Over-expression of fibroblast growth factor-2 causes defective bone mineralization and osteopenia in transgenic mice
Abstract
Over-expression of human FGF-2 cDNA linked to the phosphoglycerate kinase promoter in transgenic (TgFGF2) mice resulted in a dwarf mouse with premature closure of the growth plate and shortening of bone length. This study was designed to further characterize bone structure and remodeling in these mice. Bones of 1–6 month-old wild (NTg) and TgFGF2 mice were studied. FGF-2 protein levels were higher in bones of TgFGF2 mice. Bone mineral density was significantly decreased as early as 1 month in femurs from TgFGF2 mice compared with NTg mice. Micro-CT of trabecular bone of the distal femurs from 6-month-old TgFGF2 mice revealed significant reduction in trabecular bone volume, trabecular number (Tb.N), and increased trabecular separation (Tb.Sp). Osteoblast surface/bone surface, double-labeled surface, mineral apposition rate, and bone formation rates were all significantly reduced in TgFGF2 mice. There were fewer TRAP positive osteoclasts in calvaria from TgFGF2 mice. Quantitative histomorphometry showed that total bone area was similar in both genotypes, however percent osteoclast surface, and osteoclast number/bone surface were significantly reduced in TgFGF2 mice. Increased replication of TgFGF2 calvarial osteoblasts was observed and primary cultures of bone marrow stromal cells from TgFGF2 expressed markers of mature osteoblasts but formed fewer mineralized nodules. The data presented indicate that non-targeted over-expression of FGF-2 protein resulted in decreased endochondral and intramembranous bone formation. These results are consistent with FGF-2 functioning as a negative regulator of postnatal bone growth and remodeling in this animal model. © 2005 Wiley-Liss, Inc.
Basic fibroblast growth factor (FGF-2) is an important regulator of bone cell function [Hurley et al., 2002]. FGF-2 stimulates osteoblast replication [Globus et al., 1988], decreases differentiation markers such as alkaline phosphatase and type 1 collagen [McCarthy et al., 1989; Rodan et al., 1989; Hurley et al., 1993] and stimulates osteoclast formation and bone resorption [Kawaguchi et al., 1995; Hurley et al., 1998; Nakagawa et al., 1999]. Intermittent treatment stimulates bone formation in vitro [Canalis et al., 1988] as well as in vivo [Mayahara et al., 1993]. FGF-2 is expressed by osteoblasts and stored in the extra cellular matrix [Hauschka et al., 1986; Globus et al., 1989; Saksela and Rifkin, 1990]. FGF-2 mRNA and protein levels in osteoblasts are regulated by hormones and local factors including parathyroid hormone, transforming growth factor β, prostaglandins and interleukin-1 [Hurley et al., 2002].
FGF-2 is encoded by a single copy gene [Abraham et al., 1986]; however, multiple FGF-2 protein isoforms produced from alternative translation start sites in have been described [Florkiewicz and Sommer, 1989; Stachowiak et al., 1994]. Each isoform is a primary translation product with no precursor-product relationship [Florkiewicz and Sommer, 1989; Bugler et al., 1991; Quarto et al., 1991a; Stachowiak et al., 1994]. Studies have shown that selective expression of isoforms of FGF-2 can modulate cell phenotype and function [Baldin et al., 1990; Quarto et al., 1991; Hill et al., 1992; Hurley et al., 2002]. We previously reported that global knockout of all isoforms of FGF-2 protein resulted in decreased bone mass in older mice that was associated with decreased proliferation of osteoprogenitor cells [Montero et al., 2000].
Although constitutive over-expression of FGF-2 cDNA linked to the phosphoglycerate kinase promoter in transgenic (TgFGF2) mice enhanced apoptosis of chondrocytes in the proliferative, prehypertrophy, and hypertrophic zones, and caused premature closure of the growth plate and shortening of bone length of femurs and humeri. [Coffin et al., 1995; Sahni et al., 2001] resulting in murine dwarfism [Coffin et al., 1995], the effects on postnatal bone formation and bone remodeling were not investigated. In this study, we assessed the effect of FGF-2 over-expression on bone structure and function in vivo in TgFGF2 mice harboring the full-length human FGF-2 coding sequence.
