The angiogenic effects of Angelica sinensis extract on HUVEC in vitro and zebrafish in vivo
Abstract
Angiogenesis plays an important role in a wide range of physiological processes such as wound healing and fetal development. Many diseases are associated with imbalances in regulation of angiogenesis, in which it is either excessive or there is insufficient blood vessel formation. Angelica sinensis (AS), commonly used in the prescriptions of Chinese medicine, is a potential candidate for curing such diseases. However, biological effects of AS on angiogenesis and underlying mechanisms are yet to be fully elucidated. This investigation describes the angiogenic effects of AS extract on human endothelial cells (HUVEC) in vitro and zebrafish in vivo. The extract was demonstrated, by XTT assay and microscopic cell counting, to stimulate the proliferation of HUVEC; in addition, flow cytometry analysis indicated that the extract increased the percentage of HUVEC in the S phase. The wound healing migration assay illustrated that a dramatic increase in migration could be measured in AS extract-treated HUVEC. Meanwhile, the number of invaded cells and the mean tube length were significantly increased in AS extract treatment groups. The extract was also demonstrated to promote changes in subintestinal vessels (SIVs) in zebrafish, one feature of angiogenesis. In addition, AS extract was found by real-time PCR to enhance vascular endothelial growth factor (VEGF) mRNA expression. In a bead-based immunoassay, higher levels of p38 and JNK 1/2 expression were also observed in effusions compared with control cells. All results suggest that Angelica sinensis extract can promote angiogenesis, and that the angiogenic effects involve p38 and JNK 1/2 phosphorylation. J. Cell. Biochem. 103: 195–211, 2008. © 2007 Wiley-Liss, Inc.
Abbreviations used:
As, Angelica sinensis extract; BP, butylidenephthalide; CBA, cytometric bead array; EC, endothelial cell; ECM, extracellular matrix; ERK, extracellular signal-regulated protein kinases; FA, ferulic acid; FGF, fibroblast growth factor; Flt-1, fms-like tyrosine kinase; HUVEC, human umbilical vein endothelial cell; JNK, c-Jun N-terminal kinase; KDR/Flk-1, kinase-domain region/fetal liver kinase-1 in mice; MAPK, mitogen-activated protein kinases; PCR, polymerase chain reaction; PI, propidium iodide; RT-PCR, reverse transcription PCR; SIVs, subintestinal vessels; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; ZL, Z-ligustilide.
Angelica sinensis (Oliv.) Diels, named danggui in Chinese, is a key component of traditional Chinese medicine. In traditional systems of folk medicine, it has been used for the treatment of dehydration, lumbago, abnormal menstruation, menopausal symptoms (including hot flashes), hypertonia, nervous disorders, anemia, chronic hepatitis, and cirrhosis of the liver. In addition to its common usage in Asia, Angelica sinensis is used as a health food product for women's care in Europe and America. Therefore, the demand for Angelica sinensis is enormous [Sarker and Nahar, 2004]. In order to control the quality of Angelica sinensis and protect the safety of the consumers, we conducted a study to compare quantitatively several chemical components of different types of Angelica sinensis [Lao et al., 2004]. Identification of bioactive components in Angelica sinensis is very important not only for the quality control of crude botanical drugs but also for elucidating the therapeutic principle. With the aim of dissecting the molecular pharmacological actions of active ingredients originating from botanical materials, novel approaches have been adopted [Lam et al., 2006]. Moreover, we have used a new approach combining biomembrane extraction and high performance liquid chromatography to identify ferulic acid (FA), ligustilide, senkyunolide H, and senkyunolide I, which are some potentially bioavailable components in Angelica sinensis [Dong et al., 2005]. Other studies have identified compounds such as 3-butylphthalide, ligustilide and FA as potential bioactive components in Angelica sinensis using bio-extraction chromatography and immobilized liposome chromatography [Mao et al., 2002; Li et al., 2006].
Herbal formulae containing Angelica sinensis have protective or ameliorative potential for memory dysfunction [Kou et al., 2005; Lin et al., 2005]. Other studies have shown that Angelica sinensis can significantly inhibit platelet activation, repair vascular EC injury, and improve microcirculation in ulcerative colitis [Dong et al., 2004]. Angelica sinensis can inhibit rabbit aorta atherogenesis through gradual reduction of the serum triglyceride concentration [Xiaohong et al., 2000; Zhui et al., 2000]. All these studies demonstrate the potential biological activities of Angelica sinensis on the vascular system. In addition, some studies have demonstrated that a polysaccharide extracted from Angelica sinensis has therapeutic effects. The polysaccharide from Angelica sinensis was found to promote the migration of gastric epithelial cells in vitro over an artificial wound, stimulate [3H] thymidine incorporation in cells in a dose-dependent manner [Ye et al., 2001], and accelerate ulcer healing in an animal model [Ye et al., 2003].
Hertig first named the proliferation of ECs and formation of new capillaries “angiogenesis” in 1935 [Folkman, 1972]. Under normal conditions, tiny vessels do not increase in size or number, except in wound healing, embryonic development and development of the corpus luteum. In fact, many diseases are associated with imbalance in regulation of angiogenesis, in which it is either excessive or insufficient blood vessel formation occurs. Sengupta et al. [2004] demonstrated that Rg1 and Rb1, the two prevalent constituents of Ginseng, have two opposing effects in modulating angiogenesis. The potential effects of Angelica sinensis were also demonstrated in these studies. Angelica sinensis has a promoting effect on the formation of capillaries in chick embryo chorioallantoic membrane, as well as promoting proliferation of human umbilical vein endothelial cells (HUVEC) and the expression of ICAM-1 [Wu and Zhu, 2001; Lei et al., 2003; Gao et al., 2005]. However, the biological effects of Angelica sinensis on angiogenesis, and the underlying mechanisms, are still unclear. This investigation describes the angiogenic effects of Angelica sinensis extract on HUVEC in vitro and zebrafish in vivo.
MATERIALS AND METHODS
Chemicals and Reagents
The Angelica sinensis extract (batch No: XD-020114) was obtained from the Huirun chemical company, Guangdong, China. FA was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) and 3-butylidenephthalide (BP) (96+%) was purchased from Aldrich (Milwaukee, WI). Z-ligustilide (ZL) was purchased from Chroma-Dex (Santa Ana, CA). Kaighn's modification of Ham's F12 medium (F-12K), fetal bovine serum (FBS), phosphate-buffered saline (PBS), penicillin–streptomycin (PS), and 0.25% (w/v) trypsin/1 mM EDTA were purchased from Invitrogen (Carlsbad, CA). Endothelial cell growth supplement (ECGS), heparin, and gelatin were supplied by Sigma (St Louis, MO). Growth factor reduced (GFR) Matrigel™ basement membrane matrix, vascular endothelial growth factor (VEGF), BioCoat™ Matrigel™ 24 well plates and the BioCoat™ Matrigel™ invasion chamber were obtained from BD Biosciences (Bedford, MA).
