Paxillin modulates squamous cancer cell adhesion and is important in pressure-augmented adhesion
Abstract
Paxillin is an adapter protein regulating signaling and focal adhesion assembly that has been linked to malignant potential in many malignancies. Overexpression of paxillin has been noted in aggressive tumors. Integrin-mediated binding through the focal adhesion complex is important in metastatic adhesion and is upregulated by extracellular pressure in malignant colonocytes through FAK and Src activation. Neither head and neck cancers nor paxillin have been studied in this regard. We hypothesized that paxillin would play a role in modulating squamous cancer adhesion both at baseline and under conditions of increased extracellular pressure. Using SCC25 tongue squamous cancer cells stably transfected with either an empty selection vector or paxillin expression and selection vectors, we studied adhesion to collagen, paxillin, FAK, and Src expression and phosphorylation in cells maintained for 30 min under ambient or 15 mmHg increased pressure conditions. Paxillin-overexpressing cells exhibited adhesion 121 ± 2.9% of that observed in vector-only cells (n = 6, P < 0.001) under ambient pressure. Paxillin-overexpression reduced FAK phosphorylation. Pressure stimulated adhesion to 118 ± 2.3% (n = 6, P < 0.001) of baseline in vector-only cells, similar to its effect in the parental line, and induced paxillin, FAK, and Src phosphorylation. However, increased pressure did not stimulate adhesion or phosphorylate paxillin, FAK, or Src further in paxillin-overexpressing cells. Metastasizing squamous cancer cell adhesiveness may be increased by paxillin-overexpression or by paxillin activation by extracellular pressure during surgical manipulation or growth within a constraining compartment. Targeting paxillin in patients with malignancy and minimal tumor manipulation during surgical resection may be important therapeutic adjuncts. J. Cell. Biochem. © 2006 Wiley-Liss, Inc.
Paxillin, a 68-kDa focal adhesion protein, has been implicated in diverse cellular events, including cellular motility [Petit et al., 2000], cell spreading [Liu et al., 2002], embryogenesis [Turner, 1991], intracellular signaling pathways [Turner, 2000], and apoptosis [Chay et al., 2002]. Paxillin is also involved in focal adhesion complex assembly and linkage of the focal adhesion complex to integrins and to the cytoskeleton, an important system in metastatic adhesion [Basson et al., 2000]. For instance, paxillin can bind in vitro to peptides mimicking the cytoplasmic domain of beta-integrins [Schaller et al., 1995], and may interact with actopaxin [Nikolopoulos and Turner, 2000] and vinculin [Turner et al., 1990], which link focal adhesions to the cytoskeleton. Paxillin also functions as an adapter protein, which is phosphorylated by FAK and Src after integrin engagement and in turn binds to other downstream proteins, facilitating their recruitment into the signal cascade [Bellis et al., 1995; Brown and Turner, 2004]. In addition, cell adhesion to matrix proteins collagen and fibronectin can induce paxillin phosphorylation [Burridge et al., 1992; Sanders and Basson, 2000].
Some studies suggest that paxillin may be important in tumor biology. For instance, head and neck cancers, including those of tongue origin, that have metastasized to lymph nodes exhibit increased paxillin expression over their non-metastatic counterparts [Nagata et al., 2003]. Elevations of paxillin have also been noted in highly metastatic human osteosarcomas [Azuma et al., 2005].
One important aspect of tumor metastasis is the adhesion of shed cancer cells to matrix proteins and endothelial cells at other sites. Although the effect of paxillin expression on cancer cell adhesion has not previously been characterized, recent observations suggest that other signaling molecules within the focal adhesion complex, known to interact with paxillin, can modulate integrin-mediated cancer cell adhesion in colon cancer cells. At 15 mmHg above ambient pressure, malignant colonocytes exhibit a 20–120% increase in binding to various extracellular matrix proteins and to endothelial cells in vitro [Thamilselvan and Basson, 2004], and to surgical wounds in vivo [van der Voort van Zyp et al., 2004], mediated by FAK and Src activation at the focal adhesion complex. Shear stress and turbulence exert a similar effect [Thamilselvan et al., 2004; van der Voort van Zyp et al., 2004]. This phenomenon may have important clinical implications. The stimulation of adhesion by surgical manipulation or wound irrigation, or even growth within a constraining compartment may ultimately increase local wound recurrence or strengthen endothelial binding of metastasizing tumor cells [von Sengbusch et al., 2005], with potentially deleterious consequences in patients. Clinical observations also suggest that physical forces may contribute to carcinogenesis in head and neck cancers [Gray and Titze, 1988; Courey et al., 1996; Niimi et al., 2001]. Since FAK and Src both interact with paxillin [Brown and Turner, 2004] and a different physical force, repetitive deformation, stimulates paxillin phosphorylation in other cell types [Li et al., 2001; Cuvelier et al., 2005], we hypothesized that paxillin might interact with other inside-out signal proteins to modulate the integrin-mediated adhesion of head and neck cancer cells and their response to pressure, and facilitate metastasis.