Endochondral bone growth at the epiphyseal plate requires growth, replacement, differentiation, and programmed-death of chondrocytes. Recently, several gene families have been implicated in the intricate regulation of chondrocyte differentiation as they progress from the resting zone to the hypertrophy zone. These include transcription factors, growth factors, and hormones that interact with a variety of the extra cellular matrix proteins to form cartilage and, ultimately, mature bone [Mundlos and Olsen, 1997]. FGFs are important growth factors that regulate endochondral bone growth [Patstone et al., 1993; Trippel et al., 1993; Muenke and Schell, 1995; De and Dickson, 1997; Hurley et al., 2002]. Human skeletal disorders have been mapped to the fibroblast growth factor receptors (FGFRs) [Muenke and Schell, 1995; De and Dickson, 1997]. These mutations are autosomal dominant, sporadic point mutations clustered around the third Ig domain, the transmembrane domain and the tyrosine kinase domain of the FGFRs. Mutation of the FGFR1 causes Pfeiffer's syndrome, [Muenke et al., 1994] and different mutations of FGFR2 cause Jackson-Wiess, Crouzon, and Alperts syndrome [Jabs et al., 1994; Reardon et al., 1994]. Various mutations of FGFR3 cause Achondroplasia, Hypochondroplasia, and Thanadophoric dysplasia [Shiang et al., 1994; Tavormina et al., 1995]. All of these mutations are believed to result in aberrant or amplified signal transduction from the tyrosine kinase domain of the FGFRs [Naski et al., 1996; Schmermund et al., 1997]. Some of the syndromes resulting from FGFR1 and FGFR2 mutations primarily affect the axial skeleton, particularly the facial and skull bones, while mutation of FGFR3 mostly affects the long bones [Muenke and Schell, 1995].
Previous studies showed that targeted deletion of FGFR1 [Deng et al., 1994; Yamaguchi et al., 1994] and FGFR2 in mice resulted in embryonic lethality [Arman et al., 1998; Xu et al., 1998]. However, targeted deletion of FGFR3 in mice showed that loss of FGFR function resulted in excessive longitudinal bone growth [Colvin et al., 1996; Deng et al., 1996]. Interestingly, while transgenic over-expression of wild-type FGFR3 caused no phenotype in mice, over-expression of FGFR3 containing a G380R mutation common to human dwarfism resulted in achondroplasia and dwarfism [Naski et al., 1998]. Knock-ins of the G380R mutation into the endogenous FGFR3 gene reproduced the mouse dwarf/achondroplasia [Chen et al., 1999].
Mapping of the human dwarfisms as sporadic, autosomal dominant phenotypes put the TgFGF2 related dwarfism in mice in a biological context. Since the human mutations are gain of function, it follows that over-expression of the ligand produces a similar result where FGF-2 is a negative regulator of bone growth. This study demonstrates that FGF-2 over-expression not only reduces the size of the mouse skeleton but also reduces bone mass and bone formation.
MATERIALS AND METHODS
Animal Breeding and Genotyping
Transgenic mice (in bred FVB/N background) over expressing all isoforms of human FGF-2 were previously described [Coffin et al., 1995] and are maintained in an inbred FVB/N background. These mice were bred and housed in the transgenic facility in the Center for Laboratory Animal Care at the University of Connecticut Health Center. Hemizygous TgFGF2 males were mated with non-transgenic (NTg) FVB/N females to produce 1:1 litters of hemizygous TgFGF2 and NTg mice. Mice were weighed prior to sacrifice. Euthanasia was carried out by CO2 anesthesia followed by cervical dislocation. Genotyping of mice by PCR of tail DNA was performed using primers as previously described [Coffin et al., 1995].
Western Blot Analysis and ELISA
The expression of FGF-2 protein isoforms was determined by Western blot analysis [Coffin et al., 1995]. Bones were harvested from mice of both genotypes, washed with ice cold phosphate buffered saline (PBS) and homogenized in 1× lysis buffer. Lysates were centrifuged at 10,000 × g at 4°C for 10 min and protein concentrations were determined using the BCA protein assay reagent (Pierce Chemical, Rockford, IL). Samples containing 50 μg of total protein (TP) were loaded in each lane. Following separation by SDS-polyacrilamide gel electrophoresis on 12% gels, proteins were transferred to Immobilon™ transfer membranes (Millipore, pore, 0.45 μm; Millipore Corporation, Bedford, MA). The membranes were blocked with a solution of 1×TBS/ 0.1% Tween 20 (TBST) containing 5% non-fat dry milk for 2 h then incubated with a mouse x human monoclonal antibody (Transduction Labs, Lexington, KY) recognizing all FGF-2 isoforms diluted 1:250 for 1 h. Finally, a rabbit anti-mouse secondary antibody (Amersham Pharmacia Biotech, Piscataway, NJ) diluted 1:200 in blocking solution was applied for 1 h. After incubation with antibodies, the membranes were washed four × 15 min in 1 × TBST, and then Western Lighting™ Chemiluminescence Reagent (Perkin Elmer Life Sciences, Boston, MA) was used for detection. Signal was detected by KODAK X-OMAT autoradiography. Band density was quantified densitometrically.
