c-Myc-dependent etoposide-induced apoptosis involves activation of Bax and caspases, and PKCdelta signaling
Abstract
The c-Myc transcription factor is a key regulator of cell proliferation, differentiation, and apoptosis. While deregulation of myc induces programmed cell death, defects in the apoptotic program facilitate Myc-driven tumor development. We have treated c-Myc inducible mouse cells and rat fibroblasts with different c-myc status with cytotoxic drugs to explore the effect of c-Myc on drug-induced apoptosis. We found that c-Myc overexpression potentiated etoposide-, doxorubicin-, and cisplatin-induced cell death in mouse fibroblasts. In addition, these drugs provoked a strong apoptotic response in c-Myc-expressing cells, but a weak apoptosis in c-myc null Rat1 cells. In contrast, staurosporine-induced apoptosis was c-Myc-independent, confirming a functional apoptotic pathway in c-myc null cells. Apoptosis was paralleled by c-Myc-dependent Bax-activation after etoposide and doxorubicin treatment, but not after cisplatin administration. All three drugs induced higher caspase activation in c-Myc expressing cells than in c-myc null cells. Furthermore, etoposide treatment of c-Myc expressing cells resulted in PKCδ cleavage, while inhibition of PKCδ reduced etoposide-induced apoptosis and prevented Bax activation. Taken together, these findings suggest that Bax and caspase activation, together with PKCδ signaling are involved in c-Myc-dependent etoposide-induced apoptosis. © 2006 Wiley-Liss, Inc.
Many human tumors carry amplified or deregulated expression of the myc oncogene (reviewed in Prendergast [1999]). In normal cells, Myc overexpression results in apoptosis, while mutations in the apoptotic pathway promote Myc driven proliferation and facilitate tumor development [Evan et al., 1992; Pelengaris et al., 2002]. As a member of the Myc/Max/Mad network of transcription factors, Myc requires heterodimerization with Max for specific DNA binding and downstream effects, such as transcriptional activation of target genes through recruitment of histone acetyl transferases. Myc is an important regulator of S-phase entry, proliferation, and differentiation [Henriksson and Lüscher, 1996]. However, in situations of cellular stress such as growth factor deprivation, hypoxia, ionizing radiation or cytotoxic drug treatment, and in response to Fas stimulation, c-Myc deregulation results in apoptosis [Evan et al., 1992; Hueber et al., 1997; Maclean et al., 2003]. These contrasting Myc functions have led to the proposal of a dual signal model where Myc possesses the ability to drive both proliferation and apoptosis, and where the cell fate is determined by the cellular environment [Harrington et al., 1994; Hueber and Evan, 1998]. Repression of c-Myc has also been shown to enable tumor regression, both by the antisense technique in transformed cells, and by in vivo studies in mice, where tumors arising from enforced Myc expression regressed when Myc was turned off [Smith and Wickstrom, 1998; Felsher and Bishop, 1999; Pelengaris et al., 2002].
It has been suggested that c-Myc-mediated cytochrome-c release from mitochondria to the cytosol occurs through activation or upregulation of the pro-apoptotic Bcl-2 family members Bax and/or Bak [Desagher et al., 1999; Mitchell et al., 2000; Soucie et al., 2001; Juin et al., 2002; Iaccarino et al., 2003; Brunelle et al., 2004]. The pro-apoptotic BH3 only protein Bid has been demonstrated to induce Bax activation in its full-length conformation [Tafani et al., 2002; Iaccarino et al., 2003] as well as in its cleaved form [Desagher et al., 1999; Eskes et al., 2000]. However, c-Myc may also directly affect the permeability of the mitochondrial membrane and thus circumvent any requirement for Bid or Bax for apoptosis induction [Iaccarino et al., 2003]. Bid-mediated Bax activation may require pro-apoptotic Bad to sequester the anti-apoptotic Bcl-2 and Bcl-XL proteins [Eskes et al., 2000]. In turn, Bcl-2 and Bcl-XL are indirectly stimulated by Akt-mediated Bad sequestration [Zha et al., 1996]. Akt is a mediator in the Phosphatidylinositol-3 Kinase (PI3K) signaling cascade [Vivanco and Sawyers, 2002] and its effect on Bad can be counteracted by the active catalytic fragment of protein kinase C delta (PKCδ) [Suzuki et al., 2004]. PKCδ is a pro-apoptotic enzyme that can undergo cleavage and activation in a caspase 3-dependent manner or independently of caspases [Emoto et al., 1995; Koriyama et al., 1999; Blass et al., 2002; Suzuki et al., 2004; Lewis et al., 2005]. In addition to the classical apoptotic pathways, PKCδ-induced apoptosis can also be promoted via mediators such as DNA-dependent protein kinase (DNA-PK), c-Abl, and p73 (reviewed in Brodie and Blumberg [2003]).
The cytotoxic drugs etoposide, doxorubicin, and cisplatin are currently used in cancer therapy [Fulda et al., 1997], while the protein kinase inhibitor staurosporine has been proposed as an adjuvant treatment to enhance the tumor sensitivity to other drugs [Gescher, 2000]. Etoposide and doxorubicin promote DNA damage by inhibiting topoisomerase II-mediated re-ligation of double strand breaks [Hande, 1998]. Doxorubicin possesses additional functions such as DNA intercalation, inhibition of strand separation, and formation of reactive oxygen species [Hande, 1998]. Cisplatin is a highly reactive, non-selective alkylating agent that interacts with DNA, RNA, and proteins [Pérez, 1998]. Several studies have defined the apoptosis pathways induced after drug treatment in relation to mitochondria and Fas signaling [Fulda et al., 1997; Desagher et al., 1999; Juin et al., 1999, 2002; Mitchell et al., 2000; Teitz et al., 2000; Soucie et al., 2001; Brunelle et al., 2004]. In this study, we have used etoposide, doxorubicin, and cisplatin in c-Myc inducible mouse fibroblasts, and in rat fibroblasts with different c-Myc status, to characterize the role of c-Myc in drug-induced apoptosis.
MATERIALS AND METHODS
Cell Culture, Transfections, and Media
NIH3T3 mouse fibroblasts with conditional c-Myc expression (Tet-Myc) were generated as previously described [Bejarano et al., 2000]. Briefly, cells stably transfected with a tet-repressor expressing plasmid were selected with puromycin (Tet-control cells). For generation of Tet-Myc cells, the puromycin-selected cells were co-transfected with a tet-myc expression vector containing the human c-myc gene and a neomycin resistance (neo) plasmid. Tet-Mad1ΔN cells were generated by co-transfection of the neo plasmid and a tet-mad1 mutant lacking the region encoding the mSin3 interacting domain (SID), important for its transcriptional repression [Bejarano et al., 2000]. The tetracycline analog doxycycline (dox; Sigma, Stockholm, Sweden) was used to induce gene expression. Tet-cells were grown in Iscove's modified Dulbecco's medium (IMDM) supplemented with glutamine, penicillin/streptomycin, 10% tetracycline-free fetal calf serum (TetFCS; BD Biosciences, Stockholm, Sweden), puromycin (5 µg/ml), and G418 (500 µg/ml; both Merck Biosciences Ltd., Nottingham, UK).