We therefore sought to evaluate the potential role of paxillin in squamous cancer cell adhesion. A squamous cancer control cell line and a line in which paxillin had been stably overexpressed were evaluated to determine the effect of paxillin expression on cell adhesion. In addition, we evaluated the effect of increased extracellular pressure on the adhesion of each cell line. Finally, we characterized the effects of pressure and paxillin overexpression on paxillin, FAK, and Src phosphorylation in suspended cells prior to adhesion, to evaluate the possible role of these signals in mediating the adhesive effects that we observed.
MATERIALS AND METHODS
Cells
A squamous cell cancer cell line of primary tongue origin (SCC 25) was used for all the experiments [Crowe and Ohannessian, 2004]. The cells studied included the parental SCC25 line, a cell line transfected with an empty vector, and a line transfected with a paxillin overexpression vector. All cells were cultured in medium consisting of 90% Dulbeco's modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS), and gentamycin 40 µg/ml (400 µg/ml for the cells carrying the expression vector).
Cell Transfection
Cells were transfected using 5 µg expression vector for paxillin with a neomycin resistance plasmid, or the plasmid alone to generate the control cells. Cells were selected in 400 µg/ml G418 for 14 days, and resistant clones were expanded and characterized [Crowe and Ohannessian, 2004].
Matrix Precoating
Six-well plates used in the adhesion studies were precoated with type I collagen (Sigma, St. Louis, MO) in an ELISA coating buffer at a concentration of 12.5 µg/ml for 24 h as previously described, and were washed three times with phosphate buffered saline (PBS) prior to use [Thamilselvan and Basson, 2004].
Pressure Regulation
Extracellular pressure was controlled using a Lucite box with inlet and outlet valves, thumb screws, and an O-ring to achieve an airtight seal. The box is prewarmed to 37°C for at least 1 h before each study. Previous studies have demonstrated temperature and pressure fluctuation to only be ±2°C and ±1.5 mmHg using this method [Basson et al., 2000].
Adhesion Studies
Subconfluent flasks were lightly trypsinized and resuspended in culture media. A hemocytometer was used to count the cells to assure equal allocation of viable cells into each well, with only those cells excluding trypan blue included in the counting. (Cell viability by trypan blue exclusion routinely exceeded 90%.) Equal aliquots (20,000 cells per 2 ml) were placed into each well of a six-well plate, previously coated as described above. Once seeded with cells, the plates were placed in a 37°C incubator. The control cells were placed directly in the incubator, while the experimental group was placed in the pressure box, set at 15 mmHg above ambient pressure, which was also maintained in the same incubator. After 30 min, the plates were removed from the incubator and immediately gently washed with PBS to remove non-adherent cells. The adherent cells were then fixed with 10% buffered formalin and stained with hematoxylin. An Olympus microscope was used to count the number of adherent cells in a single high powered field. Twenty high powered fields were counted per well.