FGF-2 levels in bone TP homogenates was determined using an ELISA kit purchased from R&D Systems (Minneapolis, MN). This immunoassay is specific for human FGF-2 and does not cross react with other cytokines. The assay has a sensitivity of (0.28 pg/ml) and a normal serum sample range of (0.5–32 pg/ml).
Dual Beam X-Ray Absorptiometry
Femoral bones were harvested and stored in 70% ethanol at 4°C. Bone mineral density (BMD) and bone mineral content (BMC) were measured using a Piximus Mouse 11 densitometer (GE Medical Systems, Madison WI).
Microcomputed Tomography
The femoral cancellous bones of the distal metaphysis were analysed by micro-CT instrumentation (μCT-20, Scanco Medical AG, Bassersdorf, Switzerland) as previously reported [Reugsegger and Mueller, 1996; Montero et al., 2000]. Using two-dimensional data from scanned slices, three-dimensional morphometric analyses were performed to calculate morphometric parameters defining trabecular bone mass and micro-architecture. These include bone volume density (BV/TV), bone surface density (BS/TV), trabecular thickness (Tb.Th = 2 × BV/bone surface [BS]), trabecular number [Tb.N = (BV/TV)/Tb.Th], and trabecular separation [Tb.Sp = (1/Tb.N)−Tb.Th]. These parameters were calculated by the parallel plate model of [Parfitt et al., 1987].
Histomorphometry
Mice were weighed and injected with calcein (25 mg/kg body wt), at 7 and 3 days prior to sacrificed. Long bones and calvariae were harvested at sacrifice. The right femurs were fixed with 10% buffered formalin followed by 100% ethanol. The bones were embedded in methyl methacrylate (MMA) for analysis of parameters of bone formation. Five–micron thick midfrontal sections of the distal femur were obtained using a Reichert microtome (Reichert-Jung, Heidelburg, Germany). The left femurs were fixed with 10% buffered formalin, embedded in a mixture of MMA, hydroxyglyucol methacrylate, and 2-hydroxyehylacrylate and polymerized at 4°C. Undecalcified 5-μm sections were prepared and stained for tartrate resistant acid phophatase (TRAP). Histomorphometry was performed with a semiautomatic image analyzing system linked to a light microscope (Cosmozone 1S; Nikon, Tokyo, Japan). The area of the secondary spongiosa was analyzed excluding the region within 0.5 mm of the growth plate-metaphyseal junction. Trabecular bone volume density (BV/TV,%) was measured. Trabecular thickness (Tb.Th, μm) and trabecular number (Tb.N, /mm) were calculated by the parallel plate model [Parfitt et al., 1987]. Osteoclast surface (Oc.S/BS,%) and osteoclast number (Oc.N/BS, mm) were determined. For dynamic histomorphometry, mineralizing surface (DLS/BS,%), mineral apposition rate (MAR, μm/day), and bone formation rate (BFR/BS, μm3/μm2 per day) were also determined.
The calvariae were fixed in 10% buffered formalin at 4°C, dehydrated in progressive concentrations of ethanol, cleared in xylene, and embedded in paraffin. Five-micron sections were cut, deparaffinized and stained for TRAP. The Bioquant program for histomorphometry was utilized (Bioquant-True Color Windows, Advanced Image Analysis Software, sVGA Frame Grabber Image Processing Board, Optronics DEI-470 Video Camera). Quantitative microscopic analysis was performed by measuring primary histomorphometric indices.
Serum Biochemistry
Serum was obtained from mice of both genotypes at time of sacrifice for calcium and phosphorous measurement using kits purchased from Sigma (St. Louis, MO) according to the manufacturer's instructions.
Cell Cultures
To assess the effects of over-expression of FGF-2 on osteoblasts proliferation, cells were prepared from calvariae of NTg and TgFGF2 mice by sequential digestion with 0.1% collagenase (Worthington Biochemical Co.) and plated in 100 mm dishes in Dulbeccos modified eagles medium (DMEM) with 10% heat inactivated fetal calf serum (FCS) (FCS, Hyclone, Logan UT) in a 5% CO2 incubator at 37°C. At confluence, cells were replated at 5,000/cm2 in six-well dishes in DMEM medium containing 10% FCS for 7 days with media change every 3rd day. For labeling studies, [3H] thymidine (10 μCi/well) was added for the last 4 h of the culture to measure cell proliferation.