TGR-1 and HO15.19 Rat1 fibroblasts [Mateyak et al., 1997] were kind gifts from J.M. Sedivy (Brown University, RI) and HOmyc3 cells [Bush et al., 1998] were kindly provided by M. Cole (Dartmouth Medical School, NH). c-myc null HO15.19 cells were generated from parental TGR-1 by homologous recombination, deleting both c-myc alleles [Mateyak et al., 1997]. These cells have a two- to threefold slower cell doubling time than TGR-1 and HOmyc3 cells [Mateyak et al., 1997; Soucie et al., 2001] and were therefore treated with drug twice as long as the c-Myc expressing Rat1 cells before analysis. Reintroduction of murine c-myc into HO15.19 cells generated the c-Myc overexpressing HOmyc3 cells [Bush et al., 1998]. Bax deficient and control mouse embryo fibroblasts (MEFs) were kind gifts from S. Korsmeyer and have been described elsewhere [McCurrach et al., 1997]. Rat1 cells and MEFs were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with glutamine, penicillin/streptomycin, and 10% FCS (Sigma).
For transfection, TGR-1 cells were seeded in 12-well plates at a density of 2.0 × 105 cells per well. After 24 h, cells were washed once in Optimized minimal essential medium I (OptiMEM I) supplemented with glutaMAX I (both Invitrogen, Paisley, UK) and 10% FCS and subsequently refed with 800 µl OptiMEM before transfection by Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Briefly, 200 µl of the transfection mixture, containing 1.6 µg cDNA, was added to each well followed by 4–6 h incubation. Cells were then washed and complete DMEM was added. Eighteen hours post-transfection, cells were transferred in a 1:2 ratio onto 12-mm round cover slips (Menzel, Braunschweig, Germany) in new 12-well plates prior to etoposide treatment (2.5 µg/ml for 24 h).
Antibodies and Reagents
The mouse monoclonal α-Myc-antibody (clone 9E10) was a kind gift from G. Evan (Cancer Research Institute, University of California, San Francisco, CA). Isotype mouse control (clone MOPC-21), Armenian hamster α-Fas (clone Jo2), isotype hamster control (clone Ha4/8), FITC-conjugated mouse α-hamster (clones G70-204, G94-56), and mouse monoclonal α-Bcl-XL (clone 2H12) antibodies were from BD Biosciences. Mouse monoclonal α-Bax (B-9) and rabbit polyclonal α-PKCδ (C-20) were from Santa Cruz Biotechnology, Inc., Santa Cruz, CA; conformation-specific mouse monoclonal α-Bax (6A7) were from BD Biosciences and Trevigen, Gaithersburg, MD; goat polyclonal α-Bid (AF-860) from R&D systems Europe Ltd., Oxon, UK; and the mouse monoclonal β-actin antibody (clone Ac-15) was from Sigma. FluoroLink™Cy2-labeled donkey α-mouse, HRP-conjugated sheep α-mouse, and HRP-conjugated donkey α-rabbit antibodies were from Amersham Biosciences, Piscataway, NJ; and the HRP-conjugated rabbit α-goat antibody was from DakoCytomation, Solna, Sweden.
Cis-platinum(II)diammine dichloride (P4394), doxorubicin hydrochloride (D1515), etoposide (E1383), staurosporine Streptomyces sp (S4400), LY294002 (L9908), and rottlerin (R5648) were from Sigma. The caspase substrates Ac-DEVD-AFC (caspase 3), Ac-LETD-AFC (caspase 8), and Ac-LEHD-AFC (caspase 9), and the general caspase inhibitor zVAD-FMK were purchased from Enzyme Systems, Inc., Livermore, CA. Etoposide, staurosporine, caspase substrates, and inhibitors were dissolved in DMSO.
cDNA encoding human wild-type PKCδ, or the dominant negative form of PKCδ (PKCδ-DN), fused into the N-terminal enhanced green fluorescent protein (EGFP) vector pEGFP-N1 (BD Biosciences), together with the pEGFP-N1 vector were kind gifts from Christer Larsson (Malmö University Hospital, Malmö, Sweden) and have been described elsewhere [Zeidman et al., 1999; Ling et al., 2004].
Western Blotting
Culture media was aspirated from plates and cell extracts were prepared. For whole cell lysates, plates were incubated with F buffer (10 mM Tris-HCl pH 7.05, 50 mM NaCl, 30 mM Na-pyrophosphate, 50 mM NaF, 5 µM ZnCl2, 100 µM Na3VO4, 2.5 µg/ml pepstatin A, 2.5 µg/ml leupeptin, 0.15 mM benzamidin, 2 µg/ml aprotinin, and 1 mM dithiothreitol) for 5 min on ice, whereafter cells were scraped off and placed on ice another 10 min before vortexing for 45 s. Alternatively, 105 trypsinized cells were resuspended in 10 µl boiling 2× sample buffer (80 mM Tris-HCl pH 6.8, 10% glycerol, 5% SDS, 4% β-mercaptoethanol, and bromphenol blue), denatured for 5 min at 95°C, and used for Western analysis. To extract cytosolic proteins, cells were incubated with NP-40 lysis buffer (100 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40, and 1 mM PMSF), scraped off and placed on ice for 30 min with occasional vortexing. Extracts were cleared by centrifugation at 14,000 rpm for 15–20 min at 4°C and the protein concentration was determined by the Bradford method (Bio-Rad protein assay reagent; Bio-Rad Laboratories AB, Sundbyberg, Sweden). The proteins were separated on 10–15% sodium dodecyl sulphate–polyacrylamide gel electrophoreses (SDS–PAGE) and blotted to nitrocellulose membranes. To confirm equal loading and transfer, membranes were stained with Ponceau red (Sigma). The membranes were blocked with 5% milk in PBS-Tween20 (PBS-T, 0.2%) for 1 h, incubated with primary antibody for 2 h at room temperature or over night at 4°C, washed twice with PBS-T, and incubated with HRP-conjugated secondary antibody for 2 h at room temperature. The membranes were then stripped and reprobed with β-actin followed by HRP-conjugated α-mouse antibody as a loading control. The blots were developed by enhanced chemiluminescence (ECL, Amersham Biosciences).