Western Blotting and Immunoprecipitation
Cells from the same flasks used for the six-well plates were also placed on pacificated bacteriologic plates. These were similarly exposed to ambient or 15 mmHg increased pressure for 30 min while in suspension. After 30 min, the cells were immediately placed in 4°C PBS, centrifuged into a pellet, and resuspended in 4°C PBS. After another centrifugation, the cells were lysed in lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% TritonX-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 50 mM NaF, 10 mM sodium pyrophosphate, 2 µg/ml aprotinin, 2 µg/ml leupeptin, pH 7.4) and centrifuged at 10,000g for 15 min at 4°C. The protein concentration of the supernatant was determined using the bicinchoninic acid reagent assay (BCA) (Pierce Chemical, Rockford, IL). Equal amounts of protein were resolved by 8% sodium dodecyl sulfate polyacrylamide electrophoresis (SDS–PAGE) and transferred to Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were blotted with specific antibodies to FAK (Upstate, Lake Placid, NY), FAK Tyr 397 (Biosource, Camarillo, CA), Src (Santa Cruz Biotechnology, Santa Cruz, CA), Src Tyr 416 (Cell Signaling, Beverly, MA), paxillin (Transduction Laboratories, San Diego, CA), paxillin Tyr 118 (Biosource, Camarillo, CA), and visualized with secondary antibody coupled to horseradish peroxidase. Enhanced chemiluminescence (ECL) (Amersham) was used for band detection, and a Kodak Image Station 440CF (Perkin Elmer, Boston, MA) used for band analysis.
Statistical Analysis
All studies were done in a paired format. Cells from each flask (parental line, etc.) were handled in an identical fashion and ultimately placed into separate six-well plates to be placed under ambient or elevated external pressure. For comparisons between cell lines, experiments were undertaken at the same time to ensure equivalent conditions. Pressure data was normalized to the mean of the control wells under ambient pressure. Statistical analysis for all data was by unpaired t-test. P < 0.05 was set a priori as the threshold for statistical significance. All data are expressed as mean ± SE.
RESULTS
Morphology and Baseline Phosphorylation of Paxillin-Overexpressing Cells
Paxillin-overexpressing cells (Fig. 1b) appeared essentially similar in morphology to empty-vector control cells (Fig. 1a) at a gross light microscopic level, except that the cells in which paxillin was overexpressed tended to exhibit more cell spreading and a larger cell surface area.
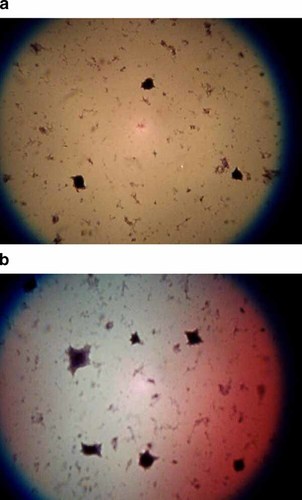
Morphology of baseline paxillin-overexpressing cells. At a gross light level, the paxillin-overexpressing cell line (a) was essentially similar morphologically to their empty-vector control counterparts (b). They did, however, grossly appear to exhibit more rapid cell spreading and a larger surface area. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
At ambient pressure, the paxillin-overexpressing cell line exhibited total paxillin levels 136 ± 8.4% (n = 12, P < 0.001) of those in the empty-vector cells (Fig. 2a). Phosphorylated paxillin was also significantly increased at ambient pressures (192 ± 18.7% of the ambient controls, n = 12, P < 0.001) (Fig. 2a). In contrast, basal levels of total FAK and total Src proteins were similar in paxillin-overexpressing cells to those in the control cells (data not shown). Although the amount of phosphorylated Src 416 was similar to the control cells, levels of phosphorylated FAK 397 were significantly reduced (55.6 ± 5%, n = 11, P < 0.001) when compared with the control cells (Fig. 2b).
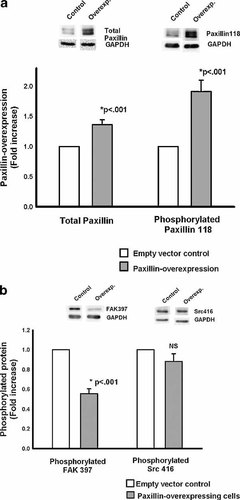
Baseline total and phosphorylated protein levels in paxillin-overexpressing cells. a: At ambient pressure, the total paxillin level in the overexpressing cells (gray bars) was significantly increased over the empty-vector controls (first two bars). Similarly, these cells displayed a level of phosphorylation of paxillin at tyrosine residue 118 that was also significantly increased over paxillin phosphorylation in control cells (n = 12, P < 0.001) (second two bars). b: At ambient pressure, the phosphorylation of Src at tyrosine residue 416 (right bars) was not statistically different in paxillin-overexpressing cells (gray bars) from that in the control cells (open bars). Phosphorylated FAK, however, did show differences at baseline (left bars). FAK tyrosine residue 397 had a phosphorylation level at ambient pressure in the overexpressing line (gray bars) that was significantly less than the control cells (open bars).