Mouse bone marrow cells were isolated using a modification of previously published methods [Montero et al., 2000]. Tibiae and femurs from 8-week-old NTg and TgFGF2 mice were dissected free of adhering tissue. Bone ends were removed and the marrow cavity was flushed with αminimal essential medium (αMEM, GIBCO-BRL, Grand Island, NY) using a sterile 25-gauge needle. Marrow cells were collected into tubes washed twice with serum free αMEM. Cells were plated in six-multi-well plates at 3 × 106 cells/well and cultured in complete media consisting of (αMEM with 10% heat-inactivated FCS, ascorbic acid (50 μg/ml); and beta-glycerophosphate (BGP 8 mM) in the presence of dexamethasone (DEX 10−8M) for 7 to 21 days. Cultures were fed every 3rd day by replacing 80% of the medium with fresh medium. To assess total cell number, cultures were harvested 7 days after plating and stained with crystal violet. Cells for alkaline phosphatase (ALP) staining were harvested on day 7, 14, and 21 of culture. ALP staining was performed with a commercial kit (Sigma Chemical Co., St. Louis MO). Dishes were scanned and then counter stained for mineral by the von-Kossa method, as previously described [Montero et al., 2000].
RNA Isolation and Northern Blot Analysis
For assessment of gene expression, total RNA [Chomczynski and Sacchi, 1987; Towbin et al., 1979] was prepared from calvariae or femurs using TRIZOL reagent according to the manufacturer's instructions. For Northern Analysis, 20 μg of total RNA was denatured and fractionated on a 1% agarose/2.2M formaldehyde gel, transferred to filters by capillary blotting and fixed to the filter by UV irradiation. After a 4-h prehybridization, filters were hybridized overnight with a [32P] cDNA probe for the mRNAs of interest. Bands were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Northern blots were quantified by autoradiography and densitometry.
Statistical Analysis
Statistics was performed using paired Student T-test or analysis of variance (ANOVA) to determine differences between groups at P < 0.05.
RESULTS
Hemizygous TgFGF2 mice appeared normal and had no significant size or weight differences compared with NTg mice. These results are consistent with our earlier report on the initial characterization of these mice [Coffin et al., 1995]. We compared FGF-2 protein levels in bones from NTg and TgFGF2 mice of different ages. Total protein was extracted from femurs of NTg and TgFGF2 mice at 4, 14, 28, 70, and 230 days post-partum (pp) and quantitated by ELISA. The concentration of FGF-2 protein (picograms/ml; pg/ml) as a fraction of TP was higher at all time points in bones from TgFGF2 mice compared with NTg mice (Fig. 1A). Western Blot analysis was performed to detect FGF-2 protein isoform expression in whole femurs from 8-week-old NTg and TgFGF2 mice. As shown in Figure 1B, femurs from TgFGF2 mice over-expressed all FGF-2 protein isoforms.
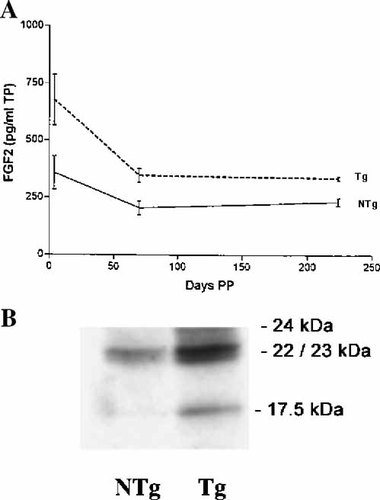
Comparison of FGF-2 protein levels in femoral bones from NTg and TgFGF2 mice of different ages. A: FGF-2 protein was measured by ELISA in bones from 4 to 230-days post partum (pp) mice. N = 4 determination at each time point, 4, 14, 28, 70, and 230 days pp. B: Western Blot analysis of FGF-2 protein isoform profile expression in femurs from 8-week-old NTg and TgFGF2 mice. Western blots analysis showed that TgFGF-2 mice over-expressed multiple FGF-2 protein isoforms in femoral bones.
To assess whether bone mass was altered in young TgFGF2 mice, BMD and BMC was measured by Piximus DEXA in femurs from 1-month-old male mice of both genotypes. As shown in Figure 2, there was a 19% decrease in BMD and a 40% decrease in BMC in bones from TgFGF2 compared with NTg mice.
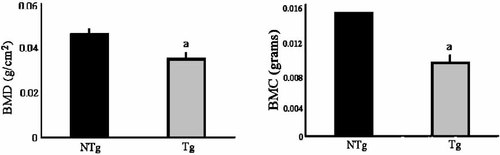
DXA analysis of bone mineral density (BMD) and bone mineral content (BMC) was determined on freshly excised femurs harvested from 1-month-old male NTg and TgFGF2 mice. aSignificantly different from NTg group; P < 0.05.