Cell Death Assays
For DNA profile analysis, 1.5 × 105 cells were plated in 6-cm dishes and treated with drugs as indicated. Floating and trypsinized cells were collected from each sample. The pellet was rinsed with PBS, fixed with 70% ice-cold ethanol, and kept at 4°C for at least 18 h prior to propidium iodide staining (50 µg/ml PI and 250 µg/ml RNAse A in PBS) for 30 min at 37°C. Samples were analyzed in a Becton Dickinson FACScan flow cytometer equipped with an argon laser. Cell doublets were excluded by electronic gating and the subG1 fraction, corresponding to fragmented DNA, was calculated using the CELLQuest software. For Fas stimulation or inhibitor experiments, cells were pretreated for 1 h with the agonistic Jo2 Fas antibody (0.5 µg/ml) or with inhibitors (10 µM LY294002 or 10 µM Rottlerin).
The “Cell death detection ELISAplus” kit (Roche Diagnostics Scandinavia AB, Bromma, Sweden) was used for detection of apoptosis in drug-treated cells. Cells (2,500) were grown in flat bottomed 96-well plates, incubated with drugs or recombinant Fas Ligand for indicated times and processed for analysis according to the manufacturer's instructions. Briefly, after discarding the supernatant, attached cells were lysed and 10% of the lysates were transferred to a streptavidin-coated microplate, incubated with a biotin-conjugated α-histone antibody and a peroxidase-conjugated α-DNA antibody, and washed before substrate addition. The peroxidase activity was determined photometrically at 405 nm with 490 nm as a reference wavelength and the results are presented as apoptosis (absorbance units).
Cell death was also evaluated by trypan blue exclusion, evaluating at least 100 cells to obtain the percentage of blue cells with permeabilized cell membranes. For Hoechst staining, cells grown on coverslips were treated with drug for indicated times, washed with PBS, and fixed with 2–4% paraformaldehyde (Merck Biosciences Ltd.). Chromatin was then stained with Hoechst 33342 (1:1,500, Sigma) for 10 min. After additional washes, coverslips were mounted (100% glycerol, 500 mM Tris-HCl pH 8.0, and 25% DABCO) for microscopy. At least 200 cells were examined and the percentage of cells with condensed chromatin and nuclear fragmentation (apoptotic nuclei) was assessed. For the EGFP transfectants (PKCδ-DN, PKCδ, and vector), apoptosis was scored as percentage of EGFP positive cells with condensed nuclei. In each experiment at least 100 green cells were counted by two independent investigators. All images were recorded on a DAS microscope Leitz DM RB with a Hamamatsu dual mode cooled CCD camera C4880 and processed in ImageProPlus software and Adobe Photoshop.
Intra- and Extra-Cellular Stainings
Fas surface expression was analyzed in 1.5 × 105 Tet-Myc cells plated in 6-cm dishes and treated with drugs as indicated. c-Myc expression was induced by dox addition (1 µg/ml) 18 h prior to drug treatment. Floating and trypsinized cells were collected and washed with cold staining buffer (0.1% NaN3 and 1% BSA in PBS). After incubation on ice for 30 min with the Jo2 Fas antibody (1 µg/ml) or the isotype hamster control antibody, cells were washed twice with cold staining buffer, incubated with FITC-mouse α-hamster (1 µg/ml), and washed again. Fixation with 1% paraformaldehyde was followed by an additional wash and resuspension in PBS prior to analysis by flow cytometry. Cell debris and dead cells were excluded by electronic gating. The Fas-specific median fluorescence intensity (MFI) was calculated by subtracting the MFI of the isotype control antibody from that of the Fas antibody.
To evaluate Bax activation, 8 × 105 cells were plated in 10-cm dishes 1 day prior to drug addition. Following treatment, cells were gently detached by scraping in pre-warmed cell dissociation solution (CDS, Sigma), washed in PBS, and fixed in 0.25% paraformaldehyde. After 30 min incubation at room temperature with the Bax-conformation-specific antibody (6A7, 5 µg/ml) or the isotype control antibody diluted in PBS:digitonin (100 µg/ml, Sigma), cells were washed twice in PBS and incubated with the FluoroLink™Cy2-labeled donkey α-mouse antibody (10 µg/ml). After washing, cells were resuspended in PBS and analyzed by flow cytometry where cell debris and dead cells were excluded by electronic gating. For blocking experiments, the Bax antibody was pre-incubated for 15 min at room temperature with blocking peptide or nonspecific peptide (6A7 blocking set, BD Biosciences) in a 1:10 ratio before staining.
For immunofluorescence analysis of Bax activation, 105 cells were grown on coverslips in 6-well plates over night, followed by 5 h incubation with drug. After washes with PBS, cells were fixed with 1% paraformaldehyde for 20 min at 4°C. Coverslips were then washed three times with PBS and stained with antibodies according to the procedure for flow cytometry, with the exception that Hoechst 33342 (1:1,500) was added together with the secondary antibody.
Analysis of Caspase Activation
To analyze caspase activity 5 × 105 cells were plated in 10-cm dishes and incubated with drug. In Tet-Myc cells, c-Myc expression was induced by addition of 1 µg/ml dox 18 h before drug addition. When indicated, cells were pre-incubated for 1 h with the general caspase inhibitor zVAD-FMK.
Floating and trypsinized cells were collected and counted. Pellets containing 4.5 × 105 live cells were washed with PBS and snap frozen in liquid nitrogen. For fluorometric detection of caspase activation, pellets were lysed with NP-40 lysis buffer (50 mM Tris-HCl pH 7.4, 0.5% NP-40, 150 mM NaCl, and 5 mM EDTA) for 15 min on ice, cleared at 14,000 rpm for 10 min at 4°C, and transferred in three equal aliquots to black flat bottomed 96-well plates (FluoroNunc polysorp, VWR International AB, Stockholm, Sweden). Substrates specific for caspase 3, 8, and 9 were diluted in reaction buffer (100 mM HEPES, 20% glycerol, 0.5 mM EDTA, and 5 mM DTT) and added to the samples at a final concentration of 20 µM in 100 µl. The plate was placed on ice for analysis in a SpectraMax Gemini XS fluorescence spectrometer (Molecular Devices, Sunnyvale, CA) at 37°C with 400 nm excitation and 505 nm emission wavelengths. Fluorescence intensity was registered every 10 min for 120 min giving a total of 13 readouts. In each experiment, the specific substrate cleavage was calculated by normalizing the readout from each sample to the substrate-emitted fluorescence. Caspase-specific activity was then plotted as relative fluorescence units, as emitted at 60 min.