Identification of the Extract and Determination of the Polysaccharide Content
Angelica sinensis extract (2.5 g) was diluted in 100 ml Milli-Q water. The solution thus obtained was filtered through a 0.45 mm PTFE syringe filter and injected into the Agilent 1100 series LC/MSD VL trap system (Agilent) for identification. An Agilent trap mass spectrograph was interfaced to the HPLC system using an electrospray ionization (ESI) source. The methanolic solutions, containing known concentrations of standards, FA (0.1 mg/ml), ZL (0.01 mg/ml), and z-butylidenephthalide (3 mg/ml), were prepared and subjected to the LC/MSD system for comparison. The detection wavelength of DAD was operated at 284 nm, and HPLC-MS data were then acquired using the program data analysis for LC/MSD Trap Version 3.2 (Build121), which corresponds with the LC/MSD Trap software 5.2 (Build 382). Meanwhile, the quantitative measurement of polysaccharide was conducted by the phenol-sulfuric acid method [Saha and Brewer, 1994].
Preparation of Reagents and Culture
For the investigation of angiogenic effects of Angelica sinensis extract on HUVEC in vitro, the stock solution of the extract (100 mg/ml) was prepared in PBS (containing 10% DMSO). For investigation of angiogenic effects of Angelica sinensis extract on zebrafish in vivo, the stock solution of the extract (1 mg/ml) was prepared in sterilized Milli-Q water (containing 0.5% DMSO). The stock of VEGF (100 µg/ml) was dissolved in sterilized Milli-Q water as described.
HUVEC Culture
HUVEC (ATCC, Manassas) were cultured in Kaighn's modification of Ham's F12 medium with 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 100 µg/ml heparin, 30 µg/ml EC growth supplement and 10% FBS at 37°C in a humidified atmosphere of 5% CO2. Tissue culture flasks were pre-coated with 0.1% gelatin.
Maintenance of Zebrafish
The transgenic zebrafish line TG (fli1: EGFP), in which ECs express eGFP, was kindly provided by ZFIN (Oregon) and maintained as previously described [Kwan et al., 2006]. Briefly, it was maintained in a controlled environment at a temperature of 28°C on a 14 h: 10 h light/dark cycle (lights on at 08:00) as described in the zebrafish handbook. Zebrafish were stored in five gallon tanks, continuously supplied with filtered reverse osmosis H2O. The fish were fed twice daily with brine shrimp in the morning and afternoon, and also with general tropical fish food occasionally.
Collection of Zebrafish Embryos
Embryos were generated by natural pair-wise mating when the fish were 3–12 months old. The filters were switched off and breeding boxes were placed into the tanks, followed by the presence of light. The fish were left undisturbed for 15–30 min. Breeding boxes were collected and the embryos were transferred into clean Petri dishes via a fine fishing net. The embryos were maintained in Milli-Q water. Healthy, limpid, and regular embryos were picked out at the 1–4 cell stage and were distributed into a 24-well microplate, with 6–8 embryos per well depending on the assay.
HUVEC Viability by XTT Assay
The HUVEC were trypsinized and seeded at 104 cells/well in 96-well gelatin coated plates. After 24 h of incubation, the complete medium was removed and replaced with low serum (0.5% FBS) medium. Cells were then incubated for a further 24 h to achieve a quiescent state. After these pre-incubations, the low serum (0.5% FBS) medium was replaced with medium containing different concentrations (11.1 to 100 µg/ml) of the Angelica sinensis extract. Cells receiving DMSO (0.1%) served as a vehicle control, and were equivalent to no treatment. In addition, cells cultured in 20 ng/ml VEGF served as a positive control. After 48 h of treatment, cell proliferation was assessed by XTT for 4 h. The spectrophotometric absorbance of each well was measured using a Multilabel counter (Perkin Elmer, Singapore). The wavelength for measuring absorbance of the formazan product was 490 nm, and the reference wavelength was 690 nm. Cell viability data were expressed as the percentages compared with vehicle control.
Viability of HUVEC by Cell Counting
The effect of Angelica sinensis extract on cell number was also assessed. The HUVEC were seeded in gelatin-coated 24-well tissue culture-treated plates at 5 × 104 cells/well. Cells were incubated in complete medium for 24 h at 37°C. Cells were further incubated with Angelica sinensis extract as described above. Cells were then washed and trypsinized at the end of treatment. The cell suspension from each well was collected in a 1.5 ml tube and centrifuged at 250 × g for 5 min. Following this, 800 µl supernatant was discarded and the cell pellet was resuspended. The cell number was determined by counting on a hemocytometer.
Invasion Assay on HUVEC
The effect of Angelica sinensis extract on HUVEC invasion was measured using the 10 mm tissue culture insert (transwell) with polycarbonate membrane (8 µm pores) and 24-well companion plate. The upper side and lower side of the membrane were pre-coated with 1:30 (v/v) and 1:100 (v/v) of Matrigel, respectively. The HUVEC (5 × 104 cells) were resuspended in low serum medium (300 µl) and seeded onto the culture inserts in triplicate. They were then deposited into the 24-well companion plate with 500 µl of low serum medium containing 33.3 or 100 µg/ml of Angelica sinensis extract. In addition, the wells of the companion plate, containing DMSO (0.1%) and 20 ng/ml VEGF, served as a vehicle control and positive control, respectively. The inserts were removed after 8 h of incubation and were then washed with PBS. Non-invasive cells on the upper surface of the membrane were removed by wiping with cotton swabs. The inserts were fixed in paraformaldehyate, stained with calcein AM and mounted on microscope slides. Images of the invasive cells were captured at 50× magnification using a fluorescent inverted microscope (Axiovert 200, Carl Zeiss, HK) and a CCD camera (AxioCam HRC, Carl Zeiss, HK). Following this, HUVEC invasion was quantified by counting the number of cells per insert with the software Metamorph Imaging Series (Molecular Devices, Tokyo, Japan).
