NGX6 gene inhibits cell proliferation and plays a negative role in EGFR pathway in nasopharyngeal carcinoma cells†
Lili Wang and Jian Ma has contributed equally to this work.
Abstract
Nasopharyngeal carcinoma (NPC) is a common cancer in South China but is rare in other parts of the world. A novel NPC-related gene was isolated by location candidate cloning strategy, whose expression was down-regulated in NPC. This gene was designated human NGX6 (Genbank accession AF188239) and encoded a predicted protein of 338 amino acids that harbors an EGF-like domain. The effects of NGX6 on cells from human NPC cell line HNE1 were investigated. The cells transfected with NGX6 had a markedly high expression of NGX6, leading to significant decrease in cell proliferation and the capability to form colonies in soft agar, delaying the G0–G1 cell cycle progression. Flow cytometry assay indicated that the expression of cyclin D1 significantly decreased in NGX6-transfected HNE1 cells as well as cyclin A and E. There was a delay in tumor formation and a dramatic reduction in tumor size when cells transfected with NGX6 were injected into nude mice. In another way, we found NGX6 played a negative role in EGFR Ras/Mek/MAPK pathway. We propose that NGX6, as an EGF-like domain gene, could delay cell cycle G0–G1 progression and thus inhibit cell proliferation by negatively regulating EGFR pathway in NPC cells and down-regulating the expression of cyclin D1 and E. © 2005 Wiley-Liss, Inc.
Nasopharyngeal carcinoma (NPC) is an epithelial tumor with an exceptionally high incidence in South China. The molecular events leading to the development of the tumor are still not well understood [Simons et al., 1977; Parkin and Muir, 1992]. Microsatellite analysis have shown high frequency of LOH on 9p (61%–85%) in NPC [Huang et al., 1994; Mutirangura et al., 1997; Chen et al., 1999; Hui et al., 1999; Fan et al., 2000; Lo et al., 2000; Shao et al., 2000; Chien et al., 2001; Fang et al., 2001; Shao et al., 2001; Wong et al., 2003].
In the previous study, a novel gene related to NPC at 9p21 was cloned by location candidate cloning strategy [Yang et al., 2000]. It was named NGX6 (Genbank accession AF188239). Its mRNA expression level at NPC cells was significantly lower than normal nasopharyngeal cells. Bioinformatics investigation revealed that, the full-length of NGX6 cDNA was 2,134 bp, the ORF of NGX6 was from 1,056 to 2,072 bp, encoding a polypeptide of 338 amino acids with a predicted molecular weight of 37 kDa. NGX6 protein included two transmembrane regions: one was from 234 to 256 amino acid, the other was from 269 to 291 amino acid. There was an EGF-like domain in the extracellular region of NGX6 protein from 185 to 221 amino acid. Searching for the NGX6-related protein. Database revealed a high amino acid sequence similarity between NGX6 and human M83 protein, which was a five-span transmembrane molecule and contains a tyrosine residue in the cytoplasm. The exact function of M83 is still unclear. NGX6 and M83 may define a new subfamily of the multi-span transmembrane protein superfamily [Motohashi et al., 2000].
EGF-like domain is involved in the interaction between receptors and ligands, some cell adhesion-related molecules and extracelluar matrix components contain this domain. This domain encompasses three of the six conserved cysteine residues and contains additional residues important for tertiary structure stabilization and receptor binding [Maida et al., 2002]. In mammals, the EGF-like domain family members can bind to several EGF superfamily ligands, and induce tyrosine phosphorylation of EGF receptors and thus activate the downstream signaling cascades, among which Ras-MAP kinase pathway is an important cascade and is implicated in diverse cellular responses including cell growth, differentiation, and apoptosis [Li et al., 2001; Bergmann et al., 2002]. Studies have shown that aberrant activation of the EGF ligand/receptor pathway is a frequent molecular event in human cancers and the deregulated EGF ligand/receptor pathway plays a leading role in tumor progression [Esparis-Ogando et al., 2002; Kanno et al., 2002; Segrelles et al., 2002; Strelkov and Davie, 2002].