In order to assess the structural parameters of trabecular bone, we performed micro-CT of the distal metaphysis of femurs harvested from male mice of both genotypes. Three-dimensional images of the femoral metaphysis of 6-month-old male NTg and TgFGF2 mice are shown in Figure 3A. There was reduction of the plate like structure of the trabecular bone from TgFGF2 mice compared with NTg mice. Quantitation of structural parameters of bones from 6-month-old male TgFGF2 mice (Fig. 3B) revealed that trabecular bone volume (BV/TV) and trabecular number (Tb.N) were reduced by 32% and 28%, respectively (P < 0.05). Trabecular separation (Tb.Sp) was significantly increased by 34% compared with NTg mice (P < 0.01). Trabecular thickness (Tb.Th) was not significantly altered when compared with NTg mice.
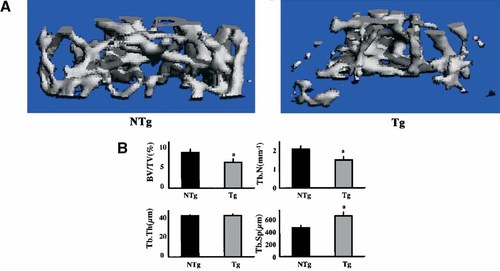
Morphologic study by micro-CT scanning of the trabecular bone of distal metaphysis of femurs of 6 month male NTg and TgFGF2 mice. A: Note that the amount of trabecular bone is reduced in the TgFGF2 mice compared with NTg mice. B: Three-dimensional microstructural parameters calculated using two-dimensional data obtained from micro-CT of femurs of 6-month-old male NTg and TgFGF2 mice. Calculated morphometric indices included bone volume/trabecular volume (BV/TV); Trabecular thickness (Tb.Th); Trabecular number (Tb.N), Trabecular separation (Tb.Sp). aSignificantly different from NTg group; P < 0.05. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Static and dynamic histomorphometry was performed to assess parameters of bone structure and function at the metaphysis of distal femurs of 6-month-old male NTg and TgFGF2 mice. As shown in Figure 4A, static histomorphometry confirmed the results that were obtained by micro-CT. BV/TV was significantly reduced by 37% (P < 0.05) and Tb.Sp was also significantly increased by 38% compared with NTg mice (P < 0.05). The results of dynamic histomorphometric parameters of bone formation at the metaphysis of distal femurs are shown in Figure 4B. There was a 21% decrease in osteoblast surface (Ob.S/BS), a 38% reduction in double-labeled surface (D-LS/BS); a 21% reduction in mineral apposition rate (MAR); and a 49% reduction in bone formation rate (BFR/BS), (P < 0.05) in bones from TgFGF2 mice.
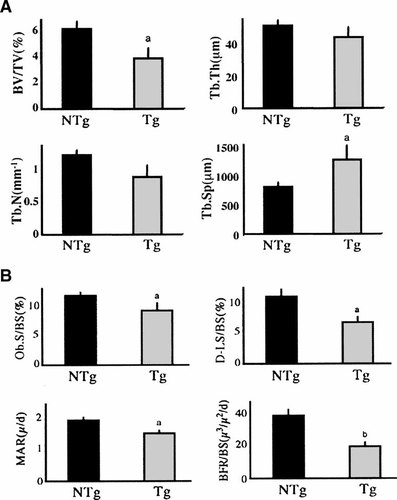
Static histomorphometric parameters of bone structure of the metaphysis of distal femurs of 6-month-old male NTg and TgFGF2 mice. A: BV/TV; Trabecular bone volume density, Tb.Th; Trabecular thickness, Tb.N; Trabecular number, Tb.Sp; Trabecular separation were measured. aSignificantly different from NTg group; P < 0.05. B: Dynamic histomorphometric parameters of bone formation at the metaphysis of distal femurs of 6-months-old male NTg and TgFGF2 mice. D-LS/BS; Double-labeled surface, MAR; Mineral apposition rate, BFR/BS; Bone formation rate, Ob.S/BS; osteoblast surface were measured. Significantly different from NTg group; P < 0.05. aSignificantly different from NTg group; P < 0.01.
To examine effects of FGF-2 over-expression on osteoclast number and activity, TRAP staining and static histomorphometric analysis was performed on calvariae from 6–8-week-old male mice. As shown in Figure 5A there were fewer TRAP positive osteoclasts (OCL) in calvaria from TgFGF2 mice. Quantitative histomorphometry, (Fig. 5B) showed that total bone area (TA/TTA), was similar in both genotypes, however percent osteoclast surface (% Oc.S), and osteoclast number/ bone surface (Oc.N/BS) were significantly reduced in TgFGF2 mice compared to NTg mice.