RESULTS
Apoptosis Induced by Etoposide, Doxorubicin, and Cisplatin is c-Myc-Dependent and can be Enhanced by c-Myc Overexpression
To evaluate how c-Myc influences the cellular fate after treatment with cytotoxic drugs, we used NIH3T3 mouse fibroblasts with doxycycline (dox) inducible c-Myc expression (Tet-Myc) [Bejarano et al., 2000] as well as TGR-1, HO15.19, and HOmyc3 Rat1 cells. The HO15.19 c-myc null cells were generated from parental TGR-1 cells by homologous recombination [Mateyak et al., 1997]. Wild-type murine c-myc was introduced into HO15.19 cells generating HOmyc3 cells [Bush et al., 1998] that express a two- to threefold higher level of c-Myc compared to TGR-1 cells [Adachi et al., 2001].
In order to quantify the cell death induced by etoposide, doxorubicin, or cisplatin, we performed DNA profile analysis on propidium iodide stained nuclei. The drug concentrations were titrated to reach a low cell death (subG1 fraction) in uninduced Tet-Myc cells (data not shown). At these low drug concentrations, c-Myc overexpression enhanced the cell death induced by all three drugs in a concentration-dependent manner (Fig. 1a). We also observed that c-Myc overexpression alone induced a low level of cell death in full serum (Fig. 1a). The dox-induced c-Myc overexpression in the Tet-Myc cells was not affected by drug treatment (Fig. 1b). The possible toxicity of dox was ruled out by performing similar experiments in Tet-control NIH3T3 cells, as well as in Tet-Mad1ΔN cells ([Bejarano et al., 2000] and data not shown). When exploring cell death in drug-treated Rat1 cells, HO15.19 cells were analyzed at the same time as TGR-1 and HOmyc3 cells, but also twice as long as they have a longer cell doubling time. We found that etoposide and doxorubicin induced a concentration-dependent increase in cell death that was higher in the c-Myc expressing TGR-1 and HOmyc3 cells than in the c-myc null HO15.19 cells. In contrast, the cell death induced by cisplatin treatment was equally high in c-myc null as in c-Myc expressing cells (Fig. 2a).
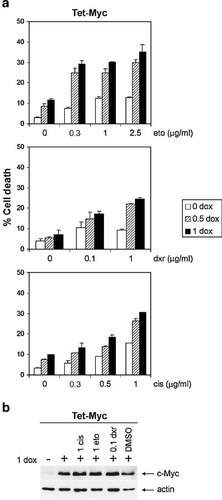
Cell death and c-Myc expression in drug-treated Tet-Myc cells. a: DNA profile analysis of propidium iodide stained cells after treatment with etoposide (eto) or doxorubicin (dxr) for 24 h, or with cisplatin (cis) for 48 h, in the absence (open bars) or presence of doxycycline (dox; 0.5 µg/ml, striped bars or 1 µg/ml, filled bars) for c-Myc induction. Results are shown as the subG1 fraction (% cell death). Bars represent the mean cell death from triplicate samples and error bars indicate the standard deviation. Data are from one representative of five independent experiments. b: Western blot analysis of c-Myc in whole cell extracts obtained after 18 h pretreatment with (+) or without (−) 1 µg/ml dox followed by 24 h incubation with drug in the concentrations indicated (µg/ml). After separation by 10% SDS–PAGE, the membrane was probed with c-Myc antibody, stripped, and reprobed with β-actin antibody as a loading control. One representative blot from two separate experiments is presented.
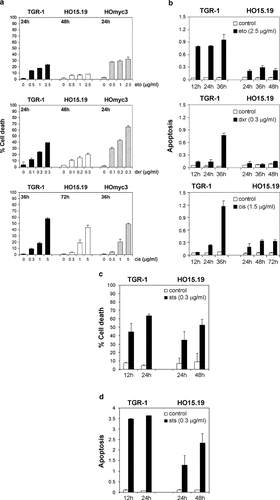
Cell death and apoptosis in drug-treated Rat1 cells. a: c-Myc expressing TGR-1 (filled bars), HOmyc3 cells (striped bars), and c-myc null HO15.19 cells (open bars) were treated with indicated drug concentrations. After 24 h (eto and dxr) or 36 h (cis), TGR-1 and HOmyc3 cells were stained with propidium iodide and the subG1 fraction (% cell death) was quantified by flow cytometry. HO15.19 cells were treated twice as long to compensate for their slower proliferation rate. Bars represent the mean of triplicate samples with error bars indicating standard deviation. Data from one of at least three separate experiments. b: TGR-1 and HO15.19 cells were treated with (filled bars) or without (open bars) drugs as indicated, and apoptosis was quantified by the apoptosis-specific ELISA kit. One representative of three independent experiments is presented as the mean absorbance units and standard deviation from duplicate samples. c: TGR-1 and HO15.19 cells were treated with (filled bars) or without (open bars) 0.3 µg/ml staurosporine (sts) and the cell death (subG1 fraction) was evaluated after the indicated times. Results are shown as the mean and standard deviation from three separate experiments. d: Sts-induced apoptosis in TGR-1 and HO15.19 cells was quantified by the apoptosis-specific ELISA. Data from untreated cells (open bars) or cells treated with 0.3 µg/ml sts (filled bars) represent duplicate samples in one of two independent experiments.
To discriminate apoptosis from necrosis, we used an apoptosis ELISA and found that etoposide, doxorubicin, and cisplatin induced apoptosis in TGR-1 cells, whereas a weak effect was observed in HO15.19 cells (Fig. 2b). These results indicated that cisplatin induced mainly necrosis in HO15.19 cells. To corroborate this finding, we compared the trypan blue positive cell count to the percentage of Hoechst stained nuclei with condensed DNA. In TGR-1 cells, cisplatin treatment generated 41% trypan blue positive cells and 38% with nuclear condensation, while in HO15.19 cells the corresponding numbers were 49% and 12%, respectively (Table I). In summary, our results demonstrate that etoposide, doxorubicin, and cisplatin induced apoptosis in a c-Myc-dependent manner.
| Cells | TGR-1 (%) | HO15.19 (%) | ||||
|---|---|---|---|---|---|---|
| cis treatmenta | 0 h | 24 h | 48 h | 0 h | 48 h | 72 h |
| Trypan blue positive | 5 | 19 | 41 | 3 | 25 | 49 |
| Condensed nuclei (Hoechst staining) | 2 | 18 | 38 | 4 | 14 | 12 |
| sts treatmentb | 0 h | 12 h | 24 h | 0 h | 24 h | 48 h |
| Trypan blue positive | 10 | 45 | 48 | 8 | 28 | 53 |
| Condensed nuclei (Hoechst staining) | 10 | 50 | 53 | 8 | 38 | 46 |
- a cis, cisplatin; 5 µg/ml.
- b sts, staurosporine; 0.3 µg/ml.