Tube Formation Assay on HUVEC
The effects of Angelica sinensis extract on HUVEC differentiation were examined by in vitro tube formation on Matrigel [Merchan et al., 2003]. Confluent HUVEC were harvested and diluted (9 × 104 cells) in 500 µl of low serum medium, containing 33.3 to 100 µg/ml of Angelica sinensis extract. The harvested cells were then seeded on 1:1 Matrigel (v/v) coated 24-well plates in triplicate at 37°C for 7 h. Cells receiving DMSO (0.1%) served as a vehicle control and were equivalent to no treatment. In addition, cells cultured in 40 ng/ml VEGF served as a positive control. The network-like structures were examined under an inverted microscope (at 50× magnification). Tube-like structures were defined as endothelial cord formations that were connected at both ends. The mean tube length in three random fields per well was quantified by software Metamorph Imaging Series.
Migration Assay on HUVEC
Migration of HUVEC was performed by the wound healing method [Sato and Rifkin, 1988]. The HUVEC (3 × 105 cells) were seeded into each well of a 24-well plate, and incubated with complete medium at 37°C and 5% CO2. After 24 h of incubation, cells were starved for another 24 h by low serum (0.5% FBS). The HUVEC were then scraped away horizontally in each well using a P100 pipette tip. Three randomly selected views along the scraped line were photographed on each well using a fluorescent inverted microscope and the CCD camera attached to the microscope at 50× magnification as soon as the medium was changed to fresh medium with or without Angelica sinensis extract. After 13 h of incubation, another set of images were taken by the same method.
Image analysis for signs of migration was performed by Metamorph Imaging Series. The average scraped area of each well under each condition was measured and subtracted from that of the before-treatment condition. The change in area of the experimental condition was compared with that of the control. A reduction in the scraped area indicates a sign of migration.
Cell Cycle Analysis of HUVEC
The cell cycle analysis of Angelica sinensis extract-treated HUVEC was carried out by flow cytometry. Vehicle-treated control cells and cells treated with Angelica sinensis extract for 4 h were harvested, adjusted to a concentration of 106 cells per ml, and fixed in 70% ethanol (4°C) overnight. The fixed cells were washed twice with cold PBS, and then incubated for 30 min with RNase (8 µg/ml) and propidium iodide (PI) (10 µg/ml). The stained cells were analyzed using a Flow Cytometer (BD FACSCanto™ BD Sciences, San Jose, CA). The fluorescent signal was detected through the FL2-H channel and the proportion of DNA in G1, S, and G2/M phases was analyzed using ModfitLT Version 3.0 software (Verity Software House, Topsham).
Zebrafish Embryos Treated With Angelica sinensis Extract
Healthy, limpid, and regular embryos were picked out at their 1–4 cell stage and distributed into a 24-well microplate. Following this, Milli-Q water was blotted from the microplate wells, and different concentrations (50 µg/ml to 400 µg/ml) of Angelica sinensis extract solution were added to the wells as soon as possible. Embryos treated in individual wells were then incubated at 28°C for 72–120 h, depending on the assay. Embryos receiving DMSO (0.2%) served as a vehicle control and were equivalent to no treatment. All of these experiments were repeated at least three times, and with 30 embryos per group.
Zebrafish Embryos Microinjected With VEGF Serving as Positive Controls
VEGF was injected either into the yolk ball or into the perivitelline space between the yolk and the periderm. These two sites for injection were chosen because proteins in the yolk are often taken up by the embryo, and the second location is in the path of venous return; therefore, after injection, proteins end up in the circulation of the embryo. Embryos were collected as described above. The embryos were then sorted into holding ramps made of 1% agarose in Milli-Q water, and oriented with the yolk ball projecting upwards. The injection was performed as follows: 100 µg/ml solution of VEGF was back-filled into a pulled-glass micropipette; the micropipette was then attached to a micromanipulator and a picospritzer (Harvard/Medical Systems PLI-90 Picoliter Injector) which was attached to a nitrogen tank. Using the micromanipulator, the tip of the micropipette was inserted into the embryo and a small volume of protein solution was expelled from the tip using positive pressure. While the volume expelled was variable, we injected approximately 10–15 nl of solution (approximately 1.0–1.5 ng VEGF), based on the size of the droplet expelled from the pipette tip at the onset of the injection.
Visual Screens of Zebrafish Embryos Using Fluorescence Microscopy
After the addition of Angelica sinensis extract, the embryos were maintained in individual wells of microtiter plates at 28°C until 72 h post-fertilization (hpf). After 24 and 48 h, embryos were visually inspected for viability, gross morphological defects, heart rate, and circulation. Observation of changes in morphology of blood vessel growth and development in embryos were routinely performed using fluorescence microscopy (Olympus IX70, Tokyo, Japan) and a digital camera (Nikon Coolpix 4500, Japan) (at 40× and 100× magnification). Images were analyzed with Adobe Photoshop 7.0 and ACDSee 7.0.
Analysis of mRNA Expression by Real-time PCR
Using “chemistry” on the ABI PRISM® 6100 Nucleic Acid Prepstation according to the manufacturer's instructions, total RNA was extracted from the HUVEC cells under study. The quantity of RNA was measured by spectrophotometric analysis at 260 nm (Beckman Coulter DU® 640, Fullerton, CA). The quality and integrity of the extracted RNA was assessed by both spectrophotometric analysis at 260 nm/280 nm and gel electrophoresis in 1.0% agarose Tris-Acetate-EDTA (TAE) gels visualized by ethidium bromide staining under ultraviolet (UV) light. The extracted RNA (0.8 µg) was converted to single-strand cDNA using the SuperScript™ III First-Strand Synthesis System. Each RT-PCR reaction consisted of denaturation at 65°C for 5 min, annealing at 25°C for 10 min, cDNA synthesis at 50°C for 50 min, termination of the reaction at 85°C for 5 min, and removal of RNA by RNase H at 37°C for 20 min, carried out using the Gene Amp® PCR System 9700 (Applied Biosystems, Singapore). mRNA expression levels of VEGF, kinase-domain region/fetal liver kinase-1 (KDR/Flk-1), fms-like tyrosine kinase (Flt-1), and GAPDH were quantified by real-time PCR. TaqMan® probes and primers for VEGF, KDR/Flk-1, Flt-1, and GAPDH were Assay-on-Demand gene expression products (Roche, Foster City, CA). The cDNA product derived from 0.02 µg of RNA was used in a 20 µl PCR reaction containing 10 µl 2× PCR reaction mix (TaqMan® Universal PCR Master Mix, Branchburg, NJ) and 250 nM each of the primers. The PCR was performed in the ABI 7500 Real-Time PCR system (Applied Biosystems) with the following amplification profile: hold at 50°C for 2 min, hold at 95°C for 10 min, and 40 cycles at 95°C for 15 s, 60°C for 1 min. The reaction without cDNA product served as a negative control. The relative expression of VEGF, KDR/Flk-1, Flt-1 mRNA was normalized to the amount of GAPDH in the same cDNA by using the relative quantification method as described by the manufacturer.
