunarelli et al., 2000; Venanzoni et al., 2003; Chen et al., 2005; Slattery et al., 2005]. Chen & al showed that, in malignant ovarian carcinoma cells, apoE was highly expressed, whilst being undetectable in benign counterparts [Chen et al., 2005]. This group also showed that apoE-expressing ovarian cancer cell line stopped proliferating and entered apoptosis when apoE expression was inhibited using an apoE-specific siRNA, suggesting that apoE has an anti-apoptotic function.
This association between apoE and apoptosis has also been identified in human fibroblasts and THP-1 macrophages, in which apoE is upregulated during apoptosis [Chen et al., 2005]. Conversely, Elliot & al also showed that macrophage apoE knockdown activated caspase-3, which is involved in apoptosis pathway [Elliott et al., 2008]. The contribution of apoE expression to cell proliferation and apoptosis is also supported by recent data showing that apoE localized in the nucleus of apoE-transfected CHO cells and level of apoE in the nucleus increased with serum deprivation [Kim et al., 2008]. Similarly, we found that high expression of endogenous apoE decrease apoptosis induced by serum deprivation. These data provide new evidence that intracellular apoE, a fortiori when over-expressed, may be targeted to the nucleus to modulate adipocyte differentiation.
SW872 liposarcoma cells are adipocytes [Izem and Morton, 2001, 2007; Jiang et al., 2001; Huang et al., 2006a], which, as many sarcoma, continuously proliferate to reach confluence. Our results show that SW872 adipocytes expressing high amount of apoE never stop proliferating, even after reaching confluence, thus fail to undergo differentiation because of a drastic downregulation of PPAR-γ1 and PPAR-γ2 gene expression. Since intracellular apoE regulates lipid metabolism and distribution, one more provocative hypothesis could be an implication of intracellular apoE on lipid-mediated activation of the PPAR-γ genes. Indeed, the PPAR-γ are transcription factors involved in adipocyte differentiation and known to bind lipid metabolites [Na and Surh, 2003; Schopfer et al., 2005; Mazid et al., 2006; Monroy et al., 2007]. As apoE has been described to bind cellular lipid [Lucic et al., 2007], we think that apoE could act intracellularly to impair the PPAR-γ pathway.
In summary, we report that high expression of apoE in SW872 human adipocytes inhibited adipocyte differentiation and promoted cell proliferation. If the absence of apoE suppresses lipid accumulation in adipocytes, an increase of intracellular apoE appears to also be a signal to prevent further lipid accumulation, confirming the potential role for apoE as a homeostatic response of adipose tissue to hypertrophy. These results bring new evidence of apoE-involvement in metabolic disorders at the adipocyte level. Further investigations are needed to explore the role of apoE at the adipose tissue level.
Acknowledgements
This work was supported by the Ministère de l'Enseignement Supérieur et de la Recherche the Ministère de l'Outre-Mer, the Conseil Régional de La Réunion and the Université de La Réunion, and in part by grant # 69097 from the Canadian Institutes for Health Research. E.T. is a recipient of a fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche. The authors acknowledge the help and support of Catherine Bouchard, Geneviève Dubuc, Lucie Boulet, Hélène Jacques, Claudia Rodriguez, Michel Tremblay and Hanny Wassef. We also thank Eric Massicotte and Martine Dupuis of the Cytofluorometry Core at IRCM for their technical support with flow cytometry and CFDA-SE fluorescent-dye cell labeling and division assay.




