Androgen axis in prostate cancer
Abstract
Endocrine therapy for advanced prostate cancer is based on androgen ablation or blockade of the androgen receptor (AR). AR action in prostate cancer has been investigated in a number of cell lines, their derivatives, and transgenic animals. AR expression is heterogenous in prostate cancer in vivo; it could be detected in most primary tumors and their metastases. However, some cells lack the AR because of epigenetic changes in the gene promoter. AR expression increases after chronic androgen ablation in vitro. In several xenografts, AR upregulation is the most consistent change identified during progression towards therapy resistance. In contrast, the AR pathway may be by-passed during chronic treatment with a nonsteroidal anti-androgen. AR sensitivity in prostate cancer increases as a result of activation of the Ras/mitogen-activated protein kinase pathway. One of the major difficulties in endocrine therapy for prostate cancer is acquisition of agonistic properties of AR antagonists observed in the presence of mutated AR. Enhancement of AR function by associated coactivator proteins has been extensively investigated. Cofactors SRC-1, RAC3, p300/CBP, TIF-2, and Tip60 are upregulated in advanced prostate cancer. Most studies on ligand-independent activation of the AR are focused on Her-2/neu and interleukin-6 (IL-6). On the basis of studies that showed overexpression and activation of the AR in advanced prostate cancer, it was suggested that novel therapies that reduce AR expression will provide a benefit to patients. There is experimental evidence showing that prostate tumor growth in vitro and in vivo is inhibited following administration of chemopreventive drugs or antisense oligonucleotides that downregulate AR mRNA and protein expression. J. Cell. Biochem. 99: 373–381, 2006. © 2006 Wiley-Liss, Inc.
Androgenic hormones, in particular dihydrotestosterone and testosterone, exert their effects in prostate gland through binding to the androgen receptor (AR), whose structure was elucidated in 1990s by several research groups. This protein's organization is similar to that of other steroid receptors; well-conserved sequences are present in the ligand- and DNA-binding domains, whereas the N-terminal region contains a number of polyglutamine and polyglycine repeats. AR homodimerizes, binds to DNA response elements, and initiates synthesis of proteins responsible for regulation of prostate cell proliferation, apoptosis, and differentiation.
Improved diagnostic methods in prostate cancer led to an increase of the percentage of tumors detected in early stages. These tumors are frequently subjected to radical prostatectomy although treatment policies vary in different countries. With an increase in proportion of elderly men in the Western world, it is expected that the number of individuals who will need either radical or palliative treatment will also rise in the future. Prostate cancer endocrine treatment is based on discoveries made by Huggins and Hodges in 1940s [Huggins and Hodges, 1941. Those scientists demonstrated that prostate cancer cell growth is dependent on androgens and that recognition was a fundament for development of various endocrine therapies in the 20th century. These therapies are based either on androgen ablation or pharmacological blockade of the AR. The main goal of this review is to provide an interested reader with current concepts of androgen action and anti-androgen therapy in prostate cancer and to discuss some of the mechanisms that are responsible for failure of endocrine therapy.
MODELS FOR STUDIES ON ANDROGEN RECEPTOR IN PROSTATE CANCER
Initial studies on AR in prostate cancer were carried out by ligand binding assays in a limited number of rat and human cell lines. In general, they showed a decrease of AR expression in cells with a growth advantage [Quarmby et al., 1990. Those early findings led to the assumption that AR levels decrease in patients who fail endocrine therapy. Once AR antibodies became available, it was possible to investigate receptor expression in tissue samples obtained from patients. In contrast to some expectations, those studies clearly demonstrated presence of the AR in tissues from nearly all patients including those who presented with tumor progression. The AR is thus detectable in lymph node and visceral metastatic lesions from individuals suffering from prostate cancer [Hobisch et al., 1995. However, it should be noted that in some patients a portion of cells lacks AR expression due to hypermethylation of the promoter of the AR gene [Kinoshita et al., 2000. This mechanism was first detected in the DU-145 cell line. On the basis of these discoveries in vivo, it became clear that novel models for studies on androgen responsiveness should be developed. Thus, studies on androgen axis in prostate cancer were extended in several ways; established cell line LNCaP originally derived from a lymph node metastasis from a patient who did not respond to endocrine therapy was subjected to steroid withdrawal or prolonged anti-androgen treatment, novel cell lines have been generated, and transgenic mouse model has been applied in studies on androgenic action in prostate cancer.