Cell Signaling Analysis of Phospho ERK 1/2, Phospho JNK 1/2, and Phospho p38 by Cytometric Bead Array (CBA)
The cell signaling analysis of Angelica sinensis extract-treated HUVEC was assessed using a bead-based immunoassay, combined with flow cytometry. Briefly, according to the manufacturer's instructions for the cell signaling master buffer kit (BD™ CBA), vehicle control cells and Angelica sinensis extract (4 h)-treated cells were harvested and lysed with denaturation buffer at 100°C for 5 min. After lysis of the cells, the supernatant was isolated by centrifugation. Protein was quantified using the Bradford [1976] method with bovine serum albumin as standard. Using the assay diluent, each cell lysate sample was diluted to 10 µg per 50 µl. In addition, cell signaling flex set capture beads (including phospho ERK 1/2 (T202/Y204), phospho JNK 1/2 (T183/Y185), and phospho p38 (T180/Y182) capture beads) and PE detection reagents were diluted to their working concentrations, and added to each assay tube. The diluted samples were then transferred to the appropriate assay tubes and incubated for 4 h at room temperature (protected from direct exposure to light). The samples were washed with 1.0 ml of wash buffer and centrifuged. Finally, wash buffer (300 µl) was added to each of the samples and the samples were analyzed using a flow cytometer (BD FACSCanto™, BD BioSciences). Three CBA flex set standard curves, phospho ERK 1/2 (T202/Y204), phospho JNK 1/2 (T183/Y185), and phospho p38 (T180/Y182), were prepared to perform the assays. The CBA data were analyzed using FCAP Array™ version 1.0 software (Soft Flow).
Statistics
Data were expressed as mean ± SEM of control. Student's t-test was used to analyze the data, with P < 0.05.
RESULTS
Identification of Chemical Composition of the Angelica sinensis Extract
Methods have been developed using high-performance liquid chromatography for qualitative determination of the characteristic components of Angelica sinensis. FA, ZL, and BP are common reference standards for identification of Angelica sinensis according to the Pharmacopoeia Commission of PRC [2005]. A peak indicating FA could be directly identified in the extract by HPLC-DAD and this peak coincided with its corresponding reference chemical standard (FA) (data not shown). Moreover, the mass components for peaks representing ZL and BP were determined by mass spectrometry (data not shown) and compared with the mass/mass spectra of the peaks and spectrometric data obtained by Lu et al. [2005]. The results suggested that ZL and BP were present in the extract. Thus, this extract was from Angelica sinensis. In addition, we used the phenol-sulfuric acid method to determine the polysaccharide content. There was approximately 60% polysaccharide in the extract.
Effects of Angelica sinensis Extract on Proliferation of HUVEC
The effects of Angelica sinensis extract on proliferation of HUVEC were evaluated by both XTT assay and the direct cell counting method. The number of HUVEC was determined by counting with a hemocytometer. Proliferation of the HUVEC exhibited a dose-dependency, with a 20% increase in cell number at the high dosage (100 µg/ml) condition (Fig. 1a). Metabolic rate, as an indirect indicator of cell number, was measured with XTT assay. After 24 h of starvation, the HUVEC were cultured with 11.1 to 100 µg/ml of Angelica sinensis extract for 48 h. The amount of the formazon product in the cell culture medium also indicated that the Angelica sinensis extract promoted cell proliferation in a dose-dependent manner (Fig. 1b).
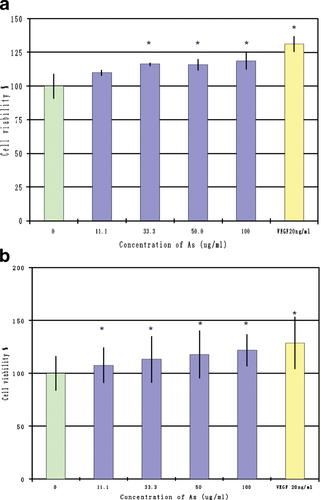
Effects of Angelica sinensis extract on proliferation of HUVEC. a: HUVEC were seeded at 5 × 104 cells/well in a gelatin-coated 24-well plate and cultured with different concentrations (11.1 to 100 µg/ml) of the Angelica sinensis extract medium. After 48 h, the total viable cells in each well were counted using the Trypan blue exclusion method. The results showed that proliferation of HUVEC increased in a dose-dependent manner; in addition, a 20% increase in cell number was observed in the 100 µg/ml treatment group. Data expressed as mean ± SEM from three individual experiments. *P < 0.05 versus cell control. b: HUVEC were seeded at 104 cells/well in 96-well plates and incubated with Angelica sinensis extract as described above. After 48 h, cell proliferation was assessed using the XTT assay. The amount of the formazon product in the cell culture medium indicated that Angelica sinensis extract promoted cell proliferation in a dose-dependent manner. Data expressed as mean ± SEM of duplicate experiments. *P < 0.05 versus control.
Effects of Angelica sinensis Extract on the Cell Cycle Progression of HUVEC in S-Phase
After starvation on 0.5%-FBS, HUVEC were found to accumulate in the G1 phase (87.9 ± 0.8%), with a concomitant decrease in S phase cells (2.4% ± 0.5%). Treatment with 33.3 µg/ml and 100 µg/ml of Angelica sinensis extract induced G1 to S progression, as shown by a statistically significant increase in the proportion of S phase cells (3.4 ± 0.7% for 33.3 µg/ml and 5.9 ± 1.5% for 100 µg/ml). This result indicated that Angelica sinensis extract stimulated proliferation of HUVEC and increased HUVEC in the DNA synthesis phase (Fig. 2).
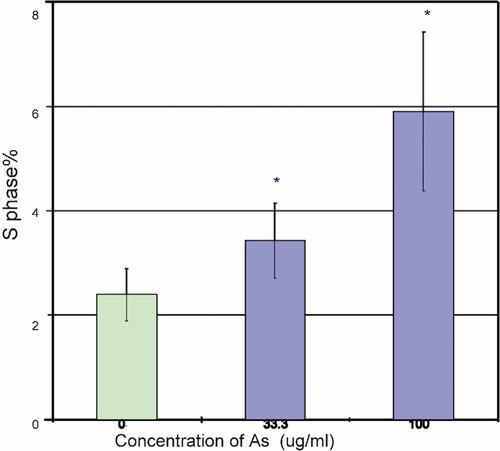
Effect of Angelica sinensis extract on the cell cycle. By starvation with 0.5%-FBS, HUVEC were found to accumulate at G1 phase (87.9 ± 0.8%), with a concomitant decrease in S phase cells (2.4% ± 0.5%). After 4 h incubation with Angelica sinensis extract, treatment with 33.3 µg/ml and 100 µg/ml Angelica sinensis extract induced G1 to S progression, as shown by the significant increase in the count of cells in S phase. Data expressed as mean ± SEM from three individual experiments. *P < 0.05 versus control.
