Splice variant 3, but not 2 of receptor protein-tyrosine phosphatase σ can mediate stimulation of insulin-secretion by α-latrotoxin
Abstract
α-latrotoxin (α-LTX) binds to several cell surface receptors including receptor protein-tyrosine phosphatase σ (RPTPσ). Here we demonstrate that transient overexpression of the short splice variant 3 conferred α-LTX induced secretion to hamster insulinoma (HIT-15) cells. In contrast, the long splice variant 2 containing four additional extracellular fibronectin-III domains was inactive in secretion or in a single cell assay. Toxin-sensitive (MIN6) and toxin-insensitive (HIT-T15) insulinoma cell lines as well as PC12 cells expressed similar amounts of endogenous short RPTPσ splice variant suggesting that this receptor does not play a role for toxin-sensitivity. J. Cell. Biochem. © 2006 Wiley-Liss, Inc.
Abbreviations used:
αLTX, α-latrotoxin; eGFP, enhanced green fluorescent protein; FNIII, fibronectin type 3 domains; KRB, Krebs-Ringer buffer; LDCV, large dense core vesicle; LPH, latrophilin; NxIα, Neurexin Iα; PBS, phosphate buffered saline; PKC, protein kinase C; RPTPσ, receptor protein-tyrosine phosphatase sigma.
The black-widow spider venom α-latrotoxin (α-LTX) induces massive exocytosis from synaptic and from large dense core vesicles (LDCVs). This property has been extensively exploited to investigate the molecular mechanisms underlying exocytosis [Henkel and Sankaranarayanan, 1999; Sudhof, 2001; Ushkaryov et al., 2004]. Toxin action requires first the binding to a surface receptor and three classes of receptors have been identified: neurexin Iα and β [Sudhof, 2001], the latrophilins or CIRL [Krasnoperov et al., 1997; Lelianova et al., 1997], and the receptor-like protein-tyrosine phosphatase σ (RPTPσ) [Krasnoperov et al., 2002]. Whereas latrophilin belongs to the adhesion receptor group of G-protein coupled receptors [Frederiksson et al., 2003], neurexins and RPTPσ contain only a single transmembrane domain and the intracellular domains are not required for the action of the toxin [Krasnoperov et al., 2002]. RPTPσ belongs to the class I cysteine-based protein tyrosine phosphatases [Alonso et al., 2004]. They play a role in neuronal axon elongation and in the differentiation of neuroendocrine as well as endocrine cells although its precise physiological role is unknown [Batt et al., 2002; Johnson and Van Vactor, 2003; Chagnon et al., 2004]. The extracellular sequence of RPTPσ is endowed with three immunoglobulin domain cell adhesion domains (IgCAM) followed by eight fibronectin type 3 domains (FNIII). The main difference among the four identified splice variants concerns the absence or presence of FNIII domains four to seven [Pulido et al., 1995]. These variations may define specific molecular interactions and distinct localizations have been reported for homologs of RPTPσ in C. elegans [Ackley et al., 2005]. The binding-site for α-LTX has been mapped to the second and third FNIII domain common to RPTPσ splice variants [Krasnoperov et al., 2002].
Pancreatic β-cells provide a very useful model to study the regulation of exocytosis by receptors [Lang, 1999]. We have previously demonstrated that α-LTX receptors are expressed and functional also in endocrine pancreatic β-cells secreting the peptide hormone insulin [Lang et al., 1998; Lajus et al., 2005], namely primary cells and the clonal cell line MIN6. In contrast, the hamster insulinoma cell HIT-T15 is largely insensitive to the effects of α-LTX and the sensitivity to the toxin was paralleled by the expression of latrophilin, but not of neurexin Iα or β [Lang et al., 1998]. We therefore addressed the question whether the third αLTX-receptor, namely RPTPσ, may participate in toxin-induced insulin release in native cells.
MATERIALS AND METHODS
Materials
The following primary antibodies were used: anti-VSV-G (P4D5), kindly provided by Dr. E. Le Bivic (Marseille, France) and the polyclonal anti-peptide antibody 322 directed against amino-acids 5–18 of mature RPTPσ (AAB28877), generously provided by Dr. A. Ullrich (Martinsried, Germany) [Aicher et al., 1997]. Fluorophore- or horseradish peroxidase-coupled second antibodies were purchased from Jackson Laboratories or Amersham, respectively. α-LTX was obtained from a commercial source (Calbiochem, San Diego). pcDNA3 or pRK5 plasmids endowed with a CMV promoter and encoding LPH1 [Lang et al., 1998], neurexin Iα [Volynski et al., 2000] or RPTPσ variants [Aicher et al., 1997; Suarez Pestana et al., 1999] were as published. The PKCα-eGFP construct was generously provided by Dr. C. Larsson (Lund, Sweden) [Raghunath et al., 2003].