CHRONIC ANDROGEN ABLATION AND ANTI-ANDROGEN RESISTANCE IN PROSTATE CANCER
Alterations that may occur during long-term androgen ablation were first investigated by the group of Liao and Kokontis whose observations on increase of AR expression and activity were confirmed by other researchers [Kokontis et al., 1994. Those pioneering studies are important for a better understanding of mechanisms responsible for failure of endocrine therapy. It was thus demonstrated that, during various stages of steroid depletion, changes in the proliferation pattern of LNCaP cells occur. Growth of LNCaP cells is regulated by androgens in a biphasic manner. Initially, androgen-ablated cells proliferate in response to lower concentrations of androgens. In later passages, however, only growth inhibition associated with upregulation of cell cycle inhibitors was observed. Those data generated in vivo stimulated discussions in scientific community on potential usefulness of supplementation of androgen in some cases of prostate cancer [Chuu et al., 2005. This concept might be supported by experimental results showing that androgen-insensitive phenotype is reversed following androgen administration in vivo. Although one could expect that expression of all androgen-regulated genes increases after prolonged steroid depletion, this is not the case. For example, some of the steroid-depleted sublines do not express the classic androgen-regulated gene prostate-specific antigen (PSA) due to epigenetic changes in its promoter [Gao et al., 1999. In parental LNCaP cells, the nonsteroidal anti-androgen bicalutamide antagonizes effects of androgenic hormones, in contrast to another antagonist hydroxyflutamide [Veldscholte et al., 1990. Hydroxyflutamide acts as an agonist because of a mutation in the ligand-binding domain of the AR that is responsible for activation by nonandrogenic steroids also. However, long-term steroid deprivation may cause changes in responses to bicalutamide in vitro. We thus observed a twofold stimulation of AR transcriptional activity by bicalutamide in LNCaP-abl cells established during chronic steroid deprivation [Culig et al., 1999. This change was obviously sufficient for bicalutamide-induced growth stimulation in vitro and in vivo. AR sensitvity increased in cells in which the Ras/mitogen-activated protein kinase (MAPK) pathway is chronically stimulated [Bakin et al., 2003. An important question regarding the requirement of the AR for tumor progression was addressed by Sawyers' group [Chen et al., 2004. They have performed a miroarray analysis in a number of xenografts and came to the conclusion that the most consistent change during progression towards resistance to endocrine therapy is upregulation of the AR. Changes in AR activity were associated with acquisition of agonistic properties of AR antagonists and alterations in the coactivator/corepressor ratio. Amplification of the AR gene that is one of the mechanisms leading to increased expression of the protein was discovered in patients after androgen withdrawal therapy. However, its implications have not been completely understood yet. Results of a clinical study showed a better survival in the group of patients with amplified AR gene subjected to complete androgen ablation [Palmberg et al., 2000. It is worth mentioning that AR amplification in vitro was observed also in a cell line derived from a c-myc transgenic mouse with prostate cancer [Watson et al., 2005.
Different mechanisms of therapy resistance are operative during prolonged treatment with bicalutamide; Hara and associates showed that chronic LNCaP treatment with bicalutamide leads to the appearance of the same point mutations as those reported in prostate cancer patients who received that therapy [Hara et al., 2003. A novel xenograft from a liver metastasis with a mutated AR was recently obtained from a patient who was treated with bicalutamide [Yoshida et al., 2005. Tumor volume increased after treatment with the anti-androgen. We and others have addressed the question whether the AR is required for progression of prostate cancer during chronic treatment with bicalutamide. In that study, levels of the AR were downregulated by vitamin E in control LNCaP cells and in the subline generated after bicalutamide treatment [Hobisch et al., 2006. However, inhibition of growth by vitamin E was observed only in controls thus suggesting that the AR signaling pathway is by-passed in the newly generated subline. Kokontis and associates also demonstrated that an increase in AR expression in cells subjected to treatment with bicalutamide and androgen does not confer androgen-independent growth [Kokontis et al., 2005. Since AR levels increase during prolonged androgen withdrawal, one could speculate that cyclic withdrawal and supplementation of androgen may not only modulate AR levels, but also inhibit growth of tumor cells. Intermittent androgen withdrawal is an option for prostate cancer patients who benefit from improved quality of life. Our experimental work has demonstrated that prostate cancer sublines derived as a result of intermittent androgen ablation in vitro acquire a growth advantage [Hobisch et al., 2004. These data suggest that the AR pathway has a different role during chronic or intermittent androgen ablation or treatment with anti-androgen, respectively.
