Fibroblast extracellular matrix gene expression in response to keratinocyte-releasable stratifin
Abstract
Termination of wound-healing process requires a fine balance between connective tissue deposition and its hydrolysis. Previously, we have demonstrated that keratinocyte-releasable stratifin, also known as 14-3-3 σ protein, stimulates collagenase (MMP-1) expression in dermal fibroblasts. However, role of extracellular stratifin in regulation of extracellular matrix (ECM) factors and other matrix metalloproteinases (MMPs) in dermal fibroblast remains unexplored. To address this question, large-scale ECM gene expression profile were analyzed in human dermal fibroblasts co-cultured with keratinocytes or treated with recombinant stratifin. Superarray pathway-specific microarrays were utilized to identify upregulation or downregulation of 96 human ECM and adhesion molecule genes. RT-PCR and Western blot were used to validate microarray expression profiles of selected genes. Comparison of gene profiles with the appropriate controls showed a significant (more than twofold) increase in expression of collagenase-1, stromelysin-1 and -2, neutrophil collagenase, and membrane type 5 MMP in dermal fibroblasts treated with stratifin or co-cultured with keratinocytes. Expression of type I collagen and fibronectin genes decreased in the same fibroblasts. The results of a dose–response experiment showed that stratifin stimulates the expression of stromelysin-1 (MMP-3) mRNA by dermal fibroblasts in a concentration-dependent fashion. Furthermore, Western blot analysis of fibroblast-conditioned medium showed a peak in MMP-3 protein levels 48 h following treatment with recombinant stratifin. In a lasting-effect study, MMP-3 protein was detected in fibroblast-condition medium for up to 72 h post removal of stratifin. In conclusion, our results suggest that keratinocyte-releasable stratifin plays a major role in induction of ECM degradation by dermal fibroblasts through stimulation of key MMPs, such as MMP-1 and MMP-3. Therefore, stratifin protein may prove to be a useful target for clinical intervention in controlling excessive wound healing in fibrotic conditions. J. Cell. Biochem. 98: 383–393, 2006. © 2006 Wiley-Liss, Inc.
Abbreviation used:
ECM, extracellular matrix; MMP, matrix metalloproteinase; KCM, keratinocyte-conditioned medium; KDAF, keratinocyte-derived anti-fibrogenic factor; KSFM, keratinocyte serum-free medium.
The ability to generate or repair injured tissue is vital to the continuity of human life. A fine balance between synthesis of extracellular matrix (ECM) and its degradation is a key factor in repair of injured skin as well as maintenance of its structural integrity. Epidermal–mesenchymal communication is critical in exchanging the information between keratinocytes and fibroblasts in order to maintain the balance between ECM synthesis and breakdown in integument [Johnson-Wint, 1980]. It is well established that any delay in epithelialization by keratinocytes increases the frequency of developing fibrotic conditions, as seen in hypertrophic scarring, characterized by excessive ECM deposition and altered remodeling [Ghahary et al., 2005].
Matrix metalloproteinases (MMPs) represent a group of diverse proteolytic enzymes involved in ECM turnover and connective tissue remodeling during embryonic growth and development, bone growth and resorption, tumor metastasis, and wound healing [Nagase and Woessner, 1999]. The MMP family consists of 25 zinc- and calcium-dependant proteinases in the mammalian system. According to their substrate specificity, primary structure, and cellular localization, these enzymes can be divided into at least five different subfamilies of closely-related members known as collagenases, gelatinases, stromelysins, matrilysins, and membrane-type MMPs [Murphy et al., 2002]. Collectively, MMPs are capable of degrading essentially all components of ECM [Kahari and Saarialho-Kere, 1997], including type I, III, IV collagen, fibronectin, elastin, and proteoglycans which are overexpressed in fibrotic tissues [Scott et al., 2000]. It has been shown that ECM not only acts as a support for resident cells, but also provides a reservoir for embedded growth factors and cytokines. Cell surface ECM receptors allow cells to sense their microenvironment and react to stimuli [Schenk and Quaranta, 2003]. Therefore, MMPs not only promote tissue remodeling by precise cleavage of ECM components but also indirectly influence cellular behavior by liberating bioactive fragments and changing ECM architecture [Mott and Werb, 2004]. An imbalance in expression of MMPs has been implicated in a number of pathological conditions such as dermal fibrosis [Ghahary et al., 1996], rheumatoid arthritis, atherosclerosis, pulmonary emphysema, and tumor invasion and metastasis [Birkedal-Hansen et al., 1993; Nagase and Woessner, 1999].
Epidermal–mesenchymal interaction plays a critical role in controlling the expression of MMPs during development and healing of skin. An increase in MMPs expression has been implicated with tissue degradation and remodeling during tumor invasion and wound healing. In both conditions, cell–cell interaction between either fibroblasts and tumor cells or fibroblasts and keratinocytes results in an increase in MMPs production [Folgueras et al., 2004; Ghahary et al., 2004]. Several studies on the effect of keratinocyte-conditioned medium (KCM) on dermal fibroblasts and keratinocyte-fibroblast co-culture systems revealed epidermal growth factor (EGF), interleukin-1alpha (IL-1α), and interleukin-1beta (IL-1β) as potential mediators of MMP-1 expression in fibroblasts [Mackay et al., 1992; Moon et al., 2001]. However, further studies showed that MMP-1 production in dermal fibroblasts was only partially inhibited by IL-1 receptor antagonists [Moon et al., 2001] and EGF plays a very small role in upregulation of this protease [Stoll et al., 1997; Moon et al., 2002]. Therefore, the precise nature of epidermal–mesenchymal interaction and keratinocyte-releasable factors involved in stimulation of MMPs in fibroblasts remain to be elucidated.