Effect of Angelica sinensis Extract on HUVEC Invasion In Vitro
We subsequently observed the effect of Angelica sinensis extract on HUVEC invasion using the Transwell culture insert. The VEGF-treated cells, serving as a positive control, indicated enhancement of invasion; meanwhile, the number of invaded cells in both treatment groups increased significantly. When compared with the vehicle control group, there were 48.1% and 76.2% increases at concentrations of 33.3 µg/ml and 100 µg/ml, respectively (Fig. 3).
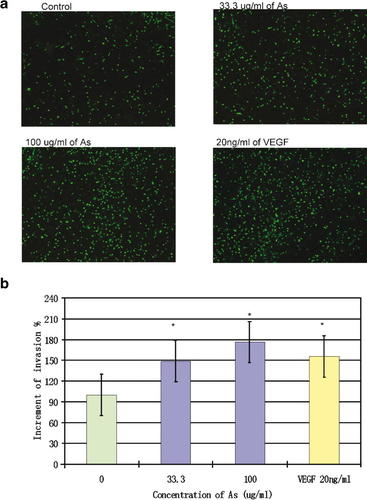
The effect of Angelica sinensis extract on HUVEC invasion. a: Observation of the effect of Angelica sinensis extract on HUVEC invasion using the Transwell culture insert. HUVEC were seeded onto the inserts and then the inserts were put into the 24-well companion plate-containing medium of 33.3 or 100 µg/ml of Angelica sinensis extract. The inserts were removed after 8 h culture and washed with PBS. Non-invaded cells on the upper surface of the membrane were removed by wiping with cotton swabs. Finally, the membranes were stained with calcein AM and captured by the fluorescent inverted microscope (at 50×). b: 48.1% and 76.2% enhancement of invasion of the HUVEC was induced by Angelica sinensis extract at concentrations of 33.3 µg/ml and 100 µg/m, respectively. The VEGF-treated cells, serving as a positive control, showed that there was also an enhancement of invasion. Data expressed as mean ± SEM from three individual experiments. *P < 0.05 versus control. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Effect of Angelica sinensis Extract on Matrigel-Induced Tube Formation by HUVEC
The processes of angiogenesis are complex, typically consisting of proliferation and alignment to form tubular structures [Risau, 1997; Isner and Asahara, 1998]. To test the ability of Angelica sinensis extract to induce EC capillary tube formation, a Matrigel model was used in this study. When HUVEC were cultured on Matrigel, a solid gel of mouse basement membrane proteins, they easily align and form hollow tube-like structures. As shown in Figure 4a, when HUVEC were plated on Matrigel in low serum medium, there was little tube formation. In the treatment groups, however, the VEGF and Angelica sinensis extract induced morphogenetic effects. As shown in Figure 4b, the mean tube length of Angelica sinensis extract treatment groups was longer than that of the vehicle control. Quantitative measurements confirmed that Angelica sinensis extract triggered a significant increase in mean tube length.
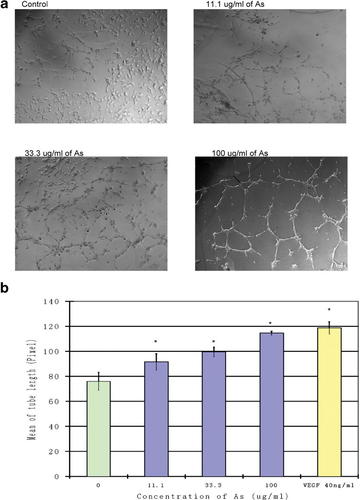
Morphological features of Angelica sinensis extract-treated HUVEC on matrigel. a: Differentiation of HUVEC in three-dimensional Matrigel cultured in treatment of Angelica sinensis extract (11.1 µg/ml, 33.3 µg/ml, and 100 µg/ml). Cells receiving DMSO (0.1%) served as a vehicle control; cells cultured in 40 ng/ml VEGF served as positive control (data not shown). b: Average of tube length in different concentrations of Angelica sinensis extract-treated HUVEC was calculated using computer software (Metamorph). The results indicated that Angelica sinensis extract could stimulate HUVEC to form longer tubes; the measurement of the mean tube length increased in a dose-dependent manner. Data expressed as mean ± SEM from three individual experiments. *P < 0.05 versus control.
Angelica sinensis Extract Enhances HUVEC Migration In Vitro
We determined the effect of Angelica sinensis extract on EC migration using the wounding-healing method. Figure 5 illustrates that at 13 h post-wounding of HUVEC, there was little migration measured in the vehicle control group, whereas a dramatic increment in migration could be measured in both treatment groups. Compared with the vehicle control group, Angelica sinensis extract at concentrations of 33.3 µg/ml and 100 µg/ml caused 38.9% and 66.0% increments in migration, respectively. Thus, Angelica sinensis extract significantly enhanced HUVEC migration in vitro (Fig. 5).
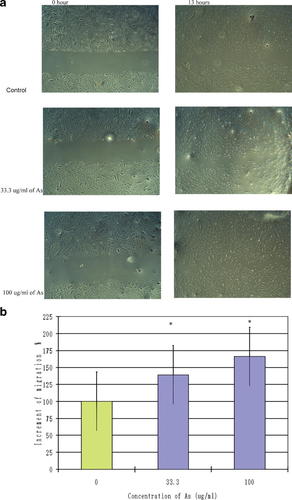
Migration assay for Angelica sinensis extract-treated ECs. a: Migration pattern of ECs after 13 h of Angelica sinensis extract (33.3 µg/ml and 100 µg/ml) treatment. b: The migration ability was analyzed by averaging the scraped area of each well under each condition for three independent experiments. *P < 0.05 versus control.