In this study, the strategy of transfecting NGX6 to human NPC cell line, HNE1 [Yao et al., 1990], was applied. We provided the data that demonstrated the efficacy of inhibiting the growth of NPC cells in vitro as well as in vivo. Flow cytometry analysis showed that overexpression NGX6 could delay the G0–G1 progression of HNE1 and down-regulate cyclin D1, E, and A expression. We also found that NGX6 could down-regulate the EGFR Ras/Mek/MAPK pathway. It suggested that NGX6 might play a role in regulating NPC cell proliferation by modulating the function of EGFR pathway and down-regulating the expression of cyclin D1 and E.
MATERIALS AND METHODS
Cell Culture and Transfection
HNE1 cell, a human NPC cell, was maintained in RPMI1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) in a humidified incubator with 5% CO2 at 37°C.
A mammal expression system using plasmid pcDNA3.1(+)/NGX6 was created by inserting the full-length human NGX6 cDNA, which was obtained from IMAGE Consortium, between the EcoRI and XhoI sites of pcDNA3.1(+) plasmid (Invitrogen). The transfection experiment was carried out with pcDNA3.1(+) plasmid containing the full-length NGX6 cDNA insert or with pcDNA3.1(+) plasmid containing no insert using Lipofectin (Invitrogen) according to the supplier's instruction. Gentamicin-G418 (500 μg/ml) (Sigma, Saint Louis, MO) was added for selection of transfectants. The stable transfectants were then isolated 3 weeks later using a ring-cloning technique. Cells grown to 80% confluence were harvested for the experiment. Northern blot and Western blot were used to detect the changes of NGX6 expression in pcDNA3.1(+)/NGX6 transfectants HNE1/NGX6(1), HNE1/NGX6(2), and pcDNA3.1(+) transfectant HNE1/vector.
Growth Curve of HNE1 Cells
Transfectant HNE1 cells were seeded at 2 × 104 cells/well in 24-well flat-bottomed plates in 0.1 ml of RPMI1640 supplemented with 0.05% fetal calf serum. The plates were incubated for 7 days in an atmosphere containing 5% CO2 at 37°C, and duplicate cultures were counted with a hemocytometer every day.
Soft Agar Colony Forming Assay
For soft agar assays, a bottom layer of 1 ml of the corresponding culture media containing 0.6% agar and 10% fetal calf serum was prepared, placed in 35-mm culture dishes, and allowed to solidify. Transfectant HNE1 Cells (2 × 104) were suspending in 50 μl of RPMI1640 containing 10% fetal calf serum and G418 (500 μg/ml). The culture medium (1 ml) containing 0.33% agarose was added to the cell suspension before seeding on the dishes. Triplicates were performed for each type of cell. Cells were incubated at 37°C in 5% CO2 atmosphere. Dishes were examined twice per week, and colonies were counted manually after 2 weeks. Results were expressed as mean ± SE.
Tumorigenicity Assay of HNE1 Cells
Suspensions of 2 × 106 transfectant HNE1 cells in PBS (0.2 ml) were injected s.c. into the flanks of 4–5 weeks old male BALB/c nude mice (each group 6 mice), and the tumor growth was estimated by the average volume of tumors. The tumor volume was calculated by the formula 4π/3 × (L/2 × W/2 × H/2; L = length, W = width, H = height of the tumor). All of the animal experiments were performed in accordance with institutional guidelines.
Northern Blot Analysis
Total RNA, 40 μg/sample, were resolved on a 1.2% agarose gel in the presence of 2 mM formaldehyde and transferred to a nylon membrane. The membrane were then prehybridized at 68°C for 3 h in ExpressHyb hybridization solution (BD, Becton Dickinson Bioscience, Palo Alto, CA) containing 100 μg/ml salmon sperm DNA (Invitrogen). The PCR-amplified probes were 32P-labeled by the random prime labeling system (Promega, Madison, WI). The hybridization was carried out at 68°C for 24 h. The filters were washed twice in 2× SSC, 0.1% SDS at room temperature for 15 min, followed by a 30 min wash in 0.1× SSC, 0.1% SDS. Then, the filters were exposed to X-ray film (Kodak, Rochester, NY) for 48 h at −70°C. The relative abundance of individual mRNA in each sample was normalized with the GAPDH mRNA band that hybridized to the 32P-labeled GAPDH probe.