Stratifin or 14-3-3 σ is a member of a large family of highly conserved dimeric 14-3-3 proteins expressed ubiquitously in all eukaryotic cells. Although more than 100 proteins have been reported to interact with intracellular members of 14-3-3 family [Pozuelo et al., 2004], functional activities of the releasable forms of these proteins in general and 14-3-3 σ, in particular, are completely unknown. In fact, the presence of 14-3-3 proteins in cerebral spinal fluid [Satoh et al., 1999] as well as KCM [Katz and Taichman, 1999] has been previously reported, without designating any function. Our group has recently identified and characterized a keratinocyte-releasable anti-fibrogenic factor (KDAF) for dermal fibroblasts, which was discovered to be a releasable form of stratifin. Both KDAF and recombinant stratifin demonstrated potent MMP-1 stimulatory activity in dermal fibroblasts [Ghahary et al., 2004, 2005]. However, the role of stratifin in regulation of other ECM factors in fibroblasts, mainly MMPs, remains unclear. In this study, we further investigated the regulatory role of keratinocyte-releasable stratifin on gene expression of large number of ECM factors using a microarray approach. Our data revealed that, in addition to upregulation of MMP-1, stratifin significantly increased the expression of other MMPs in fibroblast, including MMP-3, MMP-8, and MMP-10, which play a key role ECM degradation and scar remodeling.
MATERIALS AND METHODS
Clinical Specimens and Cell Culture
Following informed consent, skin punch biopsies were obtained from patients undergoing elective reconstructive surgery, under local anesthesia, according to a protocol approved by the University of Alberta Hospitals Human Ethics Committee. Biopsies were collected individually and washed three times in sterile Dulbecco's modified Eagle's medium (DMEM) (Gibco, Grand Island, NY) supplemented with antibiotic–antimycotic preparation (100 mg/ml penicillin, 100 mg/ml streptomycin, 0.25 mg/ml amphotericin B) (Gibco). Specimens were dissected free of fat and minced into small pieces less than 0.5 mm in diameter, washed six times with DMEM, and distributed into 60 × 15-mm Petri dishes. Cultures of fibroblasts were established as previously described [Karimi-Busheri et al., 2002]. Upon reaching confluence, the cells were released by trypsinization, split for subculture at a ratio of 1:6, and reseeded into 75-cm2 flasks. Fibroblasts at passages 3–7 were used in all experiments conducted in this study.
To establish cultured keratinocytes, the procedure of Rheinwald and Green [1975] was used for cultivation of human foreskin keratinocytes using serum-free keratinocyte medium (Gibco) supplemented with bovine pituitary extract (50 mg/ml) and EGF (5 ng/ml) [Rheinwald and Green, 1975]. These additives were used only to establish keratinocytes in cultures. Thus, to eliminate any effects of EGF and/or pituitary extract on our findings, obtained from co-culturing keratinocytes with fibroblasts, keratinocyte serum-free medium (KSFM) supplemented with EGF and pituitary extract was exchanged with our test medium consisting of 49% DMEM, 49% KSFM, and 2% FBS with no additives, a medium in which keratinocytes undergo differentiation with time. In this system, both keratinocytes and fibroblasts remain viable and any factor released from keratinocytes can diffuse through the 0.4-mm porous membrane separating the two chambers. Primary cultured keratinocytes at passages 3–5 were used.
Keratinocyte–Fibroblast Co-Culture System
In order to study the effect of 14-3-3 proteins on fibroblasts during keratinocyte–fibroblast cross-talk, a previously established [Karimi-Busheri et al., 2002; Ghahary et al., 2004] keratinocyte/fibroblast co-culture system in which keratinocytes and fibroblasts were grown in the upper and lower chambers of the system, respectively was used. For the co-culture experiments, 30-mm Millicell-CM (Millipore) culture plate inserts with 0.4-mm pore size were coated with fetal bovine skin collagen (3 mg/ml). Subsequently, 0.5 × 106 keratinocytes were seeded on the collagen-coated inserts with KSFM supplemented with BPE (50 mg/ml) and EGF (5 mg/ml). In a separate experiment, 0.5 × 106 fibroblasts were seeded in each well of a six-well culture plate containing DMEM with 10% FBS. The cells were incubated in a cell culture incubator for 24 h and the conditioned medium was collected and cells were washed with phosphate-buffered saline (PBS). The co-culture system was then assembled, with the upper chamber being the collagen-coated insert with keratinocytes and the bottom chamber being the fibroblasts grown on a plastic six-well plate. The controls were inserts either alone, with keratinocytes, or with fibroblasts. Each chamber received 2.5 ml of our test medium consisting of 49% KSFM without additive and 49% DMEM plus 2% FBS. As only a permeable membrane separates these cells, fibroblasts in the lower chamber can be exposed to any soluble factor that may be released from keratinocytes. As an index for the anti-fibrogenic effects of keratinocyte-derived factor(s) on dermal fibroblasts, total RNA was extracted from fibroblasts grown in the lower chamber and the expression of collagenase mRNA was evaluated by Northern analysis.