Proangiogenic Effect of Angelica sinensis Extract in Zebrafish
Stimulation of angiogenesis with VEGF as positive control
VEGF, a selective mitogen for ECs, is known to be important growth factor for vascular development and angiogenesis. Because of this, human VEGF was injected into the yolk sacs of zebrafish as a control for angiogenic stimulation. After injection, the angiogenic phenotype (the appearance of long and more spikes projecting from the subintestinal vessel (SIV) basket) was observed (Fig. 6) and no apparent vascular changes were observed in other regions. The percentages of abnormal phenotypes at SIV basket are given in Table I.
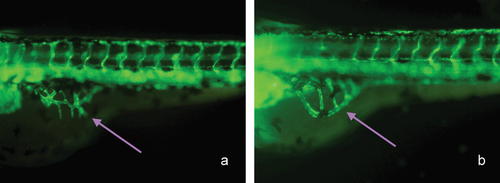
The effects of VEGF on blood vessel formation in SIVs (subintestinal vessels, white arrow) of Tg (fli1: EGFP) zebrafish embryos. (a) treated with 1.5 ng VEGF (b) control with DD H2O. (a) and (b) were observed under a fluorescent microscope at 100× magnification (lateral views, anterior is to the left) at 96 hpf. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
| Drug extent of increase in angiogenic phenotype in SIVs | VEGF 1.5 ng (1 mg/ml) | AS 50 (µg/ml) | AS 100 (µg/ml) | AS 200 (µg/ml) | AS 400 (µg/ml) | Ctrl |
|---|---|---|---|---|---|---|
| + | 25% ± 5 | 18% ± 7 | 18% ± 4 | 20% ± 5 | 33% ± 6 | 0 |
| ++ | 37% ± 9 | 28% ± 7 | 27% ± 1 | 22% ± 5 | 25% ± 5 | 0 |
| +++ | 12% ± 5 | 2% ± 3 | 2% ± 3 | 4% ± 4 | 7% ± 6 | 0 |
| Total | 74%% ± 6 (P < 0.01) | 48% ± 9 (P < 0.01) | 47% ± 4 (P < 0.01) | 46% ± 2 (P < 0.01) | 64% ± 5 (P < 0.01) | 0 |
- Frequency of zebrafish embryos with different degrees of change of blood vessel formation in SIVs was shown in the Table I.
- The extents of increased angiogenic vessel formation were represented by number of “+.”
- + is an estimated arbitrary unit of increase in SIVs. (n = 30 per group, repeated three times).
- AS represents the effects of Radix Angelicae Sinensis. VEGF is vascular endothelial growth factor.
Changes in vessels after Angelica sinensis extract treatment
In zebrafish, angiogenic vessel development does not begin until 20 h post fertilization (hpf). Therefore we detected changes in SIVs after 72 hpf. At this time point, SIVs showed a very clear pattern, which facilitated visual identification of aberrations in blood vessel formation [Seng et al., 2004]. Figure 7 shows the results of treatment with different concentrations of Angelica sinensis extract. The drug treatments ended at 72 hpf but the blood vessel formations were observed and recorded at 96 hpf and 120 hpf. The results indicate that Angelica sinensis extract stimulates angiogenesis in the SIVs of zebrafish and no apparent vascular changes were observed in other regions. The frequency of embryos with varying degrees of increased blood vessel formation in the SIVs (percentage of abnormal phenotypes) is shown in Table I and Figure 8. All experiments were repeated at least three times, with 30 embryos per group.
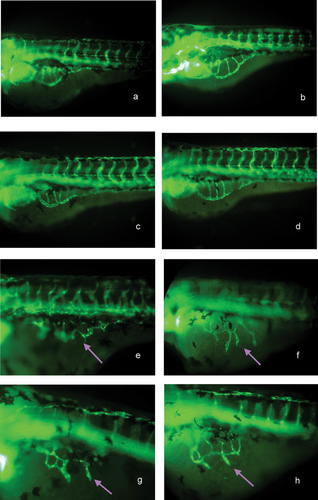
The effects of Angelica sinensis extract on blood vessel formation in SIVs (white arrow) of Tg (fli1: EGFP) zebrafish embryos. (a) and (b) show control without any treatment at 78 hpf and 96 hpf. (c, d) were treated with 0.2% DMSO and at 78 hph and 96 hpf, showing no effect on vessel formation. (e, f) were treated with extract of Angelica sinensis at 50 µg/ml, (e) is at 78 hpf, and (f) is the same fish at 98 hpf. (g, h) were treated with extract of Angelica sinensis at 100 µg/ml, (g) is at 78 hpf, and (h) is the same fish at 96 hpf. (a–h) are representative embryos from each treatment group (n = 30 per group, repeated three times) (lateral views, anterior is to the left). Arrowhead indicates the abnormal phenotype of blood vessel formation in SIVs. A summary of the quantitative analysis of embryos showing different degrees of change in blood vessel formation is shown in Table I. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
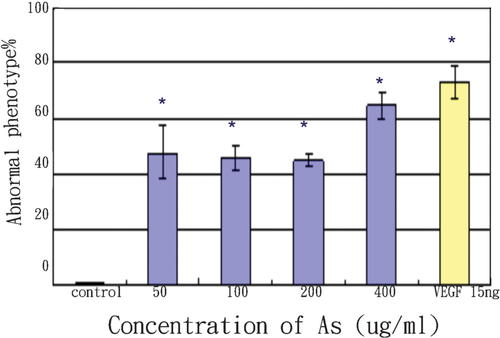
The percentage of phenotypes after drug treatment. Data expressed as mean ± SEM. n = 30 per group, repeated three times. *P > 0.01 versus control.
Because the drug was routinely prepared in a carrier or solvent, such as DMSO, the tolerable concentration of the solvent for the zebrafish model was determined. It was found that the growth and development of fish was not affected by 0.2% DMSO (Fig. 7c, d). The percentage of abnormal phenotypes observed is shown in Table I and Figure 8.
Detection of mRNA Expression in Angelica sinensis Extract Treated HUVEC
In order to identify the molecular targets of the angiogenic effects of Angelica sinensis extract on HUVEC, we measured mRNA expression using real-time PCR. As shown in Table II, Angelica sinensis extract could significantly upregulate the gene expression of VEGF in a dose-dependent manner (2.1 ± 0.8-fold at the low concentration; 4.0 ± 0.9-fold at the high concentration). Figure 9 shows that Angelica sinensis extract has a trend of a slight and statistically insignificant increase in mRNA expression of KDR/Flk-1 in a dose-dependent manner. In contrast, Angelica sinensis extract causes an apparent dose-dependent decrease in mRNA expression of Flt-1.
| Concentration of As gene name | Control | 33.3 µg/ml | 100 µg/ml | Upregulated (↑) or downregulated (↓) |
|---|---|---|---|---|
| VEGFRQa | 1.0 ± 0.1 | 2.1 ± 0.8* | 4.0 ± 0.9* | ↑ |
| VEGFFCc | 1.0 | 2.1 | 4.0 | |
| KDRRQ | 1.0 ± 0.2 | 1.5 ± 1.2 | 2.2 ± 1.3 | ↑ |
| KDRFC | 1.0 | 1.5 | 2.2 | |
| Flt-1RQ | 1.0 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.2 | ↓ |
| Flt-1FC | 1.0 | −1.4b | −1.7 |
- The values represent the fold change of the samples with their direction. Data expressed as mean ± SEM from three individual experiments.