Most data obtained on AR signaling in prostate cancer reflect the situation in LNCaP cells and their sublines. In recent studies, researchers have utilized LAPC-4 cells that express the wild-type AR and MDA PCa cells whose receptor contains a double mutation and is increasingly activated by glucocorticoid hormones [Zhao et al., 2000. This mechanism of receptor activation may be physiologically relevant. Since tissue levels of testosterone and AR optical density are similar in benign prostate and relapsed cancer [Mohler et al., 2004, it could be concluded that androgen axis is functional in patients with a progressive disease.
ANDROGEN RECEPTOR MUTATIONS IN PROSTATE CANCER SPECIMENS
Initial studies on frequency of AR point mutations in prostate cancer did not yield unequivocal results. Problems in interpretation of data may occur because of different percentage of cells expressing a point mutation and contamination of specimens with stromal cells. More recent experimental work revealed that there is an increase in the percentage of these mutations during tumor progression [Marcelli et al., 2000. There might be different implications of AR mutations in prostate cancer tissue; most interesting mutations are those that show a gain of function. In some cases, there was no change in functional properties of the receptor and diminished function was also reported. Most interestingly, AR mutations have been detected in patients who received treatment with anti-androgens. A subgroup of patients who received treatment with hydroxyflutamide showed a temporary response to the anti-androgen bicalutamide [Taplin et al., 1999. This is the reason why “second line” endocrine treatment may be beneficial in some cases. It does not seem that a single mechanism is responsible for the anti-androgen withdrawal syndrome, a condition in which discontinuation of endocrine treatment is associated with a paradoxical decrease in PSA levels and, in some patients, improvement in clinical status. Specific point mutations may, however, contribute to this syndrome. Most AR mutations studied were detected in the conserved domains. However, patients who were subjected to treatment with estramustine presented with AR mutations in the N-terminus [Hyytinen et al., 2002. Mutated AR may be increasingly activated by adrenal androgens and products of dihydrotestosterone metabolism, compounds considered as weak androgenic hormones. Such mechanism was demonstrated for the mutations, which were first characterized in prostate cancer tissue, Val715Met and Val730Met [Newmark et al., 1992; Culig et al., 1993. Although these are the same amino acid changes located in the close proximity, the first one was detected in a specimen representing metastatic tumor and the second one in an organ-confined cancer. A direct oncogenic role for an altered AR was reported for a murine receptor that contains a mutation in the N-terminal region [Han et al., 2005. This might be a rare event and most AR gene mutations generate proteins that show hyperresponsiveness during endocrine therapy.
IDENTIFICATION OF ANDROGEN RECEPTOR COACTIVATORS INVOLVED IN PROSTATE CANCER PROGRESSION
There is a broad spectrum of coregulatory proteins that interact with one or more domains of the AR. The first coactivator detected in prostate cancer is ARA70 that increases AR activation by steroids and anti-androgens [Yeh and Chang, 1996. However, there is no consensus regarding its expression at various stages of prostate carcinogenesis. Recent studies are focused on investigation of alterations in expression and aberrant cofactor function in prostate cancer models (Fig. 1).
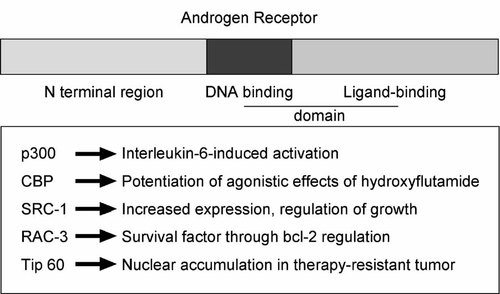
Summary of action of important AR coactivators in prostate cancer.