Preparation of Human Recombinant Stratifin (14-3-3 σ)
Procedures of human recombinant 14-3-3 σ protein preparation were established as described previously [Ghahary et al., 2004]. Briefly, the cDNA of 14-3-3 σ from human keratinocytes was cloned into pGEX-6P-1 expression vector (Amersham/Pharmacia Biotech, Piscataway, NJ) and transformed into protein expressing bacteria, BL-21 (DE3) (Novagene, Madison, WI). To purify the protein, bacteria were centrifuged and lysed with 50 mM Tris-HCl (pH 7.4) containing 10 mM EDTA, 5 mM EGTA, protease inhibitor cocktail (Sigma, St Louis), 1% Triton X-100, and 0.5% IGEPAL CA630. Glutathion-S-transferase (GST)-fused 14-3-3 σ was purified by using a glutathione sepharose-4B affinity column and subsequently digested using PreScission protease according to the manufacturer's procedure (Amersham/Pharmacia Biotech). To evaluate the efficacy of our recombinant protein, confluent culture of fibroblasts were treated with various concentrations (0, 0.5, 1.0, 2.0, 4.0 µg/ml) of GST-fused 14-3-3 σ, GST-free 14-3-3 σ, and GST alone. Cells were then harvested at 24 h and the expression of MMP-1 mRNA was evaluated by Northern blot analysis according to procedure described here. Furthermore, colorimetric methyl thiazolyl tetrazolium (MTT) assay was used to evaluate the effect of recombinant 14-3-3 σ on fibroblasts' proliferation. Experiments were performed at least three times with similar results.
RNA Isolation and Northern Blot Analysis
Fibroblasts were harvested with 400 ml of 4 M guanidium isothiocyanate (GITC) solution, and total RNA from each group was isolated by the acid–guanidium–phenol–chloroform method [Ghahary et al., 1998]. Total RNA from each individual fibroblast culture was then separated by electrophoresis (10 µg per lane) and was blotted onto a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). Following 2 h incubation in prehybridization solutions, blots were hybridized using MMP-1, stromelysin-1 (MMP-3), or 18S ribosomal RNA radioisotope labeled cDNA probes. Autoradiography was performed by exposing a Kodak X-Omat film to nitrocellulose filters at −20°C in the presence of an intensifying screen. Each experiment was performed at least twice to ensure reproducibility of the results. The cDNA probes for MMP-1 and 18S ribosomal RNA were obtained from the American Type Culture Collection (Rockville, MD). The cDNA probe for stromelysin-1 was obtained by extracting fibroblast total RNA and was amplified by RT-PCR. The PCR product was then purified and ligated into a pGEX-6P-1 vector (Amersham/Pharmacia Biotech). The ligated products were then transformed to competent DH5α cells with the regular heat-shock transformation method. Positive clones were identified by the size of restriction enzyme-digested products. DNA sequence was confirmed by fluorescence dNTP sequence analysis.
Detection of Extracellular 14-3-3 σ and MMP Proteins by Western Blotting
To determine whether the release of stratifin (14-3-3 σ) is unique to keratinocytes, fibroblasts, or both, conditioned media from fibroblast/fibroblast (F/F), keratinocyte/fibroblast (K/F), and keratinocyte alone (K) co-culture systems were collected for 48 h. It should be noted that the original KSFM media supplemented with BPE and EGF was replaced with test medium without FBS. FBS was removed as serum proteins interfere with electrophoresis of proteins present in conditioned medium.
To evaluate MMP-1 and MMP-3 production in dermal fibroblast, original conditioned media was replaced with FBS-free DMEM following overnight incubation and cells were treated with various concentrations of recombinant stratifin for 24 h and conditioned medium collected for analysis. All samples of conditioned medium were centrifuged to pellet the debris and passed through a Centricon filter device (Millipore) with molecular weight cut of 5 kDa. Protein content of concentrated conditioned medium was determined and an equal amount (50 µg per sample) of total protein was subjected to SDS–PAGE analysis with 12% (wt/vol) acrylaminde gel, and electro-transferred onto polyvinylidine difloride membranes (Millipore). Non-specific proteins on membranes were blocked in 5% skim milk powder in PBS–0.1% Tween-20 overnight. Immunoblotting was performed using polyclonal goat anti-human 14-3-3 σ (Santa Cruz Biotechnology, Santa Cruz), monoclonal mouse anti-human MMP-1 (R&D Systems, Minneapolis), and monoclonal mouse anti-human MMP-3 (R&D Systems) antibodies. The membranes were then incubated with the appropriate secondary horseradish peroxidase-conjugated anti-goat IgG (Sigma) or anti-mouse IgG (Bio-Rad Laboratories) antibodies (1:2,500 dilution). Immunoreactive proteins were then visualized using the ECL + plus Western blotting detection system (Amersham Biosciences, Buckinghamshire, England).