Western Blot Analysis
Transfectant HNE1 cells were washed with cool PBS and lysed at 4°C for 30 min in lysis buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 1 mM phenylmethanesulfonyl fluoride, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, and 1% protease inhibitor cocktail). The lysate were then centrifuged at 12,000g for 10 min to pellet large cellular debris. Protein concentrations were measured using the BCA kit (Pierce, Rockford, IL). Equal amount of samples (30 μg) were mixed with an equal volume of SDS sample buffer, heated at 90°C for 5 min then loaded onto 6%–12% SDS–PAGE for electrophoresis. Protein was transferred to PVDF membrane (Invitrogen, Carlsbad, CA) and incubated in PBS containing 5% non-fat dry milk at 37°C for 2 h, then incubated with the primary antibody at 4°C overnight. After incubation with the primary antibody the membrane were washed with PBS for 30 min and then incubated for 2 h at room temperature with anti-mouse (or rabbit) IgG-horseradish peroxidase-conjugated antibody (USBiological, Swampscott, MA), as recommend by the manufactures. To visualize protein, blots were incubated in Super signal west pico chemiluminescent substrate reagent (Pierce) according to the manufacturer's instruction and exposed to Kodax film. α-Tublin (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a control. Antibody of NGX6 protein was purchased from Boster (Hubei, China). Other antibodies were purchased from the Santa Cruz: anti-EGFR, p-EGFR (Tyr 1131), k-Ras, Mek-1, and c-Jun.
Flow Cytometry Analysis of Cell Cycle Distribution and Cyclins Expression in HNE1 Cells
For cell cycle distribution, transfectant HNE1 cells were collected, washed with PBS, and fixed in 70% (v/v) ethanol overnight. Cells were centrifuge at 1,000g for 10 min, resuspended in 50 μg/ml propidium iodide (Sigma) in PBS, and immediately subjected to flow cytometry analysis using a FACStar (Becton-Dickinson, Mountain View, CA). Appropriate settings of forward and side scatter gates were used to examine 10,000 cells per experiment. Data were analyzed with Modfit software (Verity Software House, Topsham, ME).
For cyclins expression, transfectant HNE1 cells were subjected to indirect immunofluorescence staining for the expression of cyclins, using murine mAb against human cyclin D1, A, E, and B. Cells were grown to subconfluence and then harvested from the tissue-cultured flask with 1 mM EDTA. Cells were fixed for 30 min at 4°C with 2.5% paraformaldehyde, washed, and then blocked with 20% goat whole serum. The cells were then incubated with anti-cyclin D1, A, E, and B (purchased from Santa Cruz), respectively, for 45 min at room temperature and again washed three times with PBS. Finally, the fluorescence intensity was analyzed by FACStar. The results are expressed as mean fluorescence intensity of positive cells.
In Vitro MAPK Kinase Assay
For detection of p-EGFR and in vitro MAPK kinase activity, cells were serum-deprived for 24 h and then incubated with EGF stimulation medium RPMI1640 containing 25 mM HEPES (pH 7.4) and 100 ng/ml recombinant human EGF (Sigma) for 15 min. The stimulation medium was subsequently removed. To stop stimulation, cells were washed with ice-cold PBS and kept on ice. Transfectant HNE1 cells total protein extracts were prepared as described above. The protocol was according to the manufacturer's instruction (MAPK immunoprecipitation kinase assay kit, Upstate, Lake Placid, NY). One milligram of protein were immunoprecipitated using 10 μl anti-MAPK kinase 1/2 agrose. Washed immunoprecipitates were resuspended in 25 μl of kinase buffer (50 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 0.5 mM Na3VO4, 0.1% 2-mercaptoethanol, 1% Triton X-100, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 10 mM sodium β-glycerol phosphate, 0.1 mM PMSF, 1 μg/ml of aprotinin, pepstatin, leupeptin, and 1 μM Microcystin). Ten micrograms dephosphorylated myelin basic protein (MBP) as an exogenous substrate, ATP, MAPK kinase inhibitor, ADB buffer also be added. After incubated for 10 min at 30°C, the reaction was stopped by adding SDS sample buffer and resolved on 12% SDS–PAGE. The primary antibody was anti-phospho MBP, or using anti-MBP as a control.
Statistical Analysis
Data were given as mean values ± SEM, with “n” denoting the number of experiments. Student's t-test was applied as appropriate. A value of P ≤ 0.05 was considered significant.