Adhesion in Paxillin-Overexpressing Cells
Malignant squamous cells overexpressing paxillin exhibited increased adhesion to collagen in comparison to the empty-vector transfected cells at ambient pressures. At ambient pressure, these paxillin-overexpressing cells exhibited adhesion to type I collagen that was 121 ± 2.9% (n = 6, P < 0.001) of the control cells (Fig. 3).
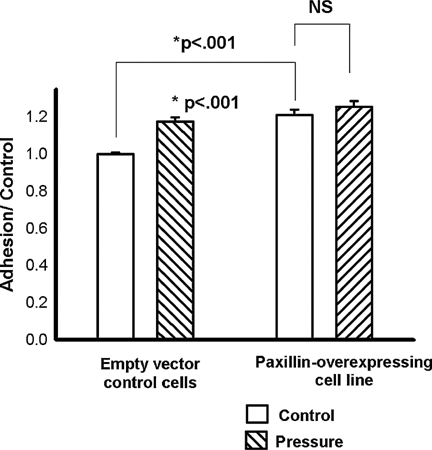
Adhesion in the paxillin-overexpressing cells. Malignant squamous cells overexpressing paxillin (right set of bars) exhibited a significantly increased level of adhesion at ambient pressures (cross-hatched bars) (n = 6, P < 0.001) compared with the control cells (open bars). In contrast to the control group, however, the cells overexpressing paxillin did not display augmentation of adhesion in response to increased extracellular pressure (cross-hatching).
Effects of Increased Extracellular Pressure on Parental Line and Empty-Vector Control Cells
Pressure augmented malignant squamous SCC25 cell adhesion to type I collagen. In the parental cell line, pressure stimulated adhesion to 136 ± 2.9% (n = 3, P < 0.001) of baseline (Fig. 4). Similarly, pressure stimulated adhesion to 118 ± 2.34% (n = 6, P < 0.001) in the empty-vector control cells (Fig. 4).
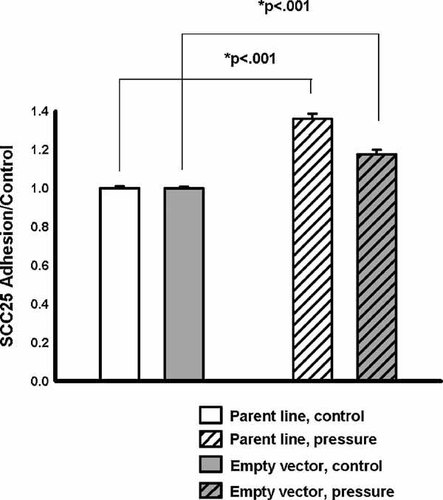
Effects of increased pressure on parental line and empty-vector control. When placed under 15 mmHg increased pressure (cross-hatching) for 30 min, SCC25 cells (white) displayed significantly increased adhesion to collagen I (n = 3, P < 0.001). The empty-vector control cell line (gray) exhibited a similar augmentation of adhesion in response to increased pressure (n = 6, P < 0.001).
FAK and Src Signaling in Response to Increased Pressure in Control Cells
Consistent with previous observations of the effects of pressure on FAK and Src in malignant colonocytes [Thamilselvan and Basson, 2004], increased extracellular pressure stimulated phosphorylation of the focal adhesion proteins FAK and Src on tyrosine residues FAK 397, and Src 416, respectively, associated with activation of these proteins. In particular, pressure increased the phosphorylation of FAK at tyrosine residue 397 to 124 ± 7.6% (n = 11, P = 0.005) of the baseline level (Fig. 5b, first two bars). Similarly, pressure increased Src phosphorylation at the tyrosine 416 residue to 119 ± 6.97% (n = 11, P < 0.05) of the baseline value (Fig. 5b, second two bars).