Gene Expression Analysis by ECM-Specific Microarray
To examine whether keratinocytes-derived or recombinant stratifin induced other ECM genes in dermal fibroblasts, GEArray pathway-specific gene expression array systems were purchased from SuperArray Bioscience Corporation (Bethesda, MD). Each GEArray Q Series Human ECM and Adhesion Molecules gene array consists of 96 genes known to be involved in cell adhesion, ECM deposition, and degradation, as well as control sequences (PUC18 plasmid DNA as negative control; β-actin, PPIA, and glyceraldehydes 3 phosphate dehydrogenase (GAPDH) for loading). Using different arrays, we compared the gene expressions of keratinocyte-co-cultured, stratifin-treated, and untreated dermal fibroblasts. For these experiments, total cellular RNA was isolated by a modification of the GITC technique. The integrity of RNA was assessed by visualization of ethidium bromide-stained gels and it was ensured that 260 nm/280 nm absorbance ratio does not exceed 1.8. The microarrays were used according to the manufacturer's instructions. In brief, using the reagents provided, cDNA was prepared from total RNA by reverse transcription with MMLV reverse transcriptase, biotinylated with Biotin-16-dUTP (Roche, Indianapolis, IN), and then hybridized under precisely specified conditions to a positively-charged nylon membrane containing the arrayed DNA. The arrays were visualized using the chemiluminescent detection system purchased from GEArray (Bethesda, MD). Loading was adjusted based on the intensity of hybridization signals to the housekeeping gene, PPIA, and then gene expression was quantified by scanning densitometry.
Reversed Transcriptase-Polymerase Chain Reaction (RT-PCR)
To validate the variations observed in MMP gene expression in the mircoarray experiment, a multigene RT-PCR profiling system was utilized. Total RNA was isolated from either monolayer of untreated fibroblasts (control), cells treated with 2.0 µg/ml stratifin (14-3-3 σ), or co-cultured with keratinocytes (K/F). A260 nm/A280 nm ratio did not exceed 1.8 and integrity of RNA sample was checked by visualization of ethidium bromide 28S and 18S bands. A total of 5 µg from each sample was reverse transcribed using the first-strand cDNA synthesis kit from SuperArray (Frederick, MD). RNA samples from three separate experiments were pooled for use in each cDNA synthesis reaction. Samples were the processed for PCR using the MultiGene-12 reverse transcriptase PCR profiling kit for 11 human MMP genes plus a human GAPD housekeeping gene (SuperArray) according to the manufacturer's instructions. Following completion of PCR, 10 µl of each sample was separated by agarose gel electrophoresis and stained and scanned as a digital image.
RESULTS
Efficacy of Recombinant Stratifin on MMP-1 mRNA Expression and Fibroblast Proliferation
It was previously shown that the recombinant stratifin protein produced in bacteria is more than 95% pure and has strong MMP-1 stimulatory effect in fibroblasts [Ghahary et al., 2004]. To ensure that recombinant GST or any residual bacterial product is not contributing to MMP-1 expression, confluent cultures of fibroblasts were treated with various concentrations of GST, GST–stratifin fusion protein, or stratifin (0, 0.5, 0.1, 2.0, 4.0 µg/ml) for 24 h (Fig. 1A). The results showed a marked increase in the expression of MMP-1 mRNA in treated fibroblasts for both GST–stratifin fusion protein and purified stratifin. This increase reached significance at around 1.0 µg/ml of stratifin used. No significant changes in MMP-1 mRNA expression was observed when cells were treated with GST alone, confirming that the MMP-1 stimulation is caused by stratifin alone. In addition, proliferation of dermal fibroblasts was monitored by MTT incorporation assays. Various concentrations of recombinant stratifin did not affect the proliferation rate of fibroblasts in culture, indicating the MMP expression in cells is not stress-induced (Fig. 1B).
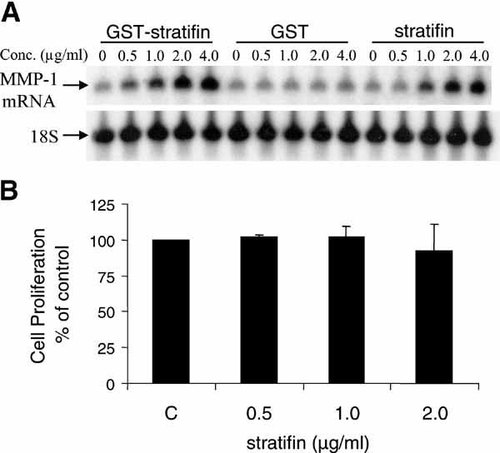
Recombinant stratifin (14-3-3 σ) protein increases the expression of MMP-1 mRNA in dermal fibroblasts. Cells were treated with various concentrations (0, 0.5, 1.0, 2.0, 4.0 µg/ml) of GST–stratifin, GST, or purified stratifin for 24 h. Expression of MMP-1 and 18S ribosomal RNA (loading control) was assessed by Northern Analysis (panel A). To evaluate any potential cytotoxicity from the recombinant stratifin, confluent cultures of dermal fibroblasts were treated with either nothing or various concentration of purified protein for 24 h. The results of an MTT proliferation assay are presented as a relative percentage of the control group (untreated cells). Data represent mean ± standard deviations for three separate experiments. Statistical significance (P < 0.05) was tested with Student's unpaired two-tailed t-test.