One of the coactivator proteins frequently studied in endocrine-related cancer is SRC-1. Although a study by Linja and associates did not suggest that SRC-1 mRNA expression changes during prostate cancer progression, it seems that the SRC-1 protein is upregulated in specimens obtained from patients who do not respond to endocrine therapy [Gregory et al., 2001; Linja et al., 2004; Agoulnik et al., 2005. Reduction of SRC-1 in prostate cancer cell lines led to an inhibition of cell growth of AR-positive LNCaP cells and their C4-2 derivative, whereas AR-negative cells were not affected. From those results, it seems that AR-SRC-1 interactions are required for cellular proliferation. Similar results were obtained with the cofactor SRC-3 (RAC-3) whose expression is also increased in patients with high tumor grade and stage [Zhou et al., 2005. Downregulation of RAC-3 by siRNA led to a decreased proliferation, reduced tumor growth in vivo, and increased apoptosis through inhibition of expression of bcl-2. Demonstration of inhibition of tumor volume in vivo after treatment with a coactivator siRNA is particularly important. It shows that there is no or minimal coactivator redundancy in the case of SRC-3. Importance of SRC-1 in prostate cancer was demonstrated in experiments on ligand-independent activation of the AR by interleukin-6 (IL-6) [Ueda et al., 2002. Another coactivator that is of critical importance for AR activation in the absence of ligand is p300 whose expression correlates with bad prognosis in prostate cancer [Debes et al., 2002. Function of p300 was studied also in the LNCaP-IL-6+ cells generated during chronic treatment with IL-6 [Debes et al., 2005. Most interestingly, although late passages of these cells lack AR protein, the target gene PSA is expressed following transfection of p300 cDNA. This mechanism may be relevant to expression of some AR-downstream genes in conditions in which the receptor itself is silenced due to hypermethylation in the promoter gene. The functional homolog of p300, CBP, a transcriptional coactivator whose expression is downregulated by androgens [Comuzzi et al., 2004. One of undesirable consequences of androgen ablation may be elevation of expression of some AR coactivators. CBP preferentially potentiates AR activation by hydroxyflutamide rather than that by bicalutamide [Comuzzi et al., 2003. Flutamide-induced AR activation was enhanced by gelsolin, a coactivator that is also upregulated following androgen withdrawal [Nishimura et al., 2003. Nuclear accumulation of Tip60 was observed in specimens from patients with refractory prostate cancer in contrast to benign prostate hyperplasia and primary prostate tumors [Halkido et al., 2003. Tip60 levels increase in LNCaP cells and in the CWR22 xenograft after androgen ablation. Another example of a coactivator highly expressed in hormone-refractory prostate cancer is cyclin G-associated kinase [Ray et al., 2006. AR activity is also under control of lysine-specific demetylase1, enzyme that demetylates repressive histones and colocalizes with the AR in benign and malignant tissue [Metzger et al., 2005. Huntingtin interacting protein 1 is a recently described AR coactivator that is capable of causing cellular transformation and enhancing AR activity [Mills et al., 2005. TIF-2 is a coactivator implicated in enhancement of AR action by epidermal growth factor whose expression is also upregulated in prostate cancer [Gregory et al., 2001, 2004.
ALTERNATIVE PATHWAYS IN ACTIVATION OF THE ANDROGEN RECEPTOR
Numerous reports have documented ligand-independent and synergistic activation of the AR by growth factors, peptide hormones, and interleukins. It is of special interest to analyze in vivo implications of the results mostly obtained in cell lines transfected with a reporter gene and receptor cDNA. In one such approach, it was demonstrated that AR activation by HER-2/neu is driving progression of the LAPC-4 xenograft towards therapy resistance [Craft et al., 1999. Conversely, androgen-induced AR transcriptional activity depends on HER-2/neu [Mellinghoff et al., 2004; Liu et al., 2005. Administration of anti-HER-2/neu antibodies or inhibitors impaired AR-mediated cellular regulations. In contrast to the tumor-promoting effect, there is an evidence that ligand-independent activation of the AR by IL-6 leads to increased expression of PSA that is inhibited by an anti-androgen [Hobisch et al., 1998. IL-6 is a cytokine that is produced by prostate cells in vitro and in vivo. IL-6 and IL-6 receptor levels increase in tissues from patients with locally confined disease and high cytokine values were measured in sera from patients with metastases [Twillie et al., 1995; Giri et al., 2001. IL-6 is a multifunctional cytokine that may cause phosphorylation of signal transducers and activators of transcription (STAT)3, MAPK, and phosphatidylinositol 3-kinase (PI 3-K). It was shown that there is a physical interaction between STAT3 and the AR that may explain the effects of IL-6 on receptor activation [Matsuda et al., 2001. In addition, MAPK phosphorylate amino acids in the N-terminal region of steroid receptors, including the AR. Divergent results are reported in the literature regarding the influence of the PI 3-K pathway on the AR. It was shown that in lower passages of LNCaP cells activation of that signaling pathway leads to decreased receptor activation, whereas in higher passages it has a positive effect on the AR [Lin et al., 2004. Similarly, opposite results on regulation of AR activity by glycogen synthase kinase-3beta that is linked to the PI 3-K were obtained [Liao et al., 2004; Salas et al., 2004. Oncostatin M, an IL-6-related cytokine that stimulates growth of prostate cancer cells in an autocrine manner, was also found to be able to induce AR activity [Godoy-Tundidor et al., 2002. AR activation by oncostatin M could not be suppressed by nonsteroidal anti-androgens.