Cell Culture, Transient Transfection, and Secretion Assays
Cell culture, transient transfection, and secretion assays were performed as described [Lang et al., 1997; Zhang et al., 1999; Boal et al., 2004; Lajus et al., 2005; Monterrat et al., 2006] except that jetPEI (PolyPlus-Transfection, Illkirch, France) was used. HIT-T15 cells [Santerre et al., 1981] were used between passages 75 and 85, MIN6m9 cells [Minami et al., 2000] were obtained from Dr. S. Seino (Chiba, Japan) and used between passage 21 and 30, PC12 cells were generously provided by Dr. B. Rudkin (ENS, Lyon, France) and used between passage 2 and 8. For secretion assays, cells were kept in Krebs-Ringer buffer (KRB; 125 mM NaCl, 5 mM KCl, 1.2 mM KH2PO4, 2 mM MgSO4, 25 mM Hepes, 3 mM glucose, 0.05% BSA, pH 7.4). The release of human insulin C-peptide as reporter gene product was measured by ELISA (Mercodia, Uppsala, Sweden) [Zhang et al., 1999]. For experiments with PKCα, cells were transfected with a mixture containing 25% plasmid coding for PKCα-eGFP and 75% plasmid of interest.
Immunoblotting and Immunohistochemistry
Cells were washed twice in PBS, detached by incubation for 10 min at 37°C with PBS containing 10 mM EDTA, centrifuged for 5 min at 4°C at 1000g and resuspended in ice-cold PBS with 1% Triton X-100. Cells were disrupted by brief sonication after incubation for 30 min on ice and homogenates stored in aliquots at −20°C. Proteins were solubilized in sample buffer for 30 min at 37°C and separated on 8% SDS–PAGE. Protein blotting, antibody incubation and detection were performed as described previously [Zhang et al., 1999] except that liquid transfer was used (8 h, 150 mA) in buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol. Quantification of immunoblots was performed as described before using different exposure times to ensure linearity of the response [Boal et al., 2004]. The same approach was used to measure total protein staining of the membranes (prior to immunological detection) and values were normalized to protein staining. Immunohistochemistry was performed as published [Zhang et al., 1999]. The antibody anti-VSV did not produce any signal in non-transfected cells at the dilution used (1:100). Imaging of fixed cells or of living cells was performed using an inverted microscope (Nikon TD300; 100× objective, 1.4 NA) coupled to a monochromator (Till Photonics) and appropriate emission filters on a filter wheel (Sutter, λ10-2) [Lajus et al., 2005; Monterrat et al., 2006]. Solution were pressure ejected (Eppendorf Femtojet; 4 psi) for 30 s into the bath (kept at 37°C) from a micropipette mounted on a micromanipulator (PCS-1000, Burleigh Instruments) and held at a distance of approximately 20 µm from the cells. Images were taken by 0.2 s exposures (binning factor 2) at 3 s intervals for 10 min and recorded by a CCD-camera (Micromax 1300Y HS, Roperts Scientific) using Metamorph software (Universal Imaging) [Lajus et al., 2005; Monterrat et al., 2006].
RT-PCR
Total RNA was prepared using Trizol (Invitrogen, Cergy-Pontoise, France) and reverse transcribed using oligo(dT)15 primers and ImPromIITM Reverse Transcriptase (Promega, Charbonnière, France) or Expand Long Template PCR system (Roche Bioscience). Amplification was performed by 30 cycles using the following primers: 5′-ACTCACGGATGTCAAGGACTCAGC-3′ (sense) and 5′-TCAGGCAGGTAGACCACGATG-3′ (antisense) corresponding to nucleotides 984–1007 and 3461–3481 of mouse RPTPσ splice variant 2 (NM_011218). Note that the corresponding rat sequence differs in one nucleotide for each primer.
Statistical Analysis
Results are presented as means±SEM from experiments performed on at least three independent cell preparations. Statistical analysis was performed by Student's two-tailed t-test for unpaired data (2p).