The Detection of Stratifin in Keratinocyte–Fibroblast Co-Culture System
To determine if 30 kDa keratinocyte-releasable stratifin in the upper chamber diffused through collagen-coated insert membrane, conditioned medium from the lower chamber was collected at 48 h and analyzed by Western blot with polyclonal antibody to stratifin. Pooled conditioned media were concentrated about 30 times through a Centricon filter (5 kDa cut-off) and 10 µl of each sample was loaded on the gel. Various amounts of recombinant stratifin (0, 0.2, 0.5, 1.0 µg) were loaded on the SDS gel as standard. As shown in Figure 2A, only keratinocytes in the upper chamber (K/F) released significant amount of stratifin with ability to reach co-cultured fibroblasts in the lower chamber through the 0.4 µm pores of collagen coated membrane. The absence of stratifin in the fibroblast-conditioned medium showed that fibroblasts are not the source of this protein in our co-culture system. Based on the density of standards, loaded volume of protein, and the dilution factor, the physiological level of stratifin was estimated to be 1.6 µg/ml of conditioned media in our co-culture system. To measure the activity of stratifin on dermal fibroblasts, total RNA was extracted from fibroblasts co-cultured with keratinocytes (K/F) or treated with 2.0 µg/ml of the recombinant protein (shown as sfn in panel B). The expression of MMP-1 mRNA was used as a marker for stratifin activity and it was evaluated by Northern blot analysis (Fig. 2B). MMP-1 mRNA expression was significantly increased in fibroblasts treated with recombinant protein or co-cultured with keratinocytes, whereas little or no detectable level of MMP-1 mRNA was found in fibroblast/fibroblast co-culture (F/F) or monolayer fibroblasts (C). To further confirm the efficacy of our recombinant protein, extracellular MMP-1 protein level in fibroblasts stimulated with different doses of stratifin (0, 0.5, 1.0, 2.0, 4.0 µg/ml) was analyzed by Western blot with a monoclonal antibody. Figure 2C shows significant increase in MMP-1 protein levels in the conditioned medium at 1.0 to 2.0 µg/ml after 24 h of treatment.
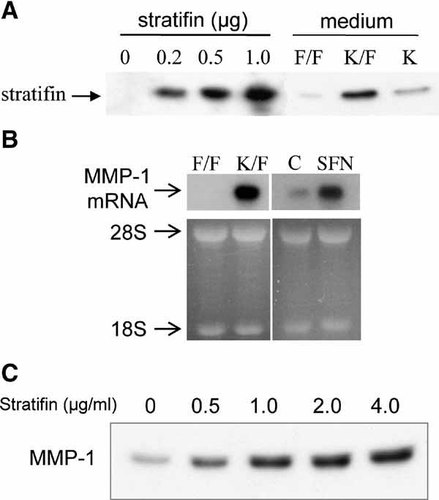
Physiological level of stratifin released by keratinocytes in a co-culture system. Conditioned medium from fibroblast (F/F), fibroblast co-cultured with keratinocyte (K/F), or keratinocyte alone (K) was collected from the lower chamber following 48 h of incubation. Purified stratifin (0, 0.2, 0.5, 1.0 µg) was used as a standard in Western blot analysis of concentrated conditioned medium (panel A). To validate the activity of releasable stratifin, expression of MMP-1 mRNA was analyzed in the co-culture system as well as fibroblasts treated with recombinant protein. As shown in panel B, fibroblasts co-cultured with keratinocytes in the upper chamber (K/F) and cells treated with stratifin (sfn 2.0 µg/ml) showed significant increase in MMP-1 mRNA expression compared with their corresponding controls (F/F and C, respectively). Experiment was repeated with three different strains of skin keratinocyte and fibroblast. Pattern of 28S and 18S ethidium bromide-stained ribosomal RNA were used as a loading control. Panel C shows Western blot analysis of MMP-1 secretion in fibroblast treated with various concentrations of stratifin. Fibroblast-conditioned medium was concentrated by 5 kDa Centricon filter and 50 µg of total protein was loaded for each sample.