- * P < 0.05 versus control.
- a Relative Quantification (RQ) is the range given for the target gene relative to the control.
- b If the RQ is less than 1, it means that this gene expression is downregulated, thus, it is calculated as 1/RQ plus a minus sign to indicate the direction of downregulation expression.
- c In contrast, if the RQ is more than 1, it means that this gene expression is upregulation.
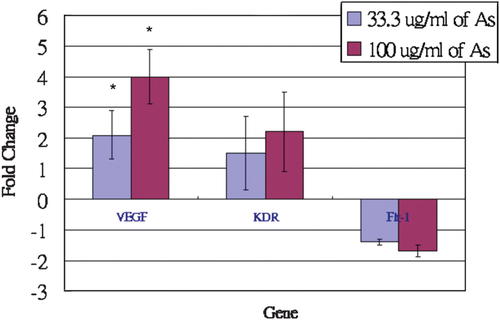
Gene expression in Angelica sinensis extract-treated HUVEC. Data expressed as mean ± SEM from three individual experiments.
Effect of Angelica sinensis Extract on the Signaling Pathway of p38, JNK 1/2, and ERK 1/2 in HUVEC
The expression of phospho ERK 1/2, phospho JNK 1/2, and phospho p38 were measured using the CBA Cell Signaling Flex Set system. The capture bead of this assay is a single bead population coded with a distinct fluorescence intensity of APC and coated with a capture antibody specific for a soluble protein analyte which is ultimately detected by PE-conjugated antibody based on a sandwich immunodetection principle. Different bead populations can be differentiated and identified by the fluorescence intensity of APC; therefore, the system allows simultaneous detection of spectrally discrete beads with different capture antibodies to create a multiplex assay.
In this assay, the capture beads (including Phospho ERK 1/2 (T202/Y204), phospho JNK 1/2 (T183/Y185), and phospho p38 (T180/Y182) capture beads), PE-conjugated detection reagent, and soluble analytes (either standards or test samples) are incubated together to form sandwich complexes. Figure 10 represents the variation of bead populations, which recognize either phospho ERK 1/2, phospho JNK 1/2, or phospho p38 in HUVEC lysates. The shift of bead populations of different phosphorylated protein targets from left to right indicate an increase in PE fluorescence intensity and an increase in the amount of the corresponding phosphorylated protein in the cell lysates. Following acquisition of sample data using flow cytometry, the results were analyzed using FCAP Array™ version 1.0 software. Figure 11 illustrates the relative expression levels of these phosphorylated protein targets in HUVEC. Approximately, 200% and 80% of phospho JNK 1/2 and phospho p38 expression increased, respectively, in HUVEC treated with 100 µg/ml of Angelica sinensis extract, compared with the control; the results did not show a statistically significant activation in the ERK 1/2 cell signaling pathway. Thus, the results suggest that Angelica sinensis extract distinctly activated the JNK 1/2 and p38 signal pathways but not the ERK 1/2 pathway.
Expression levels of phospho ERK 1/2, phospho JNK 1/2, and phospho p38 in Angelica sinensis extract-treated HUVEC. The position of each type of bead is marked on the right. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
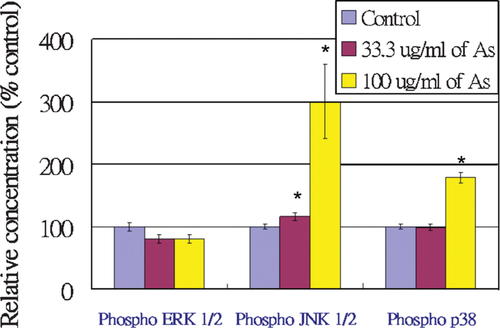
The relative expression levels of phospho ERK 1/2, phospho JNK 1/2, and phospho p38 in Angelica sinensis extract-treated HUVEC. Data expressed as mean ± SEM from three individual experiments. *P < 0.05 versus control. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
The development of new blood vessels from pre-existing ones is generally referred to as angiogenesis [Holash et al., 1999], and plays an important role in physiologic and pathologic processes, such as embryonic development, wound healing, tumor growth, metastasis, and various inflammatory disorders [Folkman, 1995]. The processes of angiogenesis typically consist of proliferation and alignment to form tubular structures [Breier and Risau, 1996]. All forms of angiogenesis are thought to share certain basic features, including migration and mitogenesis of ECs, lumen formation, connection of new vascular segments with the pre-existing circulation, and extensive remodeling of the extracellular matrix (ECM) by proteases. Interactions between ECs and ECMs, including fibrinogen, vitronectin, collagen, laminin, and von Willebrand factor (VWF), through cell surface adhesion receptors, are involved in the multiple processes of neovascularization [Stromblad and Cheresh, 1996]. Wu and Zhu [2001] have indicated that a traditional Chinese medicine formula, with Angelica sinensis as the main ingredient, could promote the proliferation of HUVEC and the expression of CD54 (ICAM-1). Thus, it is interesting to investigate whether Angelica sinensis extract affects the viability of ECs.
As shown in Figure 1, Angelica sinensis extract dose dependently increased cell proliferation. In addition, the results of the cell cycle analysis indicated that Angelica sinensis extract stimulated HUVEC proliferation and increased HUVEC in the DNA synthesis phase. These results suggest that Angelica sinensis extract can promote EC proliferation. Besides cell proliferation, endothelial migration, invasion and tube formation are essential during the angiogenic process. The wound healing migration assay, as shown in Figure 5, illustrated that at 13 h post-wounding of HUVEC, there was little migration measured in the vehicle control group, whereas a dramatic increment in migration was measured in both treatment groups. Meanwhile, the numbers of invaded cells and the mean tube length were significantly increased in both treatment groups. Angelica sinensis extract can therefore be seen to enhance the migration, invasion, and tube formation effects of HUVEC in vitro. The effect of Angelica sinensis extract on promoting angiogenesis was further assessed in vivo in zebrafish by observation of morphological changes in SIVs. The results indicate that Angelica sinensis extract can stimulate angiogenesis in zebrafish in vivo (Fig. 7).