RESULTS
Expression of NGX6 in Transfectants
Plasmids pcDNA3.1(+)/NGX6 containing full length NGX6 cDNA and the pcDNA3.1(+) vector were transfected into human NPC cell line HNE1 and stable transfectants were selected. Northern blot and Western blot analysis were used to examine the NGX6 mRNA and protein expression level among these transfectants; as shown in Figure 1A,B, the expression of NGX6 mRNA and protein were observed in NGX6 transfectants, HNE1/NGX6(1) and HNE1/NGX6(2) as compared with the empty vector transfectant, HNE1/vector.
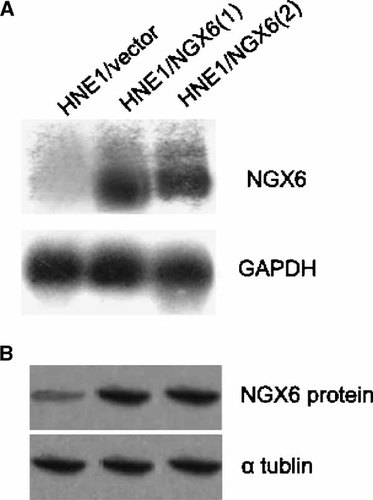
Expression of NGX6 in transfectants (A) Northern blot analysis of NGX6 transfectant and control cells. A part of NGX6 cDNA, 1.1 kb, containing open reading frame, was used as a probe. Northern blot revealed that HNE1/NGX6(1) and HNE1/NGX6(2) expressed high levels of NGX6 mRNA whereas empty vector transfectant HNE1/vector did not. The housekeeping gene GAPDH probe was used as an internal control. B: Western blot analysis of NGX6 transfectant and control cells. α-Tublin is internal control. The primary antibodies used in this experiment was anti-NGX6 (Boster). Western blot revealed that HNE1/NGX6(1) and HNE1/NGX6(2) expressed high levels of NGX6 protein.
Overexpression of NGX6 Inhibits NPC Cell Growth In Vitro
To investigate whether NGX6 re-expression affects the growth of HNE1 cells, we performed a cell growth curve assay. As shown in Figure 2, HNE1/NGX6(1) and HNE1/NGX6(2) grew significantly slower than HNE1/vector especially after the 3rd day (P < 0.01) (Fig. 2A). These results showed that overexpression of NGX6 negatively affected cell growth.
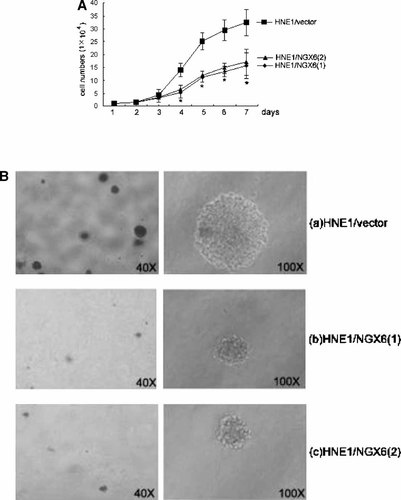
Overexpression of NGX6 inhibit HNE1 cell growth. A: Cell growth curve assay. Mock-transfected cells and NGX6-transfected cells were maintained in RPMI1640 10% fetal bovine serum (FBS) and cell number were counted on the indicated days. Each value represents mean ± SE for three observations. B: Soft agar assay. Assays were performed in parallel on all cells under identical culture conditions. a: Photograph of HNE1/vector cells. b: Photograph of HNE1/NGX6(1) cells. c: Photograph of HNE1/NGX6(2) cells.
Anchorage-independent growth in the semisolid medium of soft agar was a strong indicator of the transformed phenotype and a more stringent test of mitogenic capacity because several cycles of cell division were required to form detectable colonies. So, we performed soft agar assay to assess the clone formation ability. The size of formed colonies in soft agar was inhibited in cells from the HNE1/NGX6(1) and HNE1/NGX6(2), although HNE1/vector exhibited avid clonogenic growth under identical culture conditions (Fig. 2B). The number of colonies formed was also significantly decreased in HNE1/NGX6(1) and HNE1/NGX6(2) compared with HNE1/vector (P < 0.05) (Table I).