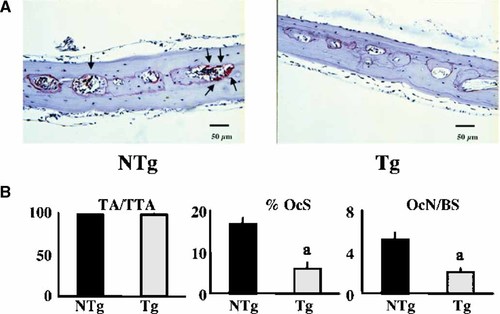
TRAP staining and static histomorphometry was performed on calvariae from 6–8-week-old NTg and TgFGF2 mice. A: There were few TRAP + OCLs (arrows) in calvarial sections fron TgFGF2 mice. B: Quantification of Static histomorphometric parameters is shown. Total trabecular bone area (TA/TTA); percent osteoclast surface, (% Oc.S); osteoclast numbers/bone surface (Oc.N/BS) was determineded. aSignificantly different from NTg group; P < 0.05. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To ascertain whether reduced bone formation was associated with systemic alteration in mineral homeostasis we measured serum calcium and phosphorus in NTg and TgFGF2. Serum levels of calcium and phosphorous were similar in both genotypes.
FGF-2 has been shown to inhibit chondrocyte proliferation but stimulate osteoblast proliferation [Hurley et al., 2002] We therefore compared the proliferative capacity of calvarial osteoblastic cells in NTg and TgFGF2 mice by measuring thymidine incorporation into DNA after 7 days of culture. Thymidine incorporation into DNA showed a significant increase of 85% in osteoblast cultures from TgFGF2 mice (Fig. 6) compared with NTg mice.
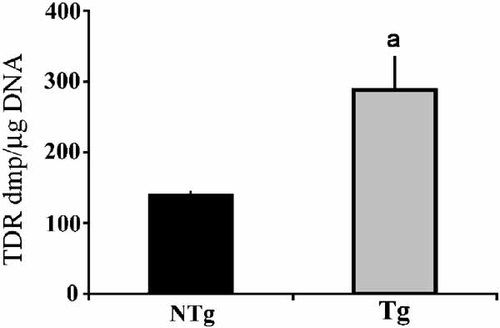
Comparison of thymidine incorporation into DNA in calvarial osteoblasts from neonatal NTg and TgFGF2 mice. Osteoblastic cells were prepared from calvariae of neonatal mice from both genotypes as described under methods and cultured in 10% FCS for 7 days and thymidine incorporation into DNA was determined. Values are the mean ± SEM for 6 determination/group. aSignificantly different from NTg, P < 0.05.
To further assess the mechanism of reduced mass in TgFGF2 mice, formation of alkaline phosphatase positive (ALP) colonies and mineralized nodules in bone marrow cultures from NTg and TgFGF2 mice was compared. As shown in Figure 7A, after 7 days of culture, crystal violet staining revealed increased numbers of cells in cultures from TgFGF2 mice. At 7th day the number of ALP positive colonies were similar in both genotypes (Fig. 7B). However, after 21 days of culture, there were fewer mineralized (vonKossa-stained) colonies in TgFGF2 cultures Figure 7C and Figure 8.
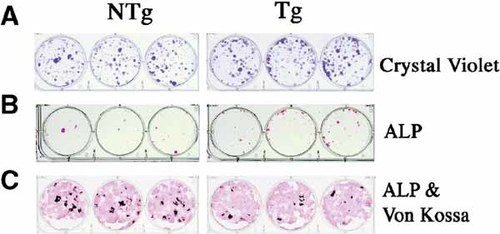
Comparison of the ability to form alkaline phosphatase positive colonies and mineralized nodules as determined by von Kossa staining in mouse bone marrow cultures from NTg and TgFGF2 mice. Cells were cultured in differentiation medium for the indicated times as described under methods. Crystal violet staining reveal increased cell number in TgFGF2 mice cultures at 7days. ALP positive staining was similar but mineralized nodules were reduced in TgFGF2 mice cultures at 21 days. This experiment is representative of 3 independent experiments. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
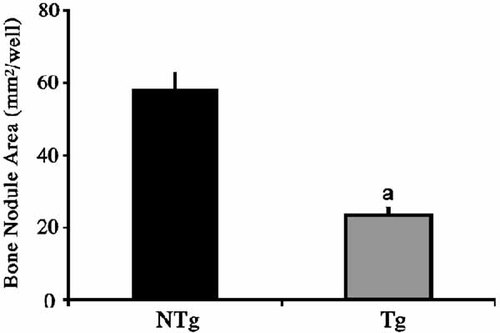
Quantitation of mineralized colony area in mouse bone marrow cultures from NTg and TgFGF2 mice cultured for 21 days in differentiation medium as described under methods. von-Kossa stained mineralized colony area was measured by NIH Image. Values are the mean ± SEM (n = 3 replicates/group). aSignificantly different form NTg group; P < 0.001.