Microarray Analysis of Fibroblast ECM Gene Expression
Figure 3 shows cDNA array results of human ECM and adhesion molecules gene expression in K/F co-culture system (panel A) and stratifin-treated fibroblasts (panel B). In both groups, total RNA was pooled from three independent experiments for cDNA preparation by RT-PCR and microarray hybridization. Final expression was normalized for cyclophilin A (PPIA). Genes with more than twofolds increase or decrease in their expression were considered significant in the selection criteria. Microarray experiments were repeated two times with the representative images shown in Figure 3. Comparison of 96 ECM genes in K/F showed increased expression of 12 genes compared with F/F, while the expression of 4 genes decreased (table in Fig. 3, panel A). Majority of upregulated ECM genes in co-cultured fibroblasts belong to the MMP family. There were 10 genes specific to key ECM components in dermal fibroblast that showed an increase by stratifin (2.0 µg/ml) treatment (Fig. 3, panel B), with 2 genes demonstrating a significant decrease. Interestingly, majority of ECM genes upregulated in stratifin-treated fibroblasts also belong to the MMP family, indicating that stratifin was the keratinocyte-releasable factor responsible for induction of these MMPs in fibroblasts. Significant increase in expression of MMP-1, stromelysin-1 (MMP-3), stromelysin-2 (MMP-10), neutrophil collagenase (MMP-8), and membrane type MMP-24 (MT5-MMP) were observed in both K/F and stratifin-treated dermal fibroblasts.
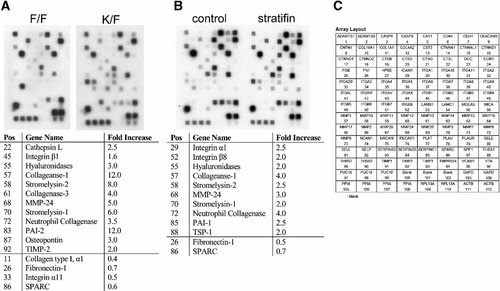
Microarray analysis of mRNA extracted from keratinocyte-co-cultured or stratifin-treated fibroblasts. Fibroblasts were either co-cultured with keratinocytes (K/F) or treated with 2.0 µg/ml of stratifin (14-3-3 σ) for 24 h in separate experiments. Fibroblast/fibroblast co-culture (F/F) or monolayer culture of fibroblast (control) were used as control for each experiment. Total RNA was extracted, reverse-transcribed, and the corresponding cDNA biotin-labeled according to the manufacturer's directions. Images in panel A (co-culture) and panel B (stratifin-treated) demonstrated the expression profile of human ECM and adhesion molecules in dermal fibroblasts. The tables below each image indicate the fold-increase of ECM gene expression relative to the control in each group. Experiment was repeated with two different stains of dermal fibroblasts. Cyclophilin A (PPIA) expression was used as negative control. A key to gene coordinates is also shown in panel C.
In order to confirm the cDNA array findings, the level of expression of 12 selected MMP genes was evaluated by RT-PCR. As shown in Figure 4, PCR analysis demonstrated significant increase in expression of MMP-1, -3, -8, and 24 in both K/F and stratifin-treated fibroblasts. MMP gene expression in untreated fibroblasts was used as experimental control and Glyceraldehyde-3-phosphate dehydrogenase (GAPD) as the housekeeping gene for normalizing expression.
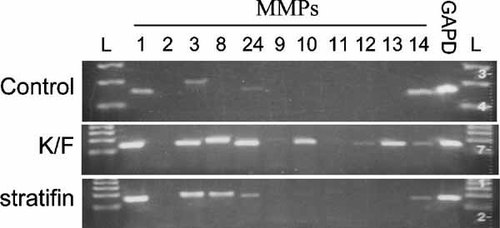
Stimulation of MMP gene expression in dermal fibroblast determined by RT-PCR. Fibroblast were treated either with nothing (control), co-cultured with keratinocyte (K/F), or stratifin (14-3-3 σ) and were then harvested for total RNA. RNA (3 µg) was characterized using a customized human MMP gene family RT-PCR profiling kit (SuperArray). The resulting PCR products were characterized by agarose gel electrophoresis. From left to right, L, ladder; 1, collagenase; 2, gelatinase A; 3, stromelysin-1; 8, neutrophil collagenase; 24, MT5-MMP; 9, gelatinase B; 10, stromelysin-2; 11, stromelysin-3; 12, macrophage elastase; 13, collagenase-3; 14, MT1–MMP; GAPD, Glyceraldehyde-3-phosphate dehydrogenase (housekeeping gene); L, ladder. Study was performed on two different strains of dermal fibroblasts with similar results.
Effect of Stratifin on Expression and Secretion of MMP-3 by Fibroblasts
To further confirm some of the findings by cDNA array and RT-PCR, we examined the effect of stratifin on stromelysin-1 (MMP-3) expression and production in fibroblasts at the protein level. Confluent cultures of fibroblasts were either treated with nothing or various concentrations of stratifin (0.5, 1.0, 2.0, 4.0 µg/ml) for 24 h. Conditioned media from untreated or treated fibroblasts were collected, concentrated, and subjected to Western blot analysis with monoclonal antibody to MMP-3. The result of the dose–response experiment showed that stratifin stimulates secretion of MMP-3 protein in fibroblasts in a concentration-dependent fashion (Fig. 5A). Significant increase in extracellular MMP-3 protein was detected in fibroblast-conditioned media starting at 0.5 µg/ml of stratifin and reached a peak at 2.0 µg/ml. Fibroblasts were also treated with 2.0 µg/ml of stratifin for different periods of time, and the release of MMP-3 into conditioned medium was determined by Western blot analysis. The results showed a marked increase in MMP-3 protein level as early as 24 h post treatment and it reached its maximum following 48 h incubation with stratifin (Fig. 5B). In another experiment, the lasting effect of stratifin in MMP-3 expression and production in dermal fibroblasts was evaluated. Following the initial treatment with 2.0 µg/ml stratifin for 24 h, original conditioned media was collected and replaced with fresh culture media. The results showed a marked increase in the expression of MMP-3 in fibroblasts at 24 h after treatment. Following removal of stratifin, the expression remained high for another 24 h and then gradually reduced to normal levels within 96 h (Fig. 6A). Level of MMP-3 protein released into fibroblast-condition medium collected at different time points after removal of stratifin demonstrated a similar pattern to its gene expression (Fig. 6B). The effect of stratifin on MMP-3 production in fibroblasts was also studied using different strains of primary fibroblasts. Four different strains of human dermal fibroblasts demonstrated higher levels of MMP-3 in their conditioned medium following treatment with 2.0 µg/ml of stratifin for 24 h (data not shown), indicating that stratifin-induction of MMPs were not isolated to a specific strain of cell.