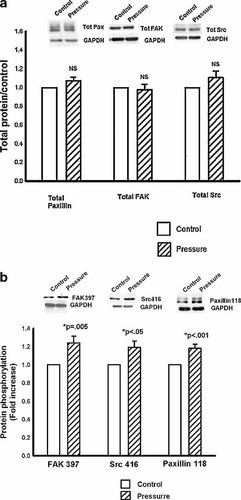
FAK, Src, and Paxillin signaling in response to pressure in control cell line. a: No changes in total FAK (first two bars), Src (second two bars), or paxillin (third two bars) were noted in response to increased extracellular pressure. b: First two bars: Increased extracellular pressure (cross-hatching) significantly, increased phosphorylation of FAK at tyrosine residue 397 (n = 11, P = 0.005) in control cells. Second two bars: Increased extracellular pressure (cross-hatching) also increased Src phosphorylation at tyrosine residue 416 in control cells. (n = 11, P < 0.05). Third two bars: Paxillin phosphorylation was similarly increased in response to increased extracellular pressure (cross-hatching) in the control line. Tyrosine residue 118, the main FAK interaction site in paxillin, was significantly more phosphorylated in response to pressure (n = 12, P < 0.001).
Paxillin Phosphorylation in Response to Pressure in Control Cells
Increased extracellular pressure also stimulated the phosphorylation of paxillin in control cells. Paxillin phosphorylation on Tyr residue 118, the primary site of FAK interaction with paxillin, was increased to 118 ± 4.6% (n = 12, P < 0.001) of the baseline in response to increased pressure (Fig. 5b, third pair of bars).
Effects of Increased Extracellular Pressure on Overexpressing Line
In contrast to the control cells, the overexpressing cell line did not exhibit significant augmentation of adhesion in response to pressure (121 ± 2.9% vs. 125 ± 3.3% of the control at ambient pressure, n = 6, P = 0.34) (Fig. 3).
FAK, Src, and Paxillin Signaling in Response to Increased Pressure in Paxillin-Overexpressing Cells
Paxillin-overexpressing cells did not exhibit any increase in paxillin phosphorylation at Tyr 118 in response to increased extracellular pressure. Furthermore, neither FAK 397 phosphorylation nor Src 416 phosphorylation were increased in response to pressure in these cells (Fig. 6).
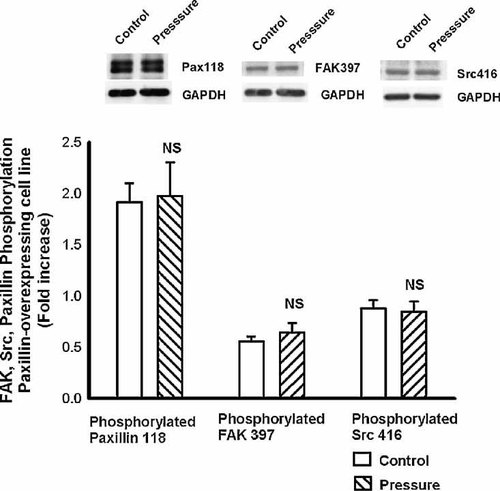
FAK, Src, and paxillin signaling in response to pressure in the overexpressing cells. Consistent with the adhesion data, paxillin-overexpressing cells did not exhibit increased phosphorylation of FAK, Src, or paxillin in response to increased extracellular pressure. There was no further augmentation in phosphorylation of FAK397 (first two bars), Src416 (second two bars), or paxillin 118 (third two bars) in response to increased extracellular pressure (cross-hatching) in this group of cells.
DISCUSSION
Paxillin overexpression was associated with increased malignant squamous cell adhesion to collagen. In control cells, extracellular pressure was associated with both increased paxillin phosphorylation and increased adhesiveness, while the paxillin-overexpressing line, in which phospho-paxillin was already increased, displayed no further increase in adhesion in response to pressure. Finally, stimulation of FAK and Src phosphorylation by pressure, which is required for pressure-stimulated adhesion in malignant colonocytes [Thamilselvan and Basson, 2004], was absent in paxillin-overexpressing cells that did not display pressure-stimulated adhesion. Thus, paxillin may be an important mediator of metastatic adhesion.