Total RNA was extracted from calvarial and femoral bones to examine the expression of genes associated with osteoblast differentiation as well as matrix proteins that may affect mineralization. As shown in Figure 9, Northern analysis of gene expression in calvariae or femur from 6-month-old mice revealed similar levels of expression of mRNA for type 1 collagen (Col1a1), osteocalcin (OCN), bone-sialoprotein (BSP), osteopontin (OPN), and runt-related transcription factor (RUNX2). We also compared the expression of these genes in femoral bones from 1 to 6-month-old mice. As shown in Table I there was similar expression of mRNA for Col1a1, OCN, OPN, and BSP and RUNX2 in femurs from both genotypes at 1 and 6 months of age. Previous studies reported defective mineralization with increased matrix gla protein (MGP) [Yagami et al., 1999]. However as shown in Table I, the mRNA for MGP was similar in bones from 1 to 6-month-old NTg and TgFGF2 mice.
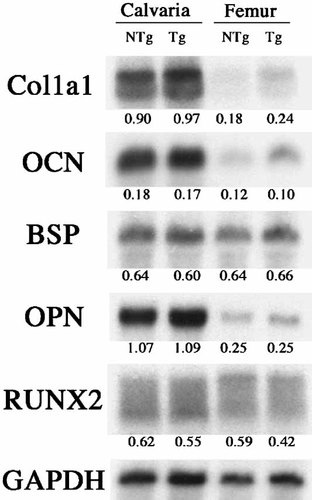
Gene expression profile in bones from 6-month-old NTg and TgFGF2 mice. Total RNA was extracted from calvariae and femoral bones. Northern Blot analysis for mRNA for osteoblast gene expression is described under methods. Filters were probed for the mRNA of interest. Filters were reprobed for GAPDH. Signals were quantitated by densitometry and normalized to the corresponding value for GAPDH.
| Age | 1 month | 6 months | ||
|---|---|---|---|---|
| Genotype | NTg | Tg | NTg | Tg |
| Col1a1 | 1.18 | 1.37 | 0.10 | 0.07 |
| OCN | 0.86 | 1.21 | 0.07 | 0.06 |
| OPN | 0.86 | 0.88 | 0.67 | 0.65 |
| BSP | 0.80 | 0.90 | 0.37 | 0.36 |
| RUNX2 | 0.59 | 0.46 | 0.49 | 0.38 |
| MGP | 0.57 | 0.61 | 0.52 | 0.51 |
Since defective bone mineralization has also been reported in mice with targeted inactivation of FGFR2 in chondrocytes and osteoblasts [Yu et al., 2003]) as well as FGFR3 knockout mice [Valverde-Franco et al., 2004], we determined whether their mRNA expression was altered. Furthermore, since FGFR1 can modulate osteoblast proliferation and differentiation [Hurley et al., 2002], we also assessed its expression. Northern blot analysis revealed no significant differences in mRNA for FGFR1, FGFR2, or FGFR3 (data not shown).
DISCUSSION
Our earlier studies demonstrated that constitutive FGF-2 over-expression impaired longitudinal bone growth resulting in murine dwarfism in the TgFGF2 mice [Coffin et al., 1995] however; there was no functional characterization of the bones of these mice. The present study was undertaken to determine the effect of FGF-2 over-expression on bone mass. We observed that BMD and BMC were decreased in TgFGF2 mice as early as 1 month of age. These results suggest a developmental defect in the acquisition of bone mass. Structural analysis of bone by micro-CT and static and dynamic histomorphometric studies demonstrated a marked reduction in bone mass as well as decreased rate of endochondral bone formation in TgFGF2 mice. The data reported here suggest that the major defect in TgFGF2 mouse bone results from decreased bone formation.