Given the fact that the c-myc null cells were relatively resistant to apoptosis, we wanted to evaluate whether the apoptotic pathway was disrupted in these cells. To this end, TGR-1 and HO15.19 cells were treated with staurosporine. DNA profile analysis revealed that the subG1 fraction was similar in TGR-1 as in HO15.19 cells upon staurosporine treatment (Fig. 2c). In this case, apoptosis was induced both in TGR-1 and HO15.19 cells, as shown by ELISA (Fig. 2d). In addition, we found 48% trypan blue positive TGR-1 cells and 53% with nuclear condensation, while the corresponding numbers for HO15.19 cells were 53% and 46%, respectively (Table I). These results demonstrated that staurosporine induced apoptosis independently of c-Myc status and that the apoptotic machinery is not disrupted in HO15.19 cells.
Drug-Induced Caspase Activation is Higher in c-Myc Expressing Than in c-myc Null Rat1 Cells
Next, we investigated the involvement of caspases in the apoptotic response to drug treatment and possible differences in the activation pattern with regard to c-Myc status. We found that caspase 3 was activated in response to etoposide, doxorubicin, and cisplatin treatment, and that activation was more pronounced in c-Myc expressing TGR-1 and HOmyc3 than in c-myc null HO15.19 cells (Fig. 3a). A higher level of caspase 8 and 9 activation was also observed in c-Myc expressing cells compared to c-myc null cells (Fig. 3a). Similarly, analysis of drug treated and untreated Tet-Myc cells revealed higher caspase activation upon induced c-Myc overexpression, correlating with the observed cell death (Fig. 1a and data not shown). As in the Rat1 cells, activation of caspase 3 was most pronounced in response to all drugs analyzed (Fig. 3a and data not shown). Pretreatment of Rat1 and Tet-Myc cells with the general caspase inhibitor zVAD-FMK completely inhibited drug-induced caspase activation. However, apoptosis was only partially blocked, indicating involvement of additional pathways (Fig. 3b and data not shown).
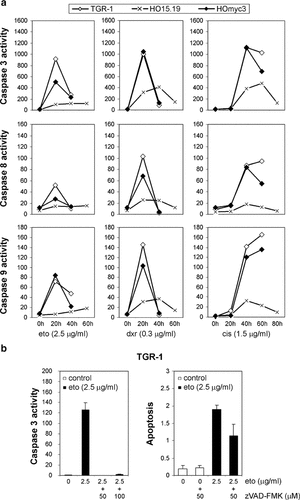
Caspase activation and drug-induced apoptosis in Rat1 cells. a: Caspase activity in Rat1 cell lysates after treatment with etoposide (eto, 2.5 µg/ml), doxorubicin (dxr, 0.3 µg/ml), or cisplatin (cis, 1.5 µg/ml) for the indicated times. The caspase-specific fluorescence emitted after 60 min incubation with the substrate is presented for TGR-1 (open diamonds), HO15.19 (×), and HOmyc3 (filled diamonds) cells. Upper panel: Caspase 3 activity, middle panel: caspase 8 activity, and lower panel: caspase 9 activity. Data are representative from at least three independent experiments. b: Caspase 3 activity and apoptosis in TGR-1 cells treated for 24 h with (filled bars) or without (open bars) 2.5 µg/ml etoposide. Where indicated, cells were pretreated for 1 h with 50 or 100 µM zVAD-FMK. The results are from duplicate samples in two independent experiments and represent either the caspase 3-specific fluorescence emitted after 60 min incubation with the substrate, or the apoptosis-specific absorbance as measured by ELISA.
Fas Stimulation Induces c-Myc-Dependent Apoptosis in Rat1 Cells
The Fas pathway has been implicated in apoptosis induced by etoposide, doxorubicin, and cisplatin, as well as in c-Myc-induced apoptosis [Fulda et al., 1997; Hueber et al., 1997]. We therefore investigated the involvement of Fas in drug-induced apoptosis in Tet-Myc cells. Our analysis revealed cell surface upregulation of the Fas receptor in response to etoposide and doxorubicin (MFI = 29.0 and 29.5, respectively) as compared with untreated control cells (MFI = 3.6) (Fig. 4a). Cisplatin also induced Fas upregulation (MFI = 6.8) but to a lesser extent than following etoposide and doxorubicin treatment. However, c-Myc overexpression did not affect Fas surface expression (Fig. 4a). Next, we stimulated Fas signaling with an agonistic antibody to analyze the effect on cell death induced by drug treatment in Tet-Myc cells with or without c-Myc overexpression. We found that Fas stimulation increased the subG1 fraction induced by all three drugs after 24 h and 48 h treatment. However, Fas activation did not further increase the drug-induced cell death in c-Myc overexpressing cells (Fig. 4b and data not shown).
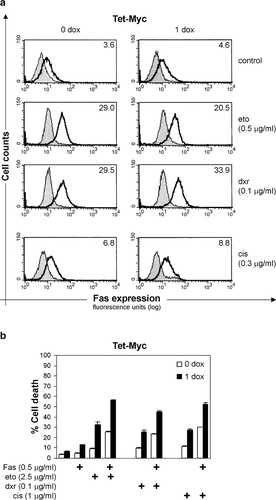
Role of Fas signaling in drug-induced cell death in Tet-Myc cells. a: Cell surface Fas expression after treatment with etoposide (eto, 0.5 µg/ml) or doxorubicin (dxr, 0.1 µg/ml) for 18 h, or with cisplatin (cis, 0.3 µg/ml) for 36 h. For induction of c-Myc expression, 1 µg/ml doxycycline (1 dox) was added 18 h prior to drug treatment (right column histograms). The histograms in the left column show uninduced cells (0 dox). Fas surface expression was determined by flow cytometry, showing the Fas antibody staining (bold line) and the isotype control antibody staining (gray peak). Specific median fluorescence intensity (MFI) is displayed in the top right corner of each histogram. Data are from one representative of three independent experiments. b: Cell death was induced by Fas stimulation (1 h pretreatment with Fas antibody) and 24 h treatment with the indicated concentrations of eto, dxr, or cis in the presence (1 dox, filled bars) or absence (0 dox, open bars) of doxycycline pretreatment (1 µg/ml for 18 h). The subG1 fraction (% cell death) was quantified by flow cytometry after propidium iodide staining of duplicate samples. One representative experiment out of two is presented.
In Rat1 cells, involvement of the Fas pathway was evaluated by treatment with recombinant Fas Ligand. While Fas stimulation generated apoptosis in TGR-1 cells, no effect was observed in HO15.19 cells (Fig. 5). Fas-induced apoptosis in the c-Myc expressing cells was paralleled by increased activities of caspases 3 and 8, but not of caspase 9 (data not shown).