RESULTS AND DISCUSSION
Expression of Endogenous RPTPσ
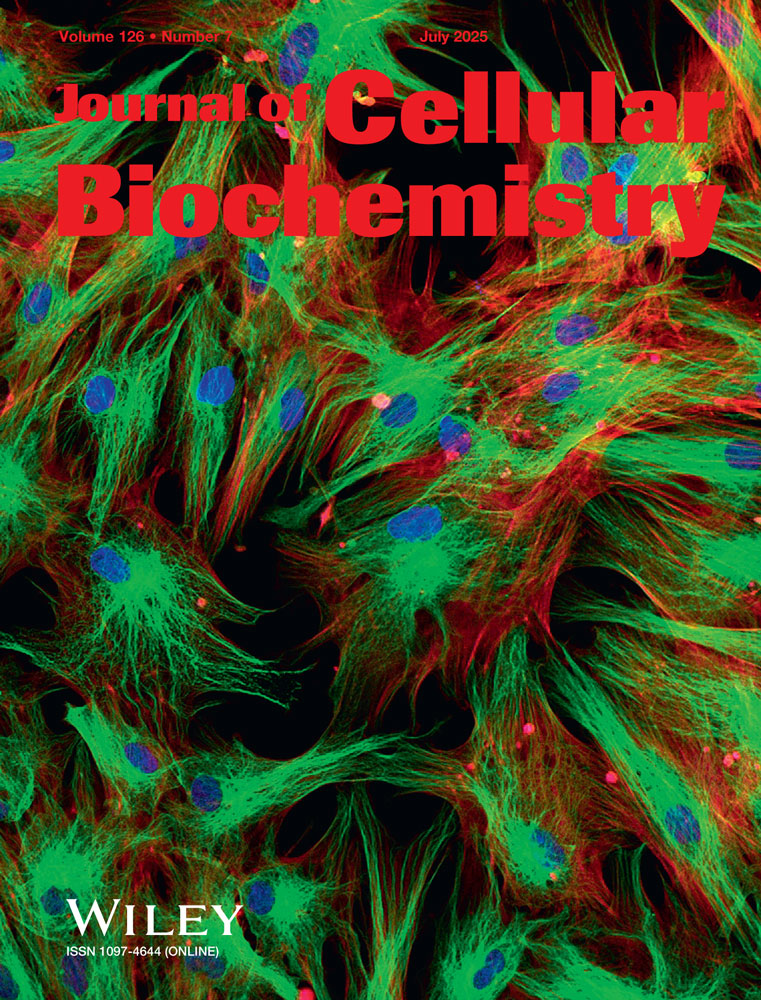
Receptor protein tyrosine phosphatases form a large and diverse protein family. The transmembrane RPTPσ (also called sometimes LAR-PTP2 or PTP9) exhibits a defined domain structure (see Fig. 1A) and cleavage in a juxtamembrane position leads to the expression of two non-covalently associated subunits derived from a pro-protein on the cell surface [Alonso et al., 2004], the extracellular E-domain and the transmembrane and intracellular P-domain. In human, four splice variants have been identified (NM_002850; NM_130853; NM_130854; NM_130855) which differ by the presence or absence of several internal fragments. The most striking difference resides in the fibronectin III-domains (FNIII) four to seven (see Fig. 1A), which are absent in splice variants 3 and 4. In rat and mouse, two splice variants have been described [Schepens et al., 1992; Ogata et al., 1994; Strausberg et al., 2002] which again contain or lack the FNIII domains four to seven and are most closely related to the human splice variants 2 and 3. The splice variants 1 and 2 of RPTPσ or LAR-PTP A encode a protein of 220 kDa. Proteolytic processing near the transmembrane domain generates an extracellular N-terminal E-domain of 130 kDa and a C-terminal P-domain of approximately 85 kDa. The short splice variants RPTPσ 3 and 4 or LAR-PTP B contain an E-domain of 95 kDa [Pulido et al., 1995; Aicher et al., 1997; Suarez Pestana et al., 1999].
Expression of RPTPσ in brain and insulin secreting cells. A: Scheme of RPTPσ and their domains (IgCAM, Immunoglobulin domain cell adhesion molecule; FN III, Fibronectin type 3 domain; TMD, transmembrane domain; PTPc, intracellular catalytic site). B: Homogenates (100 µg) from HIT-T15, MIN6 or PC12 cells were separated by SDS–PAGE, blotted and incubated with antibody 322. Left panel, immunoblot; right panel, membrane staining for proteins. Arrow, E-domain of short RPTPσ splice variant; star, full-length long RPTPσ splice variant. C: Expression levels of the E-domain of short RPTPσ splice variant (95 kDa) and of the full-length long RPTPσ splice variant (220 kDa) were determined by immunoblots and normalized to expression in HIT-T15 cells (n = 4–6 for each condition). D: Left panel: RT-PCR analysis of indicated cells, tissues or plasmids (encoding for rPTPσ2 or rPTPσ3) using primers corresponding to sequences within the third IgCam domain (sense) or distal to the eighth FNIII domain (antisense). Control, absence of RT-product. Right panel: overexposure of PCR analysis from MIN6 and PC12 cells.