- Triplicate plates were seeded with 2 × 104 cells each and colonies were counted 14 days later. Each number represents the average of triplicate determination expressed as mean ± SE.
- * Represents P < 0.05 compared with HNE1/NGX6 group.
Overexpression of NGX6 Inhibits HNE1 Cell Growth In Vivo
To directly evaluate the role of NGX6 for tumor formation in vivo, NGX6-transfected and NGX6-negative cells were injected into nude mice. As shown in Figure 3, Mice treated with HNE1/vector cells developed solid tumors with a take rate of 100% (n = 6). Tumor growth was greatly reduced when exogenous NGX6 was stably expressed in HNE1 cells. The tumor volumes of the mice at day 32 were suppressed significantly by 68% in HNE1/NGX6(1) and 58% in HNE1/NGX(2) compared with that of vector-transfected HNE1 cells (P < 0.01). All of the tumors were stained with H&E, and the existence of cancer cells was confirmed (data not shown). These observations suggested that overexpression of NGX6 resulted in a decreased tumorigenic phenotype.
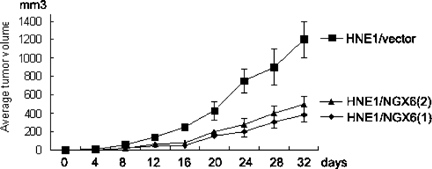
Tumor formation assay in nude mice. The average volume of six tumors were measured after injections of 2 × 106 HNE1 transfectant cells in day 0. The tumor volume was calculated by the formula 4π/3 × (L/2 × W/2 × H/2; L = length, W = width, H = height of the tumor).
Overexpression of NGX6 Increases the Length of the G1 Phase of the Cell Cycle and Influences Cyclins Expression
Because overexpression of NGX6 inhibited the HNE1 cells growth, we investigated whether cell cycle regulation was one of the mechanisms by which NGX6 exerted its growth-suppressive effect. The total cellular DNA content and the proportion of cells in various phases of cell cycle were quantified by flow cytometry analysis. Scatter profiles and the percentages of each in the various phases of the cell cycle showed that NGX6 re-expression resulted in a substantial increase in the G0–G1 phase (17%–20% increase) and a reduction in the number of cells in S phase (11%–22% decrease). Little effect was noted for the G2–M phase of the cell cycle (Fig. 4).
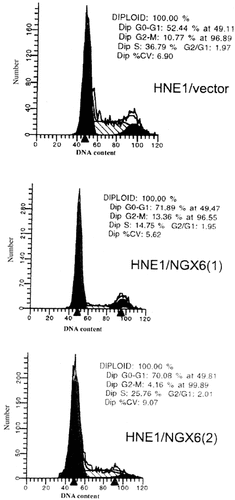
Distribution of the cells in the cell cycle. DNA contents were analyzed by flow cytometry. The percentage of cells within the G0–G1, S, and G2–M phases of the cell cycle was determined as described under “Materials and Methods.”
Because the cell cycle analysis suggested NGX6 could increase the length of the G1 phase of the cell cycle, we carried out a flow cytometry analysis for cyclin (cyclin D1, A, B, E) protein expression. The cells were subjected to indirect immunofluorescence staining using mAb against cyclin D1, E, A, and B. The NGX6 transfectants HNE1/NGX6(1) and HNE1/NGX6(2) showed reduced cyclin D1, A, and E expression as compared with HNE1/vector, while the cyclin B expression had no change (Fig. 5). So, the cell cycle distribution change induced by NGX6 transfection could be partly due to its influence on cyclin D1 and E expression.
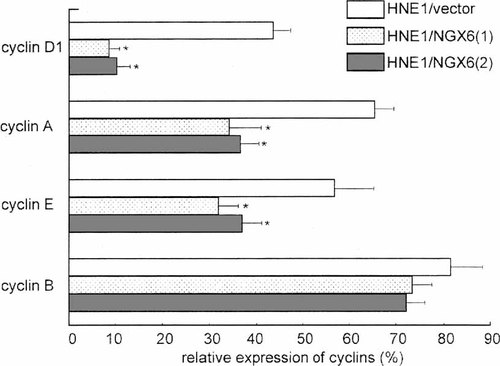
Flow cytometry analysis of cyclins expression by specific mAb in HNE1/vector, HNE1/NGX6(1) and HNE/NGX6(2) transfectants revealed that cyclin D1, A, and E were down-regulated by NGX6 re-expression (*P < 0.05 by Student's t-test as compared with HNE1/vector). Each experiment was carried out in triplicate. The data are presented as means ± SD.