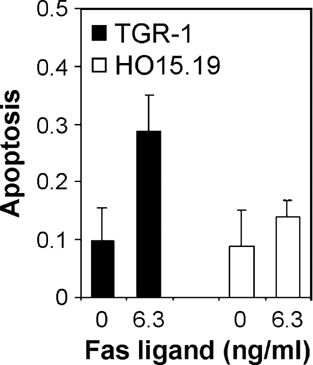
Fas Ligand-induced apoptosis in Rat1 cells. Apoptosis was analyzed by the apoptosis-specific ELISA kit in TGR-1 (filled bars) and HO15.19 cells (open bars) after 72 h treatment with or without 6.3 ng/ml recombinant Fas Ligand. Results are shown as the mean absorbance units from duplicate samples from three independent experiments.
Etoposide and Doxorubicin Induce Bax Activation in c-Myc Expressing Rat1 Cells
One mechanism for c-Myc-induced apoptosis involves the release of cytochrome-c from mitochondria [Juin et al., 1999; Iaccarino et al., 2003]. Since the pro-apoptotic Bax protein has been suggested to mediate cytochrome-c release [Desagher et al., 1999; Soucie et al., 2001; Juin et al., 2002], we evaluated its expression and activation after drug-induced apoptosis. To analyze Bax activation, we used an antibody, 6A7, specific for the active conformation of Bax. A low spontaneous Bax activation was seen in untreated Rat1 cells (Fig. 6a). Treatment of TGR-1 cells with doxorubicin or etoposide resulted in an increased Bax activation, whereas no further activation was observed in HO15.19 cells, even at late time points (Fig. 6a,b). Cisplatin treatment, on the other hand, did not result in increased Bax activation, neither in TGR-1 nor in HO15.19 cells (Fig. 6a). The specificity of the 6A7 Bax antibody was shown by blocking the staining with a Bax-specific peptide (Fig. 6c).
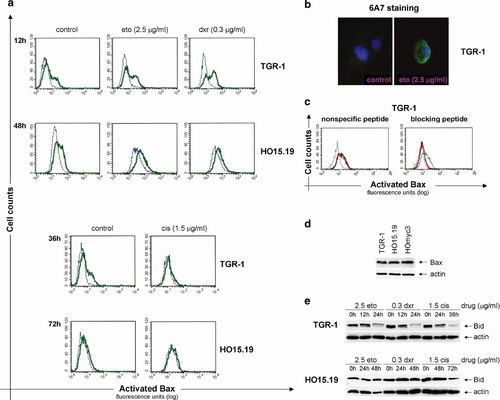
Bax expression and activation in drug-treated Rat1 cells. a: Bax activation as analyzed by flow cytometry in TGR-1 and HO15.19 cells treated with doxorubicin (dxr; 12 h and 48 h), etoposide (eto; 12 h and 48 h), or cisplatin (cis; 36 h and 72 h) as well as in untreated cells (control). The histograms display cells stained with the conformation-specific 6A7 Bax antibody (bold line), or with an isotype control antibody (dotted line), followed by a Cy2-labeled donkey α-mouse antibody. Data are representative from at least three independent experiments. b: Etoposide-induced Bax activation was analyzed by immunofluorescence in TGR-1 cells after 5 h treatment with 2.5 µg/ml etoposide (eto) and staining with the 6A7 antibody. c: Etoposide-treated TGR-1 cells (2.5 µg/ml for 24 h) were stained with 6A7 antibody with (bold line) or without (thin line) 15 min pre-incubation with nonspecific or blocking peptide to confirm specificity. The dotted line represents cells stained with the isotype control antibody. Data are representative from two independent experiments. d: Bax expression in untreated Rat1 cells with (TGR-1 and HOmyc3) or without (HO15.19) c-Myc. Cytosolic cell extracts were analyzed by Western blot using 15% SDS–PAGE. The membrane was probed with Bax antibody (B-9), stripped, and reprobed with β-actin antibody as a loading control. One representative blot from two independent experiments is presented. e: Bid expression in TGR-1 and HO15.19 cells after treatment with or without 2.5 µg/ml eto, 0.3 µg/ml dxr, or 1.5 µg/ml cis for the times indicated. Cytosolic cell extracts were separated by 15% SDS–PAGE for Western blot analysis. The membrane was probed with Bid antibody, stripped, and reprobed with β-actin antibody as a loading control. One representative blot from two independent experiments is presented. [Color figure can be viewed on the online issue, which is available at www.interscience.wiley.com.]
The lack of Bax activation in HO15.19 cells was not due to decreased levels of the protein, since the expression was similar in all three Rat1 cell lines (Fig. 6d). From these findings, we deduced that factors upstream of Bax activation were blocked in c-myc null cells and thus analyzed expression and cleavage of Bid. We observed reduced levels of full-length Bid after etoposide, doxorubicin, and cisplatin treatment in TGR-1, but not in HO15.19 cells (Fig. 6e). In agreement with a recent report, we also found an increased expression of Bcl-XL in HO15.19 as compared to TGR-1 cells ([Grassilli et al., 2004] and data not shown). We therefore assessed the possibility to enhance drug-induced apoptosis in HO15.19 cells by indirectly blocking Bcl-XL with the PI3K inhibitor LY294002 as shown for doxorubicin-induced apoptosis in melanoma cells [Panaretakis et al., 2002]. However, LY294002 did not affect apoptosis in these cells (data not shown).
Etoposide-Induced Apoptosis is Reduced by Blocking PKCδ in c-Myc Expressing Rat1 Cells
The pro-apoptotic kinase PKCδ is activated and undergoes cleavage in response to various apoptotic stimuli [Emoto et al., 1995; Koriyama et al., 1999; Blass et al., 2002; Suzuki et al., 2004]. In order to assess the involvement of PKCδ in c-Myc-dependent drug-induced apoptosis, we examined PKCδ cleavage in etoposide-treated TGR-1 and HO15.19 cells. The level of PKCδ decreased and a 40 kDa cleavage product accumulated following etoposide treatment in c-Myc expressing but not in c-myc null cells (Fig. 7a). Given the fact that PKCδ signaling has been shown to potentiate apoptosis induced by a variety of agents, we next investigated whether blocking this pathway would inhibit apoptosis. We found that the PKCδ inhibitor rottlerin reduced etoposide-induced apoptosis in TGR-1 cells, while no inhibition but rather a slight increase was observed in HO15.19 cells (Fig. 7b). To corroborate these data we transfected TGR-1 cells with constructs expressing wild-type (PKCδ) and dominant negative PKCδ (PKCδ-DN) proteins. Expression of PKCδ-DN reduced etoposide-induced apoptosis to a similar extent as rottlerin, while PKCδ or the vector control did not have any effect (Fig. 7c,d). These data indicate a role for PKCδ in Myc-dependent etoposide-induced apoptosis.