Paxillin has previously been linked to tumor biology. Primary oral squamous cell cancers, including glossal lesions, exhibit increased paxillin in association with lymph node metastasis [Nagata et al., 2003]. Paxillin upregulation also correlates with increased metastatic potential in proliferative prostate epithelium and directly correlates with HER-2 receptor expression in aggressive breast cancer cell lines and grade III human tumors [Vadlamudi et al., 1999].
To determine whether paxillin influences malignant cell adhesion, we examined a stably transfected SCC25 subclone that overexpressed paxillin and exhibited elevated levels of paxillin phosphorylated at tyrosine 118. These paxillin-overexpressing cells displayed elevated basal adhesion at ambient extracellular pressure. The increased total and phosphorylated paxillin in our cells may stimulate adhesion via downstream signals induced by paxillin phosphorylation or by its effects on subcellular organization. While paxillin functions as an adapter signal protein that facilitates cell signaling, it also links the cytoskeleton to the focal adhesion complex [Turner et al., 1990]. Non-phosphorylatable mutants of paxillin inhibit tracheal smooth muscle tension development, which requires a physical linkage between cytoskeletal components and the extracellular matrix [Tang et al., 2003]. Paxillin phosphorylation, significantly increased in our overexpressing cell line, may contribute to cytoskeletal reorganization. This occurs in RSV-transformed chick embryo fibroblasts [Burridge, 1986] and polymorphonuclear cells treated with TNF-alpha [Fuortes et al., 1994]. Pharmacologic modulation of cytoskeletal function also alters pressure-mediated adhesion [Thamilselvan and Basson, 2005]. Thus, paxillin might also influence basal and pressure-stimulated adhesion by virtue of its function in cytoskeletal organization, either in mechanotransduction or by causing integrin clustering. These alternative mechanisms await further study.
Although it would be interesting to evaluate the effects of inhibiting paxillin, no specific pharmacologic inhibitor for paxillin is currently available. Although molecular techniques might be used for this purpose, data derived from such studies might be difficult to interpret because nearly completely reducing paxillin's activity might have substantial effects on focal adhesion complex assembly as well as potentially ablating paxillin-dependent signal pathways in response to pressure and other stimuli.
To further evaluate the role of paxillin in malignant adhesion, we examined squamous cancer cell adhesion in response to pressure. We previously reported that pressure upregulates adhesion and proliferation of malignant colonocytes in vitro and in vivo [Basson et al., 2000; Walsh et al., 2003; Thamilselvan and Basson, 2004; van der Voort van Zyp et al., 2004]. Parental and empty vector control SCC 25 cell lines responded to extracellular pressure similarly to malignant colonocytes, increasing adhesion to 120–130% of baseline. In contrast, paxillin-overexpressing cells did not increase adhesion in response to pressure, but seemed maximally adhesive at baseline, presumably because of paxillin overexpression. Consistent with the adhesion data, FAK and Src phosphorylation did not change in response to pressure.
The pathway by which forces such as pressure stimulate adhesion by forces may influence tumor metastasis as well as local recurrence after surgical excision. Tumor cells are acted upon by a variety of forces, reflecting hemodynamic effects, local edema, and surgical manipulation. Teleologically, this response may be adaptive for the malignant cancer, increasing the likelihood of survival of disseminated cells acted upon by such forces. Interestingly, free tumor cells on peritoneal surfaces predict poor prognosis, independently of tumor stage [Baskaranathan et al., 2004]. Such cells may have responded to surgical forces with augmented adhesion, resistant to preclosure irrigation.
We observed phosphorylation of the focal adhesion proteins FAK and Src in response to increased extracellular pressure in the control line, suggesting their activation and raising the possibility that phosphorylation of FAK and Src might influence malignant squamous cell adhesion. Indeed, this is consistent with our previous observation that FAK and Src activation are required for the stimulation of malignant colonocyte adhesion by extracellular pressure [Thamilselvan and Basson, 2004]. The similarity in findings suggests that diverse tumor types can augment their adhesiveness in this manner.