Overexpression of NGX6 Influences the EGFR/Ras/MAPK Pathway
Although the regulatory mechanisms of NGX6-induced inhibition of HNE1 cell proliferation remain to be fully elucidated, we hypothesize that the EGF-like domain containing novel gene NGX6 may inhibit proliferation partly by attenuating EGFR activation. To test this hypothesis, HNE1/NGX6 cells were assayed for EGFR tyrosine kinase activity. In this assay, phosphorylated EGFR was detected by Western blot analysis using an antibody that recognized the phosphorylated forms of EGFR only. As shown in Figure 6, after stimulation of EGF, results revealed that the extent of phosphorylation of EGFR in NGX6-transfected cells was significantly inhibited compared with the corresponding levels in HNE1/vector. Western blot revealed no significant differences in EGFR between NGX6-transfected and control cells, indicating the inhibition of EGFR activation could not be attributed to changes in EGFR levels. We also detected expression of three molecules involving in the EGFR/Ras/MAPK pathway and found that NGX6 re-expression could down-regulate the expression level of k-ras, Mek-1, c-jun. MAP kinase is the most important target of this pathway, so we performed in vivo kinase assay to detect the kinase activity of MAP kinase using dephosphorylated MBP as an exogenous substrate. We found that NGX6 re-expression inhibited the phosphorylation level of MBP, i.e., inhibited the kinase activity of MAP kinase (Fig. 6).
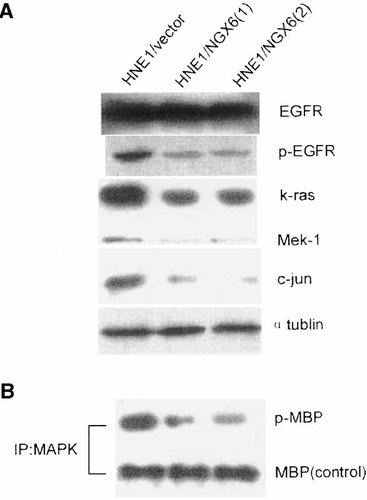
A: Western blotting analysis of phosphorylation EGFR, k-ras, Mek-1, c-jun in HNE1 transfectants. α-Tublin is internal control. The primary antibodies used in this experiment were anti-k-ras, anti-Mek-1, anti-c-jun, anti-p-EGFR (Tyr 1173) (Santa Cruz). B: In vitro MAPK kinase assay of HNE1 transfectants. Anti-myelin basic protein (MBP) is internal control.
DISCUSSION
NPC is a disease involving polygenic alteration, which is associated with many additive gene groups and certain environmental factors. Previous study provided some information about the known tumor suppressor genes such as p53, p16, and RB that might be related to the occurrence of NPC. But the relationship is not close to each other, there might be some other unknown suppressor genes which play a key role in the carcinogenesis of NPC.
NGX6 was isolated by candidate position cloning strategy, its protein contains two transmembrane regions, a EGF-like domain and several phosphorylation sites. In this study, we have identified and characterized a novel EGF-like domain containing gene NGX6 which located at the high frequent LOH region 9p21-22 of NPC. EGF-like domain is involved in receptor–ligand interaction, extracellular matrix formation, cell adhesion, cell growth, and signal transduction. An EGF-like domain retains six cysteines, which form three disulfide bonds that are required for optimum binding affinity to EGFR.
Although the functional properties of NGX6 remained to be fully elucidated, our observation of overexpression of NGX6 in HNE1 cells were associated with a marked reduction in proliferation. The mechanism that NGX6 inhibits cellular growth was unknown. However, our observation suggested that the induction of NGX6 expression was associated with inhibition of proliferation, additional support came from the observation that the cells of overexpression of NGX6 showed the lower ability to grow in soft agar. Because the latter is used to assess transformed property of cells, our observation that overexpression of NGX6 in HNE1 cells attenuated their ability to form colonies in soft agar further suggested a anti-tumorigenic property of NGX6. We have also demonstrated that there was a significant delay in tumor formation and a dramatic reduction in tumor size and weight when cells transfected with NGX6 were injected into nude mice.