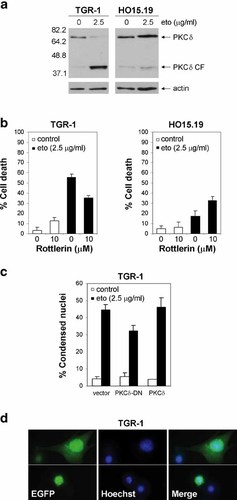
Role of PKCδ in etoposide-induced apoptosis in Rat1 cells. a: Analysis of PKCδ cleavage in TGR-1 and HO15.19 cells in the absence (0 µg/ml) or presence (2.5 µg/ml) of etoposide for 24 h or 48 h. Western blot of whole cell extracts after separation by 10% SDS–PAGE. The membrane was probed with PKCδ antibody, stripped and reprobed with β-actin as a loading control. The full-length protein (PKCδ) and the catalytic fragment (PKCδ CF) are indicated to the right and the molecular weights to the left. One representative blot from two separate experiments is shown. b: Etoposide-induced cell death in TGR-1 and HO15.19 cells with or without 1 h pretreatment with 10 µM of the PKCδ inhibitor rottlerin. DNA profile analysis of propidium iodide stained cells after treatment with (filled bars) or without (open bars) 2.5 µg/ml etoposide for 24 h (TGR-1) and 48 h (HO15.19), respectively. The mean subG1 fractions (% cell death) from duplicate samples in three independent experiments are shown. c: Apoptosis in TGR-1 cells transfected with EGFP-tagged PKCδ constructs (vector, PKCδ-DN, and PKCδ) 24 h prior to treatment with (filled bars) or without (open bars) etoposide (2.5 µg/ml) for 24 h. The percentage of EGFP positive cells with condensed nuclei, as visualized by Hoechst staining, is presented as the mean and standard deviation from three separate experiments. d: Representative images of etoposide-treated EGFP positive vector transfected cells, showing nuclear condensation (lower panel) and non-apoptotic morphology (upper panel). Displayed from left to right: EGFP fluorescence, Hoechst staining, and the merged image. [Color figure can be viewed on the online issue, which is available at www.interscience.wiley.com.]
PKCδ Signaling is Upstream of Bax Activation in Etoposide-Induced Apoptosis
Our next aim was to determine whether PKCδ activation was upstream of Bax, thus initiating the apoptosis process, or if it was a secondary event executed by active caspase 3 [Emoto et al., 1995; Blass et al., 2002; Brodie and Blumberg, 2003; Lewis et al., 2005]. To this end, we assessed the effect of rottlerin on etoposide-induced Bax activation in TGR-1 cells and observed a rottlerin-mediated prevention (Fig. 8a). In addition, we found that PKCδ was cleaved to a similar extent in Bax−/− mouse embryo fibroblasts (MEFs) as in wild-type MEFs after etoposide treatment (Fig. 8b). Taken together, these results indicate that PKCδ activation occurs prior to, and may facilitate, Bax activation.
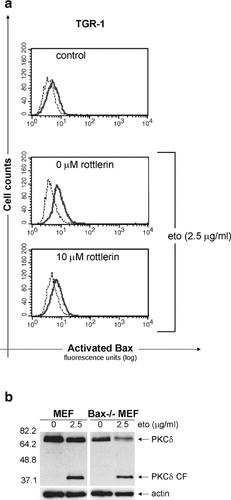
PKCδ signaling and Bax activation in etoposide-induced apoptosis. a: Bax activation in TGR-1 cells after 24 h treatment with or without 2.5 µg/ml etoposide and 1 h pretreatment with 10 µM rottlerin. Flow cytometry histograms of cells stained with the conformation-specific 6A7 Bax antibody (bold line), or an isotype control antibody (dotted line), followed by a Cy2-labeled donkey α-mouse antibody. Data are representative from two independent experiments. b: Analysis of PKCδ cleavage in mouse embryo fibroblasts (MEFs) in the absence (0 µg/ml) or presence (2.5 µg/ml) of etoposide for 24 h. Whole cell extracts (Bax−/− MEFs or control MEFs) were separated by 10% SDS–PAGE and blotted onto nitrocellulose, whereafter the membrane was probed with PKCδ antibody, stripped, and reprobed with β-actin as a loading control. The full-length protein (PKCδ) and the catalytic fragment (PKCδ CF) are indicated to the right and the molecular weights to the left. One representative blot from two independent experiments is shown.
DISCUSSION
We have used cytotoxic drugs to induce apoptosis in cell lines with either inducible c-Myc expression or with different c-Myc status. Our data confirm previous observations that apoptosis induction by several anticancer drugs is Myc-dependent [Horiguchi-Yamada et al., 2002; Desbiens et al., 2003; Gorrini et al., 2003; Grassilli et al., 2004]. We have extended these findings by further characterizing the apoptosis pathways, and found a Myc-dependent activation of Bax and caspases as well as involvement of PKCδ signaling in etoposide-induced apoptosis.
Our data suggest that cisplatin induces necrosis in the absence of c-Myc and apoptosis in its presence. HO15.19 and TGR-1 Rat1 cells have been reported to respond similarly to cisplatin [Adachi et al., 2001]. However, while these authors concluded that cisplatin induced apoptosis in TGR-1 as well as in HO15.19 cells, we observed mainly necrosis in HO15.19 cells. The discrepancies in these results may be due to the use of different techniques for apoptosis detection [Adachi et al., 2001]. It has been established by others that cisplatin and platinum derivatives induce cell death through necrosis as well as apoptosis [González et al., 2000]. Necrosis was induced when cells had low or no p53 expression [González et al., 2000; Pestell et al., 2000]. Thus, the two- to threefold deficiency in p53 induction observed in c-myc null cells, even though the protein is wild-type, may determine the cell death pathway induced by cisplatin [Marhin et al., 1997; Adachi et al., 2001]. This supports the model where c-Myc acts downstream of p53 to sensitize the cell to apoptotic stimuli [Evan and Littlewood, 1998; Adachi et al., 2001].
Our finding that staurosporine induced apoptosis in HO15.19 cells corroborates observations made in other studies [Adachi et al., 2001; Grassilli et al., 2004] and disproves the suspicion of a deficient apoptosis signaling in the c-myc null background. The exact mechanism by which staurosporine induces apoptosis remains to be elucidated, but there are reports supporting caspase-independent as well as caspase-dependent pathways. For instance, it has been suggested that the lysosomal aspartic protease cathepsin D is involved in the caspase-independent process [Janicke et al., 1998; Belmokhtar et al., 2001; Johansson et al., 2003].