In view of this striking difference we have first asked whether the distinct splice variants are expressed in insulinoma cell lines (Fig. 1B) and performed immunoblots using an antibody directed against the extracellular N-terminal portion conserved among the E-domains of RPTPσ isoforms [Aicher et al., 1997; Suarez Pestana et al., 1999]. This antibody revealed a major band of approximately 95 kDa and comparable intensity in hamster (HIT) and mouse (MIN6) insulinoma cells as well as rat pheochromocytoma cells (PC12) (Fig. 1B, arrow) compatible with the E-domain of the short splice variants of RPTPσ. In addition, a minor band was detected at approximately 220 kDa compatible with the expression of the full-length form of long splice variants (220 kDa; Fig. 1B, star). Quantification of the expression by immunoblots did not reveal any significant difference among the cell lines (Fig. 1C). Our data indicate that the short splice variants of RPTPσ were expressed and processed to similar extent.
To confirm further the expression pattern we resorted to RT-PCR using a pair of primers spanning a region which includes the four FNIII domains inserted into splice variants 1 and 2. These primers permit amplification from plasmids encoding for the RPTPσ 2 and 3 (Fig. 1D). The amplicons obtained from cellular material migrate as expected for RPTPσ 3 (or 4) and their identity was confirmed by sequencing of amplification products obtained from HIT-T15 cells. After long exposure of agarose gel, an additional band migrating as expected for amplicons from RPTPσ 1 or 2 could be detected in mouse brain (data not shown) whereas these forms could not be detected in all the cell lines tested except as a minor product in MIN6 cells These findings indicate expression of the short forms of RPTPσ in insulin-secreting cell lines and islets at the mRNA and protein level, whereas the longer splice variants are present only at minor amounts. The expression of RPTPσ has previously been reported in β-cells and other target organs for insulin although the probes chosen did not permit to distinguish between the splice variants [Norris et al., 1997; Ostenson et al., 2002].
RPTPσ and αLTX Induced Insulin Exocytosis
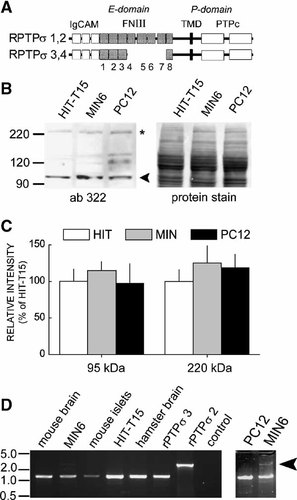
To investigate whether both forms of RPTPσ are capable to sensitize exocytosis of LDCVs from insulin-secreting cells to the action of α-LTX, we have transiently co-expressed the two splice variants in HIT-T15 cells together with a plasmid coding for human preproinsulin. Using a specific ELISA which recognizes human but not rodent insulin C-peptide, secretion from co-transfected cells only can thus be measured [Lang et al., 1997; Zhang et al., 1999]. We have previously shown that these cells express considerable amounts of neurexin Iα and β, but not of latrophilin 1, and do not respond to α-LTX by secretion of insulin [Lang et al., 1998; Lajus et al., 2005]. As expected, overexpression of latrophilin 1 or of neurexin Iα results in a major increase in insulin exocytosis upon exposure to α-LTX (see Fig. 2). As a control, cells have also been stimulated by depolarization via KCl leading to Ca2+-influx through voltage-dependant Ca2+-channels. For this stimulation no significant difference was observed between control cells and those expressing the different constructs. Transient expression of RPTPσ3 was also capable to mediate α-LTX-induced exocytosis albeit at a lower rate than latrophilin 1 or neurexin Iα. In contrast, RPTPσ2 did not confer α-LTX-induced insulin exocytosis.