As NGX6 contains an EGF-like domain, we detected the effect of NGX6 on the EGFR/Ras/MAP kinase pathway. In this study, we found that in the NGX6 transfectants, phosphorylation of EGFR was down-regulated as well as k-ras, Mek-1, c-jun. To investigate whether the final target of this pathway can be affected by NGX6, we carried out in vitro MAPK kinase assay, and found overexpression of NGX6 could inhibit MAP kinase activity in HNE1 cells. This result suggested that NGX6 might exert its anti-proliferative effect by inhibiting EGFR function.
It has been reported that overexpression of EGFR plays a key role in pathological process such as tumorigenesis and metastasis, especially epidermal carcinomas. Overexpression of EGFR has also been shown to correlate with tumor grade, tumor size, lymph node metastasis, survival and resistance to chemotherapy in cancer patients [Aaronson, 1991]. NPC occurs with a high incidence in southern China and southeast Asia. This malignancy is of epithelial origin with overexpression of EGFR [Sun et al., 1999]. In mammals, the EGFR can bind to several EGF superfamily ligands, and induce tyrosine phosphorylation of EGFR and thus activate the downstream signaling cascades, among which Ras/Mek/MAPK pathway is an important cascade and is implicated in diverse cellular responses including cell growth, differentiation, and apoptosis [Li et al., 2001; Bergmann et al., 2002]. Using differential display, Williams et al. [1999] have identified a novel mucin gene (MUC12) that is down-regulated in colorectal cancers and contains two EGF-like domains. Together with known interactions between mucin and c-erbB-2 growth factor receptors, suggested that MUC12 might be involved in epithelial cell growth regulation. So it is possible that genes containing EGF-like domain may be a negative regulator of EGFR pathway.
We also presented evidence that overexpression of NGX6 could increase the length of G1 phase of cell cycle in HNE1 cells by flow cytometry analysis and change the expression level of certain cyclins, such as down-regulating the expression of cyclin D1, A, and E. Cell cycle progression is a strictly controlled process and it needs delicate cooperation of many molecules. Ras/MEK/ERK pathway is one well-characterized serine/threonine kinase cascade. When extracellular growth factors (such as EGF) bind to their corresponding receptors on cytomembrane, the receptors are auto-phosphorylated and recruit the complex growth factor receptor bound protein 2/Son of Sevenless (GRB2/Sos), thus activating ras kinase and initiating intracellular signaling cascade ras-raf-MEK-ERK and ultimately leading to activation of nucleus transcription factors [Lopez-Ilasaca, 1998; Peyssonnaux and Eychene, 2001]. Ras/MEK/ERK pathway is strongly involved in many important cellular events such as cell cycle, differentiation, apoptosis, and senescence [Lee and McCubrey, 2002]. The relationship between Ras/MEK/ERK pathway and cell cycle has been well documented as following: activated Ras/MEK/ERK signal can increase the activity of cyclinD/cdk4 kinase as well as the transcription of cyclinD; furthermore, ras activation can down-regulate the p27kip1and results in the activation of cyclinE/cdk2 which is essential for entry into S phase. In addition, ras and raf, have also been reported to activate Cdc25A phosphatase which could remove the inhibitory phosphates from cdk2 and cdk4 and contribute to their activation [Kerkhoff and Rapp, 1998]. Therefore, ras/MEK/ERK signal promotes G1–S progression of cell cycle. In our experiments, we found that overexpression of NGX6 played a negative role in the EGFR ras/MEK/MAPK pathway and down-regulated the expression of cyclin D1 and E. These data can explain why overexpression of NGX6 increased the length of G1 phase of cell cycle in HNE1 cell.
In summary, we have identified an expressed sequence tag that encoded NGX6, a novel gene containing an EGF-like domain. Our present results suggested that the growth suppression of HNE1 cells by NGX6 was mediated through the arrest of G1 phase of cell cycle and down-regulating EGFR pathway. However, the cellular functions of NGX6 remain to be elucidated: firstly we were unable to demonstrate direct association of NGX6 with EGFR in co-immunoprecipitation and pull-down experiments, the effects of NGX6 on EGFR signaling might not involve a direct association of the two molecules; secondly the role of NGX6 in NPC carcinogenesis in vivo.