Activation of the epidermal growth factor receptor by its ligand triggers association of the protein kinase Src with the AR and estrogen receptor-beta in LNCaP cells [Migliaccio et al., 2005. This association could be prevented by steroid receptor antagonists or inhibition of their expression. Constitutive activation of Src was observed in high LNCaP passages that do not respond to dihydrotestosterone [Unni et al., 2004. These findings, taken together with previous data showing ligand-independent and synergistic activation of the AR by epidermal growth factor and related peptides, show that there is a complex interplay between the two signaling cascades [Culig et al., 1994.
NOVEL THERAPY APPROACHES AIMED TO INHIBIT ANDROGEN RECEPTOR EXPRESSION AND FUTURE PERSPECTIVES
Nonsteroidal anti-androgens hydroxyflutamide and bicalutamide prevent acquisition of a transcriptionally active form of the AR. Since increased activation of the AR was observed after chronic steroid depletion or as a consequence of a point mutation or ligand-independent activation, it was hypothesized that inhibition of AR expression may provide an improvement in endocrine therapy for prostate cancer. In addition to its role in the regulation of proliferation and survival, the AR mediates upregulation of vascular endothelial growth factor and is also important for lipogenesis [Swinnen et al., 1996; Joseph et al., 1997. There are several ways to achieve this goal. Compounds that have a chemopreventive effect such as vitamin E, selenium, or nonsteroidal anti-inflammatory drugs inhibit AR expression. A more direct way to downregulate the AR is use of antisense oligonucleotides or siRNA. Antisense oligonucleotides against polyglutamine repeat sequences in the N-terminal region are efficient tools and their effect is associated with inhibition of proliferation, in vivo growth, and PSA expression [Eder et al., 2000. A potential limitation of this kind of therapy is that it is currently not known how tumor cells will adapt to long-term ablation of the AR. It also does not distinguish between inhibition of proliferation and differentiation function. It should be kept in mind that stable expression of the AR in PC-3 cells leads to a reduced proliferation and invasion, presumably through a reduced phosphorylation of the epidermal growth factor receptor and activation of the PI 3-K pathway [Bonaccorsi et al., 2006. Importantly, in these transfected cells immunoconfocal fluorescent microscopy revealed colocalization of the AR and epidermal growth factor receptor. Thus, acquisition of a less malignant phenotype may be mediated through interaction between the two receptors. Inhibitory effects of androgen were also reported in a cell line derived from metastatic ascites of a prostate cancer patient [Zhau et al., 1996. Conversely, AR expression may decrease by a compound that stimulates growth such as stromal heparin-binding epidermal growth factor that acts through the mammalian target of rapamycin [Cinar et al., 2005. Taken together, critical evaluation of studies on androgen axis that is functional in advanced prostate cancer (Fig. 2) implies that there should be a differentiated approach in prostate cancer therapy.
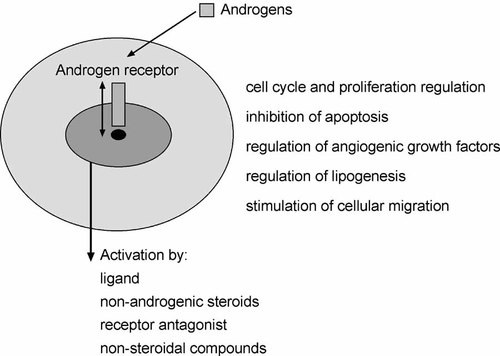
Cellular events regulated by androgenic hormones and mechanisms of activation of the androgen receptor.
Some patients will most likely benefit from AR ablation but the same treatment might be useless in other subgroups of patients.
Acknowledgements
Editorial assistance of Robert Schober is gratefully acknowledged.