Effect of α-LTX on secretion from insulinoma cells transiently expressing LPH/CIRL1, RPTPσ2, 3 or NxIα. HIT-T15 cells were transiently co-transfected with indicated constructs and a plasmid encoding human preproinsulin. Ninety-six hours later the release of human insulin C-peptide was measured in the absence or the presence of 2 nM of α-LTX or 50 mM KCl in Krebs-Ringer buffer (KRB). Values are expressed as % of intracellular hormone content. n = 6–8 for each point. * 2p < 0.05 as compared to KRB alone.
RPTPσ Overexpression and Translocation of PKCα-eGFP
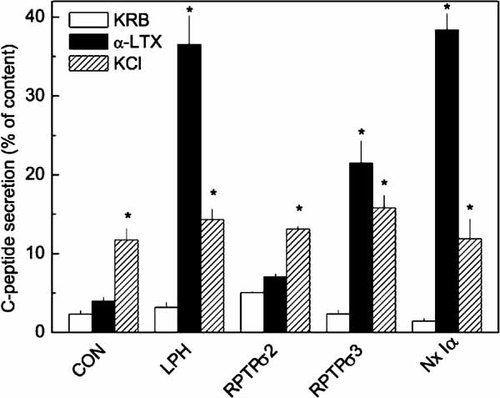
In view of the absence of a measurable effect of αLTX on RPTPσ 2, we have examined the expression of the protein and also measured the behavior of single cells. As shown in Figure 3, transient overexpression of RPTPσ 3 can be detected in homogenates from HIT-T15 cells by using the antibody 322 which reacts with the 95 kDa extracellular E-domain of RPTPσ 3. To detect the transiently expressed mouse RPTPσ 2, we took advantage of a VSV-G epitope introduced at its C-terminus. An anti-VSV antibody detected a 80 kDa band, corresponding to the intracellular P-domain, only in homogenates from transiently transfected cells. The fact that in both cases the processed forms (E or P domains) were detected on denaturing gels indicates correct processing of the transiently expressed proteins. We also examined the subcellular distribution of RPTPσ 2 using the same antibody. As shown in Figure 3B, RPTPσ 2 localises to the plasma membrane in transfected HIT-T15 cells. Non-transfected cells were not stained (see cells at upper right edge).
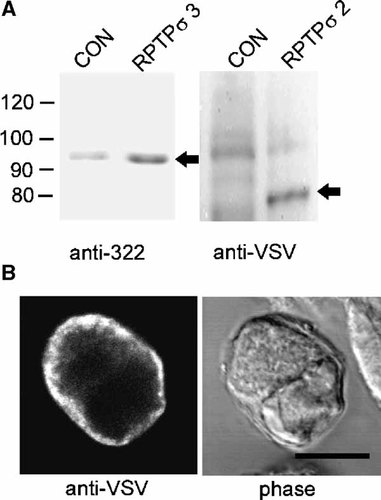
Overexpression of RPTPσ in insulinoma cells. A: Homogenates (100 µg) from HIT-T15 cells, transiently transfected with plasmids encoding for indicated constructs, were analyzed by immunoblot using the antibody 322 (1:5,000) or anti-VSV-G (1:1,000). The relevant bands are indicated by arrows. Note unspecific staining of a 96 kDa band by anti-VSV-G in control and transfected cells. B: Immunofluorescence of HIT-T15 cells transiently expressing RPTPσ2. Cells were stained with an anti-VSV-G tag antibody directed against the epitope inserted at the C-terminus of RPTPσ2. Bar = 10 µm.
To compare the toxin effects on a single cell level we took advantage of eGFP-labeled PKCα. Upon Ca2+-influx and/or stimulation of phospholipase C, this fluorescent probe efficiently translocates to the plasma membrane due to the increased affinity of its C1 and C2 domains to membrane phospholipids [Raghunath et al., 2003]. Indeed, recent observations from our and other laboratories have demonstrated that binding of α-LTX to functional receptors is accompanied by calcium influx and activation of protein kinase C [Bittner and Holz, 2000; Lajus et al., 2005; Li et al., 2005; Liu et al., 2005]. As shown in Figure 4A, translocation of the fluorescent protein to the plasma membrane was induced by exposure of cells to defined concentrations of free calcium as well as influx of calcium through the ionophore ionomycine or direct stimulation of PKC by the phorbol ester PMA. As can be seen, αLTX did not induce translocation of the probe in HIT-T15 cells co-transfected with plasmids coding for PKCα-eGFP and a control plasmid (CON, pcDNA3). In contrast, αLTX always induced complete translocation in cells expressing LPH. The same was observed for RPTPσ 3, whereas cells co-expressing RPTPσ 2 never responded to stimulation. Imaging was conducted in all experiments for 10 min but no further changes as given in Figure 4 were observed except for bleaching. These results confirm that RPTPσ3, but not RPTPσ2, confers sensitivity to α-LTX in HIT-T15 cells2.