We previously reported that FGF-2 increased osteoclast formation [Hurley et al., 1998], therefore, decreased bone mass in TgFGF2 mice could be due to increased osteoclast formation and activity resulting in increased bone resorption. Static histomorphometry of calvariae showed that cortical width and total trabecular area were not significantly different between the NTg and TgFGF2 mice, however there was a significant decrease in percent osteoclast surface and osteoclast numbers/ bone surface in TgFGF2 mice compared to NTg control mice. Thus, bone resorption appears to be decreased in the TgFGF2 mice. Thus, FGF-2 over-expression modulates both endochondrol and intramembranous bone formation.
Decreased bone formation was not due to perturbation in mineral homeostasis since serum concentrations of calcium or phosphorous both were similar between TgFGF2 and NTg mice.
The earlier onset of decreased bone mass at 1 month of age in TgFGF2 mice can be contrasted with our observation in the Fgf2−/− null mice where decreased bone mass was observed as the mice aged [Montero et al., 2000]. Reduced bone mass in Fgf2−/− mice appears to be due in part to reduced numbers of osteoblast precursors. In contrast to Fgf2−/− mice, there was increased thymidine labeling in TgFGF2 osteoblasts suggesting that there were no deficits in the number of early osteoblast precursors. Furthermore total cell number was increased in TgFGF2 marrow stromal cultures and alkaline phosphatase colonies were similar in marrow stromal cultures from both genotypes.
Previous studies found FGF-2 expression in epiphyseal plates during physiological endochondral bone formation and expression in chondrocytes during the earliest stages of differentiation [Hill et al., 1992b]. Staining for FGF-2 was also present in ossification centers in osteoblasts as well as calcified matrix [Hill et al., 1992b]. Therefore, we measured FGF-2 levels in bones from TgFGF2 and NTg mice of different ages using an ELISA that does not cross react with other FGFs. FGF-2 levels were much higher in bones from TgFGF2 mice of all ages compared with bones from NTg mice. It is possible that similar to our observation in vitro [Hurley et al., 1993], continuous exposure to high levels of FGF-2 in vivo caused decreased osteoblast differentiation resulting in decreased bone mass. In support of this possibility, we did observe fewer mineralized colonies in stromal cultures from TgFGF2 mice.
Although total colony number was similar, we observed reduced mineralization of colonies in the TgFG2 mice. Previous studies showed that FGF-2 regulates the expression of OCN [Xiao et al., 2002], OPN [Leali et al., 2003], and BSP [Shimuzu-Sasaki et al., 2001]. This is relevant to our studies since these non-collagen proteins are important in bone mineralization [Chen et al., 1992; Ducy et al., 1996; Rittling et al., 1998]. In contrast, MGP [Yagami et al., 1999; Zebboudj et al., 2002] has been associated with defective mineralization. However, we did not observe any difference in the expression of genes for non-collagen proteins between the two genotypes suggesting that they are not important in defective mineralization in the TgFG2 mice.
FGF-2 can signal via multiple FGF receptors [Ornitz and Marie, 2002]. Since defective bone mineralization has been reported in mice with targeted inactivation of FGFR2 in chondrocytes and osteoblasts FGFR2 [Yu et al., 2003], we examined FGFR2 mRNA in bones from TgFGF2 mice. We observed no significant differences in FGFR2 expression in bones from NTg and TgFGF2 mice. FGF-2 also signals via FGFR3 [Ornitz and Marie, 2002] and over-expression of FGF-2 recapitulates short-limbed dwarfism that is similar to mutations caused by constitutive activation FGFR3. In contrast to activation of FGFR3, disruption of FGFR3 did not cause dwarfism in mice, however recent studies showed reduced bone mass and impaired bone mineralization in FGFR3 null mice [Valverde-Franco et al., 1998]. Interestingly impaired bone mineralization in FGFR3 null mice was associated with increased matrix-GLA protein (MGP) that was previously shown to block mineralization [Yagami et al., 1999]. However, we did not observe any differences in either FGFR3 or MGP mRNA in bones of TgFGF2 mice, suggesting that neither MGP nor FGFR3 were important in reduced mineralization or decreased bone mass in TgFGF2 mice.
In summary, we have shown that non-targeted over-expression of FGF-2 not only regulates bone development but also modulates postnatal bone formation and bone remodeling. Targeted over-expression of FGF-2 in chondrocytes and osteoblasts should provide important information regarding the role of FGF-2 protein in dwarfism and bone formation as well as the signaling pathways that are involved. The FGF regulatory pathways for bone growth, beyond the ligands and receptors, are now under intense scrutiny. Overall, genetic and biochemical data for negative regulators such as FGF-2 suggest that both endochondral and epiphsyial bone growth is dependent on maintaining a tightly regulated balance of the growth factor regulatory molecules.
Acknowledgements
This work was supported in part by NIH grant AR-46025 (to MM Hurley).