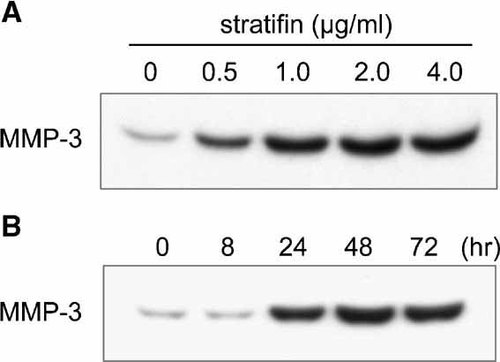
Confirmation of array results by Northern analysis and Western blot of MMP-3. Dermal fibroblasts were treated with various concentrations of stratifin for 24 h. In panel A, fibroblast-conditioned medium was collected post treatment and concentrated with a 5 kDa cut-off Centricon filter. Total protein concentration was determined using Bradford protein assay and 50 µg of each sample was subjected to SDS–PAGE analysis. In a similar experiment, fibroblasts were treated with 2.0 µg/ml of stratifin for different periods of time as indicated (panel B). The extracellular MMP-3 protein levels were determined by Western blot analysis using specific antibodies.
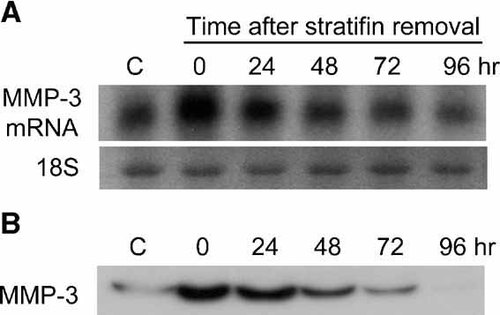
Recombinant stratifin protein increases the expression of stromelysin-1 (MMP-3) in dermal fibroblasts. To determine the lasting effect of stratifin on MMP-3 expression, fibroblasts were treated with either nothing (c) or 2.0 µg/ml of stratifin for 24 h. The medium was replaced with fresh medium without stratifin and cells were then harvested at 0, 24, 48, 72, and 96 h post stratifin removal. Fibroblast-conditioned medium was collected at each time point, concentrated using 5 kDa Centricon filter, and subjected to SDS–PAGE. The expression of MMP-3 gene and extracellular MMP-3 protein in dermal fibroblasts were then evaluated by Northern blot (panel A) and Western blot (panel B) analysis, respectively.
DISCUSSIONS
The purpose of this study was to explore the regulatory effect of keratinocyte-releasable stratifin on ECM production in dermal fibroblasts. The expression of wide range of MMPs was induced in dermal fibroblasts treated with the recombinant stratifin or co-cultured with primary keratinocytes. Furthermore, stromelysin-1 (MMP-3) gene expression and protein secretion was characterized in fibroblasts in response to stratifin. Our results showed that stratifin induces production of MMP-3 by fibroblasts in a dose- and time-dependant fashion.