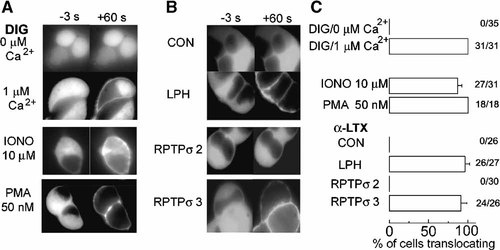
LPH and RPTPσ3, but not RPTPσ2 mediate α-LTX-induced translocation of PKCα-eGFP. HIT-T15 cells were transiently co-transfected a plasmid encoding human eGFP-tagged protein kinase Cα (PKCα-eGFP) and with indicated constructs. Ninety-six hours later living cells were exposed for 30 s to different agents and images were taken 3 s before and 60 s after starting exposure. A: Cells transiently cotransfected with PKCα-eGFP and pcDNA3 were exposed to digitonin (30 µM) and 0 or 1 µM of free Ca2+, 10 µM ionomycine or 50 nM of the phorbol ester PMA. B: Cells cotransfected with plasmids PKCα-eGFP, pcDNA3 (CON), LPH, RPTPσ2 or RPTPσ3 were stimulated with 2 nM of α-LTX. C: Percentage of translocations from three different transfections for each agent or plasmid. Number of stimulations and positive events are given.
CONCLUSIONS
RPTPσ act as receptors for cell–cell as well as cell–extracellular matrix associated cues and may intervene in nerve regeneration, actin remodeling and insulin signaling [Chagnon et al., 2004]. Currently two ligands have been identified for these receptors, heparan sulfate proteoglycans and the black-widow spider venom α-LTX [Aricescu et al., 2002; Krasnoperov et al., 2002]. The binding site for αLTX has been mapped on RPTPσ. In the case of RPTPσ 3 it encompasses the FNIII domains 2 and 3 [Krasnoperov et al., 2002] and therefore should be present in all splice variants. Interestingly, the toxin-binding domain in LPH has been mapped to a similar distance from the transmembrane domain [Krasnoperov et al., 1999]. Insertion of 400 amino acids by additional FNIII domains, such as in RPTPσ 2, may increase the distance between the toxin and the plasma membrane thereby lowering its efficacy and interaction with the bilayer.
The cellular system employed here differs from the pheochromocytoma (PC12) cells or neuronal preparations often used to examine native or overexpressed αLTX receptors as native HIT-T15 insulinoma cells do not respond to the toxin. Interestingly, the effect of overexpressed receptors or truncations can only be observed in chromaffin or PC12 cells at low nanomolar concentrations of toxin suggesting some kind of interaction of the toxin with several receptors [Sugita et al., 1998; Bittner and Holz, 2000; Krasnoperov et al., 2002]. Notably, co-immunoprecipitation of latrophilin/CIRL with RPTPσ has been reported provided that αLTX is present [Krasnoperov et al., 2002]. Our data demonstrate that upon its overexpression, RPTPσ can serve as a receptor for αLTX also in a secretion-competent system where function of other endogenous αLTX-receptors cannot be detected. However, the comparable expression levels of the endogenous short splice variant of RPTPσ in toxin-insensitive HIT-T15 cells and toxin-sensitive MIN6 cells argue against a role for endogenous RPTPσ in toxin-induced secretion in native cells. As the same is true for neurexin I, αLTX-mediated effects in native cells are most likely mediated by endogenous latrophilin as it is the only receptor whose distribution coincides with toxin sensitivity [Lang et al., 1998]. Comparison of secretion-competent cells such as HIT-T15 and MIN6 may therefore provide a very useful tool to search for putative endogenous ligands for latrophilin.
Acknowledgements
We are grateful to Dr. A. Ullrich (Martinsried, Germany) for generously providing us the antibody 322 and the expression clone for the short splice variant of RPTPσ (rat LAR-PT2 B). We also thank Dr. Boehme (Jena, Germany) for the expression plasmid of the long splice variant of RPTPσ. We thank Alexandra Milochau and Marie-Noëlle Benassy for excellent technical assistance. This work was supported by grants from the Region of Aquitaine and from the University of Bordeaux I (BQR2003 and 2004).