Early signals for fracture healing
Abstract
Fracture healing requires the cooperation of multiple molecular signaling pathways. To better understand this cascade of transcriptional events, we compared the gene expression profiles between intact bone and fractured bone at days 1, 2, and 4 using a rat femur model of bone healing. Cluster analysis identified several groups of genes with dynamic temporal expression patterns and stage-specific functions. The immediate-response genes are highlighted by binding activity, transporter activity, and energy derivation. We consider these activities as critical signals for initiation of fracture healing. The continuously increased genes are characterized by those directly involved in bone repair, thus, representing bone specific forefront workers. The constantly upregulated genes tend to regulate general cell growth and are enriched with genes that are involved in tumorigenesis, suggesting common pathways between two processes. The constantly downregulated genes predominantly involve immune response, the significance of which remains for further investigation. Knowledge acquired through this analysis of transcriptional activities at the early stage of bone healing will contribute to our understanding of fracture repair and bone-related pathological conditions. © 2005 Wiley-Liss, Inc.
Fracture repair is essentially a recapitulation of bone development that involves many different cell types including endothelial cells, fibroblasts, chondroblasts, osteoblasts, and osteoclasts and cellular processes such as adhesion, proliferation, migration, and differentiation [Lombardo et al., 2004]. Thus, it requires a temporal and spatial orchestration of many transcriptional programs for regulating hemostasis, inflammatory response, immune defense, chondrogenesis, and osteogenesis [Kunimoto, 1999]. These fracture-activated programs presumably involve hundreds of differentially expressed genes.
There is a large body of literature describing molecular basis of hard tissue healing [Bouletreau et al., 2002; Desai et al., 2003; Meyer et al., 2003; Pacicca et al., 2003; Lombardo et al., 2004]. Many signaling molecules have been shown to play an active role in the fracture healing, including members of FGF, PDGF, IGF, TGFβ, and BMP families [Rosen and Thies, 1995]. However, due to the technological limitations, those studies were only able to focus on one or at most a few genes simultaneously, thus revealing an isolated molecular event rather than a comprehensive molecular picture of bone healing. With the development of microarray technology, it becomes possible to perform transcriptome analysis of fracture healing. Hadjiargyrou et al. [2002] have reported a large-scale expression analysis using a combination of suppressive subtractive hybridization and cDNA microarray. Although this experimental design was limited to interrogate upregulated genes, and possibly missed early molecular cascades as the first time point studied was post-fracture day 3, the data clearly demonstrated the biological complexity of fracture healing.
This report focused on early events of fracture healing—inflammatory stage, which is most active in the recruitment of cells and release of various cytokines/growth factors, and thus represents a signaling stage of fracture healing. How cells program at this stage is critical for the speed and quality of overall healing. To characterize the concurrently activated molecular signals at this stage, we used the Affymetrix Rat U34A arrays, which include approximately 7,000 known genes and 1,000 EST clusters, to investigate transcriptional changes between non-fractured control and days 1, 2, and 4 post-fractured bone in the rat femur. Here, we report the global gene expression profiles of early bone fracture healing and functionally dissect characteristic features of gene expression patterns. We also report an interesting observation that early bone fracture healing shares many common molecular signals/pathways with tumorigenesis, underscoring the importance of cell proliferation in early regulation of bone repair.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley laboratory rats were obtained from Harlan (Indianapolis, IN) and housed at the research animal lab under conditions of 12 h light, 12 h darkness, ambient temperature of 20–23°C, and relative humidity of 35%–60%. Experimental animal procedures were in compliance with animal welfare regulation and approved by the OrthoLogic Research Department.
Experimental Design and RNA Extraction
Ten-month-old male rats weighing from 400 to 500 g each were used in this study (difference in age was ±1 week). Standard closed fractures of the right femur midshaft were created using the device and method by Bonnarens and Einhorn [1984]. The fractures were verified via contact radiograph using the Hewlett Packard Model no. 43855-A Faxitron Closed X-ray System. One centimeter of fractured femur, including early fracture callus and cortical bone shaft, from each group was harvested at three time points (day 1, 2, and 4) and each time point had three replicates. In addition, three intact, age-matched rat femurs (three replicates) were used as control (i.e., pin was not applied to and marrow was not removed from the control femur). The rats were euthanized by intraperitoneal injection of 2 ml Euthasol (Delmarva Labs, Midlothian, VA). Fractured femurs were carefully cleaned to ensure no muscle contaminations and midshafts were cut off using a sterile dremel saw blade and frozen in liquid nitrogen until use. Total RNA was isolated by using Trizol reagent (Life Technologies, Gaithersburg, MD) followed by RNeasy Mini column purification (Qiagen, Chatsworth, CA) according to the manufacturer's instructions. Integrity of RNA was evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). The purity/concentration was determined using a GeneSpec III (Miraibio). All RNA samples used for hybridization had an OD260/280 and OD260/230 ratio >1.8 and total RNA concentration >1 μg/ml.
Microarray Hybridization
All rat U34A gene array hybridizations were performed at the Functional Genomics Facility, University of Chicago. The target preparation protocol followed the Affymetrix GeneChip Expression Analysis Manual (Santa Clara, CA). Briefly, 10 μg of total RNA was used to synthesize double-stranded cDNA using the Superscript Choice System (Life Technologies). First strand cDNA synthesis was primed with a T7-(dT24) oligonucleotide. From the phase-log gel-purified cDNA, biotin-labeled anti-sense cRNA was synthesized using BioArray High Yield RNA Transcript Labeling Kit (Enzo Diagnostics, Farmingdale, NY). After precipitation with 4M Lithium Chloride, 20 μg of cRNA was fragmented in fragmentation buffer (40 mM Tris-Acetate, pH 8.1, 100 mM KOAc, and 30 mM MgOAc) for 35 min at 94°C and then 12 μg of fragmented cRNA was hybridized to U34 Arrays for 16 h at 45°C and 60 rpm in an Affymetrix Hybridization Oven 640. The arrays were washed and stained with streptavidin phycoerythrin in Affymetrix Fluidics Station 400 using the Affymetrix GeneChip protocol and scanned using the Affymetrix Agilent GeneArray Scanner.