Increased extracellular pressure in the control cell line was also associated with paxillin phosphorylation at tyrosine 118, the main site of FAK–paxillin interaction [Bellis et al., 1995]. Paxillin phosphorylation in response to pressure in parental SCC25 cells is consistent with reports of paxillin phosphorylation in other cells in response to forces. For example, paxillin phosphorylation is stimulated in malignant colonocytes under conditions of laminar flow [Haier and Nicolson, 2002] and in repetitively deformed Caco-2 intestinal epithelial cells [Li et al., 2001], and in human gingival fibroblasts subject to strain [Glogauer et al., 1997].
Interestingly, the paxillin-overexpressing cells displayed decreased FAK397 phosphorylation at ambient pressures without any difference in total FAK level. This decrease in FAK phosphorylation in the setting of increased basal adhesion contrasts with reports by our group and others suggesting that increased FAK phosphorylation is associated with integrin-mediated adhesion [Cary and Guan, 1999; Cary et al., 1999; Sanders and Basson, 2000; Thamilselvan and Basson, 2004]. Taken together with these previous observations, our present results raise the possibility that paxillin phosphorylation may be an important step between FAK phosphorylation and the subsequent increase in adhesiveness. The exogenous overexpression of paxillin and consequent increase in the amount of phosphorylated paxillin available could stimulate adhesion independently of FAK signaling and even stimulate a negative feedback that might inhibit FAK itself. This may be consistent with the finding that the invadopodia of invasive breast cancer cells do not contain FAK, but contain abundant paxillin [Bowden et al., 1999]. The further link between paxillin itself and adhesion has yet to be delineated.
That extracellular forces may modulate the biology of head and neck malignancies via paxillin signaling is consistent with previous clinical correlations of physical force effects and oropharyngeal biology. For instance, shear forces produced by excessive phonation may cause basement membrane changes and subsequent nodule or polyp formation [Gray and Titze, 1988; Courey et al., 1996]. Others have hypothesized that mechanical pressure between oral squamous cell carcinoma and the vasculature stimulates an inflammatory reaction that enhances vascular invasion [Niimi et al., 2001]. While such clinical observations likely reflect diverse effects of such forces, the paxillin, FAK, and Src signals and changes in adhesion with pressure that we delineate here in vitro may contribute to such effects.
Phosphorylation of proteins like FAK, Src, and paxillin has traditionally been studied in models in which integrin ligation leads to activation and formation of a focal adhesion complex linked to the cytoskeleton. However, the signals we report here in response to increased extracellular pressure, like those previously observed in malignant colonocytes [Thamilselvan and Basson, 2004] occur in suspended cells prior to adhesion. Thus, these signals originate from within the cell, in response to extracellular pressure, without the prerequisite of integrin ligation. Intracellular signals in this model influence adhesion rather than adhesion initiating intracellular signals. Cells adhering to a matrix may respond differently to extracellular pressure than cells not yet adhering. For instance, we have previously reported that malignant colonocytes display increased ERK activation in response to extracellular pressure only after adhesion has occurred [Walsh et al., 2004]. The present study focuses on the intracellular signals by which cells in suspension, prior to adhesion, respond to extracellular pressure and then subsequently adhere. It therefore may be difficult to extrapolate from our present observations to the effects of longer term pressure on adherent tumor cells in vivo in rapidly growing tumors that may experience chronic increases in extracellular pressure. Nevertheless, longer term increases in extracellular pressure, such as are encountered in tumor growth, may also exert other effects on tumor cell biology. For instance, we recently reported that increasing extracellular pressure for 4.5–24 h is mitogenic for SW620 colon cancer cells by a mechanism distinct from that which mediates the adhesive response to pressure [Walsh et al., 2004]. Increases in pressure over weeks to years may have profound effects on tumor biology that await further study.
In summary, paxillin influences squamous cancer adhesion, both at ambient pressures and under conditions of increased extracellular pressures. Paxillin-overexpression, noted in some malignancies, appears to maximally upregulate adhesive properties, even at ambient pressure. Paxillin phosphorylation may also upregulate squamous cancer cell adhesion in response to pressure. Minimal tumor manipulation and chemotherapeutic or molecular targeting of paxillin in selected tumors might reduce metastasis in patients with head and neck tumors or other malignancies that display this response paradigm.
Acknowledgements
The study was supported by NIH, contract grant numbers RO1DK06771 (MDB), NIH2 T32 GM008420 (MDB).