Following full- or partial-thickness burns, wound healing may lead to formation of hypertrophic scars and keloids in predisposed patients. These scars are generally characterized by an overabundance of dermal collagens, proteoglycans, fibronectin, and other connective tissues, which are of altered composition and organization compared to normal skin or mature scar [Swann et al., 1985; Niessen et al., 1999; Scott et al., 2000]. As fibroblasts are the main source of the excess scar tissue, most attempts have been focused on the dermal component of the scar. However, clinical evidence points toward irregularity in mesenchymal–epithelial signaling as a possible mechanism for development of hypertrophic scarring. Development of hypertrophic scars is commonly observed in wounds with delayed reepithelialization [Deitch et al., 1983; Niessen et al., 2001; Ghahary et al., 2004]. Wound repair involves cell proliferation, migration, and tissue remodeling. These highly orchestrated processes are facilitated by matrix-degrading proteases or MMPs. In search of KDAFs that mediate MMPs product is on in dermal fibroblasts, we have recently demonstrated that an extracellular form of stratifin secreted by differentiated keratinocytes acts as a strong stimulator of MMP-1 in dermal fibroblasts [Ghahary et al., 2004, 2005]. MMP-1, as all other collagenases, cleaves fibrilar collagens into ¾ and ¼ fragments, which in turn denature into gelatin at body temperature [Kahari et al., 1997]. However, MMP-1 degrades collagen type III more specifically, whereas MMP-8 and MMP-13, other members of the collagenase family, express more specificity toward type I and type II collagen, respectively [Knauper et al., 1996; Mitchell et al., 1996]. Given that hypertrophic scars demonstrate excessive production of a wide range of fibrous proteins such as type I and III collagen, proteoglycans, fibronectin, elastin, and tenascin [Scott et al., 2000], it is unlikely that augmented expression of MMP-1 alone, independent of other proteases, can lead to optimum remodeling of the scar tissue. Our findings here suggest that stratifin released from keratinocytes acts as a paracrine signaling molecule for fibroblasts with a potent MMP stimulatory activity. An increase in fibroblast expression of collagenase-1, stromelysin-1, stromelysin-2, neutrophil collagenase, and MT5-MMP, in response to stratifin, can synergistically degrade type I, II, III, IV collagen, fibronectin, and proteoglycans in scar tissue and promote tissue remodeling [Kahari et al., 1997; Wang et al., 1999; Abe et al., 2001; Dong et al., 2001].
Fibroblast stromelysin-1 (MMP-3) is a protease known to degrade mainly the noncollagenous portion of the ECM such as fibronectin, proteoglycans, and laminin [Kahari et al., 1997]. Increase in MMP-3 expression and release by fibroblasts in response to extracellular stratifin, together with MMP-1 upregulation shown previously, can initiate degradation of almost all major components of the ECM. In fact, the results from a pilot rabbit ear hypertrophic scar study show significant ECM remodeling and reduction in size of scars treated with stratifin (data not shown). Interestingly, in synovial fibroblast culture, MMP-3 and MMP-1 expression appear to be coordinately modulated [Saus et al., 1988]. Our results also support this finding, with fibroblast MMP-1 and MMP-3 expression showing dose- and time-dependent response to stratifin treatment. It is possible that promoter regions of these genes respond to stratifin stimuli through a common signaling pathway or transcription factor. We have previously characterized that stratifin modulates fibroblast MMP-1 levels through the p38 mitogen-activated protein kinase (MAPK) signaling pathway, c-fos gene transcription, and formation of AP-1 transcription factor complex [Lam et al., 2005]. The majority of inducible MMPs, including MMP-3, are regulated through AP-1 or PEA3 cis-elements in their promoter region [Gaire et al., 1994; Pendas et al., 1997]. It remains to be seen if MMP-3 or other MMPs stimulated here by stratifin are also activated through the same signaling transduction pathway as MMP-1.
Our data shows a higher expression of MMP-1 mRNA in fibroblasts co-cultured with keratinocytes compared to cells treated with recombinant stratifin. This is probably due to the fact that stratifin is not the only signaling factor released by keratinocyte with collagenase-inducing activity. It has been shown that IL-1α also stimulates matrix degradation and is mainly derived from keratinocytes during the later stages of wound healing [Maas-Szabowski et al., 2000; Moon et al., 2001]. In fact, lower epidermal IL-1α expression has been reported in hypertrophic compared with normal scars [Niessen et al., 2001]. Therefore, it is not unreasonable to postulate that absence or downregulation of keratinocyte-derived factors such as stratifin and IL-1α, leading to a reduction in MMPs, may play a key role in the development of dermal fibrotic conditions. Our results also reveal that fibroblasts co-cultured with keratinocytes or treated with stratifin show a significant reduction in mRNA expression of type I collagen and fibronectin. Excessive and uncontrolled production of these proteins is a signature characteristic in fibrous connective tissues of hypertrophic scars [Scott et al., 2000]. It will be important to determine whether 14-3-3 σ is the only factor released by keratinocytes that reduces collagen and fibronectin expression in fibroblasts and if the measured gene expression correlates with changes at the protein level.
Although presence of 14-3-3 proteins has been reported outside of the cell, they are generally considered to be intracellular protein without the amino-terminal ER signal peptides. Therefore, the mechanism by which stratifin is secreted by keratinocytes has yet to be explored. We have previously assayed for the presence of lactate dehydrogenase (LDH) in the medium, as a marker for cell lysis, and established that the release of stratifin was not the result of cell lysis [Karimi-Busheri et al., 2002; Ghahary et al., 2004]. Evidence indicate the cells can also release membrane vesicles, called exosomes, in the extracellular environment and a proteomic analysis revealed presence of various isoforms of 14-3-3 within these exosomes in dendritic cells [Thery et al., 2001]. Studies are underway to investigate the mechanism of stratifin secretion from keratinocytes and nature of its interaction and signal transduction pathway in fibroblasts.
In conclusion, the findings of this study represent an important advance in understanding the role of keratinocyte-releasable stratifin as a potent promoter for ECM degradation and scar tissue remodeling, through stimulation of MMPs in dermal fibroblasts. Characterization of stratifin levels in normal and hypertrophic scar tissue might shed further light on the role of this factor in the development of dermal fibrotic conditions.