The importance of Fas receptor ligation in promoting c-Myc-induced apoptosis has been debated [Hueber et al., 1997; Juin et al., 1999]. Suggestions that it is essential for c-Myc-induced cell death [Hueber et al., 1997] were later challenged by theories of parallel pathways for Fas- and c-Myc-induced apoptosis [Juin et al., 1999]. Our data from Tet-Myc fibroblasts supports the model where Fas and c-Myc operate in parallel pathways to amplify the apoptotic response [Juin et al., 1999]. However, our observations in Rat1 cells, where we found c-Myc-dependent Fas Ligand-induced apoptosis and drug-induced caspase 8 activation, raise the possibility that Fas signaling enhances drug-induced apoptosis in a c-Myc-responsive manner. It was recently reported that Fas Ligand-induced apoptosis was equally high in HO15.19 and TGR-1 cells [Grassilli et al., 2004]. However, these results were based on quantification of total cell death while we used an apoptosis-specific assay.
The pro-apoptotic Bcl-2 family members are involved in apoptosis signaling in most cells, and c-Myc activation has been reported to enable Bax-, or Bak-mediated cytochrome-c release from mitochondria [Juin et al., 1999, 2002; Soucie et al., 2001; Iaccarino et al., 2003; Brunelle et al., 2004]. Both etoposide and doxorubicin have been reported to target mitochondria through Bax activation [Rebbaa et al., 2001; Karpinich et al., 2002]. We extended these findings by demonstrating that this Bax activation was c-Myc-dependent. Our observation that Bax was not activated in HO15.19, in spite of similar protein levels as in TGR-1 cells with endogenous c-Myc expression, support the hypothesis that c-Myc is important for activation but not transcription, degradation, or translocation of Bax [Soucie et al., 2001]. It is not established, however, whether changes in Bax conformation occur before or after its translocation to the mitochondria. While some authors have found changes in Bax conformation prior to its translocation [Desagher et al., 1999; Iaccarino et al., 2003], Soucie et al. [2001] reported drug-induced translocation of Bax without subsequent cytochrome-c release or apoptosis in c-myc null Rat1 cells.
Bid has been reported to activate Bax as a full-length protein as well as in its processed form [Eskes et al., 2000; Iaccarino et al., 2003]. In agreement with the latter data, we found a correlation between Bid cleavage and Bax activation as induced by etoposide and doxorubicin in c-Myc expressing Rat1 cells. In the case of cisplatin-induced apoptosis in TGR-1 cells, where Bid cleavage did not correlate with Bax activation, the response may occur through activation of Bak [Mandic et al., 2001]. Bid-mediated Bax oligomerization and mitochondrial insertion is blocked by the anti-apoptotic Bcl-2 and Bcl-XL proteins, and is released upon phosphorylation of pro-apoptotic Bad by the PI3K mediator Akt [Zha et al., 1996; Eskes et al., 2000; Vivanco and Sawyers, 2002]. Our finding that the PI3K inhibitor LY294002 did not sensitize the c-myc null cells to etoposide together with the reported observation that the parental TGR-1 cells did not become drug-resistant by overexpression of Bcl-XL [Grassilli et al., 2004], suggests that the elevated Bcl-XL level in HO15.19 cells is not the sole cause of their apoptosis insensitivity. Based on this and our observation that drug-induced apoptosis was not blocked by inhibition of caspase activity, we propose that mitochondria- and caspase-independent pathways, in addition to signaling through mitochondria, are important for apoptosis induction by cytotoxic drugs. Some of these pathways appear to be deficient in the c-myc null cells.
Cleavage and activation of PKCδ after treatment with etoposide has been reported to amplify the apoptotic response [Koriyama et al., 1999; Blass et al., 2002]. Our observation that etoposide induced PKCδ cleavage in TGR-1 but not in HO15.19 Rat1 cells, indicates deficient PKCδ signaling as a partial reason for the reduced apoptosis in HO15.19 cells. This is in line with previous findings that PKCδ is activated upon c-Myc-induced apoptosis in Rat1 cells [Hotti et al., 2000]. Our results indicate that Bax, caspases, and PKCδ are involved in c-Myc-dependent etoposide-induced apoptosis, but also suggest contribution by other mediators. These may include activated serine proteases, found to promote doxorubicin-induced apoptosis in TGR-1 cells independently of mitochondria [Grassilli et al., 2004], and apoptosis-inducing factors released from mitochondria in a caspase-independent manner, such as Omi/HtrA2 [de Bruin et al., 2003; Egger et al., 2003].
The c-Myc oncoprotein is of major importance in promoting tumorigenesis [Evan and Littlewood, 1998; Hueber and Evan, 1998; Henriksson et al., 2001] and other tumor promoters, such as Bcl-2 and Ras, depend on c-Myc for cellular transformation. Thus targeting c-Myc in tumor cells or promoting its apoptosis-inducing capacity may be enough to delay progression or even cause tumor regression [Orlowski et al., 1998; Smith and Wickstrom, 1998; Felsher and Bishop, 1999; Pelengaris et al., 2002]. Myc activation itself may not be enough to induce apoptosis, but can enhance the apoptosis induced by cytotoxic drugs used in treatment of cancer patients [Peltenburg et al., 2004]. Accumulating evidence including our results demonstrate that c-Myc potentiates drug-induced apoptosis and indicate that Myc levels could be important for tailored cancer treatment with the possibility to use lower drug doses in Myc overexpressing tumors. In addition, the acquired drug resistance found in many tumor cells carrying myc amplifications, could be circumvented by reactivation of Myc-driven apoptosis.
Acknowledgements
We thank J.M. Sedivy for the TGR-1 and HO15.19 Rat1 cells, M. Cole for the HOmyc3 Rat1 cells, S. Korsmeyer for the Bax null and control mouse embryo fibroblasts, F. Chiodi (Karolinska Institutet, Stockholm, Sweden) for the Fas Ligand, G. Evan for the Myc antibody, D. Grandér (Karolinska Institutet, Stockholm, Sweden) for the PKCδ antibody, and C. Larsson for the EGFP-tagged PKCδ and control constructs. We further thank C. Mathay (Karolinska Institutet, Stockholm, Sweden) for providing experimental help, M.C. Shoshan (Karolinska Institutet, Stockholm, Sweden) and S. Linder (Karolinska Institutet, Stockholm, Sweden) for advice on analysis of Bax activation, K. Helin (European Institute of Oncology, Milan, Italy) for sharing unpublished data, and many colleagues for valuable scientific discussions. A. Albihn was supported by a training grant from the Swedish Foundation for Strategic Research, and A. Albihn, J. Lovén, J. Ohlsson, and L.M. Osorio were recipients of fellowships from the Cancer Research Institute/Concern Foundation for Cancer Research, Los Angeles.