The results of real-time PCR illustrated that Angelica sinensis extract increased VEGF expression, as well as having a tendency to upregulate and downregulate the expression of KDR/Flk-1 and Flt-1, respectively. VEGF, also known as vascular permeability factor (VPF), was originally described as an EC-specific mitogen and a potent angiogenic factor [Ferrera, 1999] and was first described as an essential growth factor for vascular ECs. The formation of new blood vessels is orchestrated by a plenitude of different proteins, also including cell adhesion molecules, ECM components, and VEGF receptors. Gene targeting experiments have provided insights into the function of the VEGF receptor [Carmeliet et al., 1996; Ferrera and Carver-Moore, 1996]. Although the inactivation of each individual VEGF receptor could cause embryonic lethality at mid-gestation, they have different functions [Fong et al., 1995; Shalaby et al., 1995]. KDR/Flk-1 is the receptor that initiates the main signaling pathways activated by VEGF in ECs. In contrast, the main function of Flt-1 in ECs appears to be to regulate binding between VEGF and KDR/Flk-1 [Park et al., 1994]. In this investigation, we found statistically significant increases in the mRNA expression of VEGF after treatment with 33.3 µg/ml and 100 µg/ml of Angelica sinensis extract. However, there was a trend of upregulating and downregulating expression of KDR/Flk-1 and Flt-1, respectively, but these were not statistically significant. In order to confirm if the VEGF and VEGF receptor, KDR/Flk-1, was involved in the proangiogenic effect of Angelica sinensis extract on HUVEC. The effect of SU5416, [3-(3,5-dimethyl-1H-pyrrol-2-ylmethylene)-1,3-dihydro-indol-2-one] which is a selective inhibitor of the VEGF receptor for KDR/Flk-1 [Bocci et al., 2004], on Angelica sinensis extract induced invasion was determined. The VEGF receptor inhibitor at 0.3 µM was found to abolish Angelica sinensis extract induced-invasion in HUVEC (data not shown). These data confirmed the predominant involvement of VEGF and KDR/Flk-1 pathway in the Angelica sinensis extract-induction pathway.
The CBA immunoassay showed that Angelica sinensis extract distinctly activated the JNK 1/2 and p38 cell signal pathways but not the ERK 1/2. Although VEGF-triggered angiogenic pathways in ECs are not fully understood, groups of signaling molecules such as Src, Akt, and mitogen-activated protein kinases (MAPKs) have been reported to be involved in VEGF signaling cascades. Three subfamilies of MAPK are known: extracellular signal regulated kinase 1/2 (ERK 1/2), c-Jun N-terminal kinase 1/2 (JNK 1/2), and p38 MAPK (p38). They are activated by growth factors, including bFGF and VEGF, and have been implicated in playing critical roles during angiogenesis [Beckner, 1999]. ERK 1/2 are usually strongly activated by growth factors and agents that stimulate cell growth, and thus are important for cell proliferation [Cobb et al., 1991]. Two other subtypes of the MAPK family, JNK 1/2 and p38, generally inhibit growth and/or induce apoptosis [Xia et al., 1995; Kyriakis and Avruch, 1996]. Moreover, recent studies have shown that they play important roles in the regulation of cytoskeleton structures, cell morphology, and motility of ECs [Guay et al., 1997; Becker et al., 2001]. VEGF-induced activation of p38 is correlated with changes in permeability and migration [Rousseau et al., 1997]. Some studies have demonstrated that an ATP-competitive inhibitor of JNK 1/2 exhibits broad-based antiproliferative activity in human endothelial and tumor cell lines. It suppresses proliferation by arresting cells in the G2/M phase of the cell cycle and inhibits EC migration [Ennis et al., 2005]. In order to identify the cell signaling mechanism involved in the angiogenic effects of Angelica sinensis extract, we measured the levels of phosphorylation of ERK 1/2, JNK 1/2, and p38 by CBA. The results showed that treatment with 100 µg/ml of Angelica sinensis extract markedly activates JNK 1/2 and p38 cell signaling pathways in HUVEC. Angelica sinensis extract probably stimulates JNK 1/2 and p38 cell signaling pathways to play multiple roles in controlling cell proliferation, viability, and morphogenesis. Moreover, these effects are achieved through the regulation of JNK 1/2 and p38 cell signaling pathways and a delicate balance between the levels of VEGF and VEGF receptors, as shown by the real-time PCR results. A schematic overview of Angelica sinensis extract acting on HUVEC is shown in Figure 12.
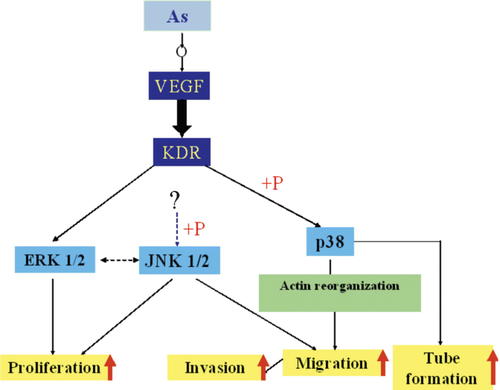
Schematic overview of Angelica sinensis extract acting on HUVEC. After Angelica sinensis extract is added to HUVEC, it activates gene expression of VEGF. Then, binding between VEGF and VEGF receptors further activates the p38 pathway. p38 is possibly phosphorylated to activate migration, invasion and tube formation in HUVEC. JNK 1/2, which is regulated by unknown upstream targets in angiogenesis, is phosphorylated to enhance proliferation as well as migration. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In conclusion, our study has demonstrated that incubation with Angelica sinensis extract can promote several features of angiogenesis in HUVEC in vitro and zebrafish in vivo. The studies have also elucidated the mechanisms of the angiogenic effects of Angelica sinensis extract on HUVEC: Angelica sinensis extract probably promotes angiogenesis through enhancing VEGF expression and stimulating JNK 1/2 and p38 phosphorylation to control cell proliferation, viability, and morphogenesis.
Acknowledgements
This study is supported by the grant from the Research Committee, University of Macau, Macau SAR (Ref. No. RG087/04-05S/LMY/ICMS/FST) and grant from the Science and Technology Development Fund, Macau SAR (Ref. No. 078/2005/A2). Ms. Hui-Chao Lin was supported by scholarship kindly provided by Guangdong Provincial Department of Science and Technology.