Data Analysis
Data analyses were performed using DNA-Chip Analyzer 1.3 [Li and Wong, 2001] with the *.CEL files obtained from MAS 5.0. We used a PM-only model to estimate gene expression level (any array with a percentage of outliers >10% was eliminated from analyses). The invariant set approach was used for normalization. For comparison analyses, thresholds for selecting significant genes were set at a relative difference >threefold and absolute difference >100, signal intensity and statistical difference at P < 0.05. Five-hundred permutations were performed to estimate the false discovery rate (FDR). In this experiment, all FDRs were zero using the thresholds set above. For the purpose of comparison between bone healing and tumor tissue, thresholds for selecting significant genes were set at a relative difference >twofold and absolute difference >100, signal intensity and statistical difference at P < 0.05 at least at one time point.
Cluster analysis was performed using D-Chip. The default clustering algorithm of genes was used [Li and Wong, 2001]. Briefly, the distance between two genes was defined as 1–r, where r is the Pearson correlation coefficient between the standardized expression values (make mean, 0 and standard deviation, 1) of the two genes. Two genes with the closest distance were first merged into a super-gene and connected by branches with length representing their distance. The expression values of the newly formed super-gene were the average of standardized expression values of the two genes across samples. Then the next pair of genes (super-genes) with the smallest distance was chosen to merge and the process was repeated n − 1 times to merge all the n genes. A similar procedure was used to cluster samples. Gene ontology (GO) analysis was performed using “classify gene” function in D-Chip. The GO terms are three structured, controlled vocabularies (ontologies) that describe gene products in terms of their associated biological processes, cellular components, and molecular functions in a species-independent manner.
Quantitative Real Time RT-PCR
The same RNA samples for microarray hybridizations were used for quantitative RT-PCR. Real-time PCR primers were selected for a representative set of genes using PRIMER EXPRESS software (Version 2.0, Applied Biosystems). Primer sequences have been published as supporting information at http://fgf.bsd.uchicago.edu/jcb. Reactions were performed in a 50-μl volume that included diluted cDNA sample, primers, and SYBR Green PCR Master mix (Applied Biosystems). Real-time PCR reactions were performed on an Applied Biosystems Prism 7000 sequence detection system. Predicted cycle threshold (Ct) values were exported directly into EXCEL worksheets for analysis. The standard Curve Method was used for the relative quantitation of expression for each gene. Ribosome 18S was used to normalize the expression data. Expression of the housekeeping gene GAPDH was not used for data normalization in this experiment because it can be changed under certain conditions [Maran et al., 2004].
RESULTS
Cluster Analyses
Compared to non-fractured controls, 234 (2.9%) genes/transcribed sequences were differentially expressed at post-fracture day 1, 225 (2.8%) at day 2, and 293 (3.7%) at day 4, which is a total of 752. Of the 752 genes/transcribed sequences, there were 411 unique. The complete lists for these three sets of genes as well as dCHIP exported full data set were published as supporting information at http://fgf.bsd.uchicago.edu/jcb. The 411 unique genes were used for subsequent cluster analysis. Cluster analysis identified several distinct expression patterns (Fig. 1). We characterized each of those patterns in relation to their function (Fig. 2). In the functional characterization, we intentionally used the same categories (wherever possible) for different gene clusters in order to reveal temporal changes in molecular activity.
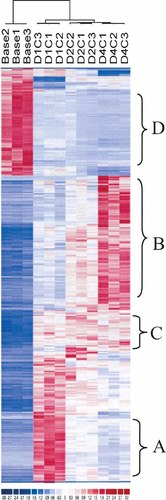
Cluster analysis of gene expression profiles. The total number of 411 genes/transcribed sequences was used for the analysis. These genes/transcribed sequences all passed our specified thresholds for differential expression as described in Methods in at least one of the time points. The result shows four major classes of gene expression patterns: A, B, C, and D. These patterns are magnified and discussed further in Figure 2.

Temporal gene expression patterns and gene classifications. A: This cluster represents immediate-response genes, and is significantly enriched in genes with binding activity, energy derivation, transporter activity, catalytic activity, and muscle development/cytoskeleton (p < 0.05). B: This cluster represents genes with continuously increased expression, and is dominated by bone formation- and matrix-related genes. C: This cluster represents genes with constantly increased expression, and is enriched with genes that are also upregulated in cancer tissues (42% of known genes). D: This cluster represents constantly suppressed genes, and is dominated by immune response genes.
1. Immediate-response genes (Group A)
We defined immediate-response genes as those significantly upregulated post-fracture day 1 (>threefold) but that quickly returned to baseline thereafter. Fifty-two genes/transcribed sequences belong to this category (Fig. 2A), 10 of which are transcribed sequences. The remaining 42 known genes fall into five broad functional categories: (i) binding activity representing the largest group (52%). ATP-binding genes are particularly prominent in this group, including cell division cycle 2 homolog A, topoisomerase 2 alpha, serum/glucocorticoid regulated kinase, ATPases (Ca++ and Na+K+ transporting), and myosin heavy chain polypeptide 6 and 7; (ii) transporter activity (16%). Genes in this group primarily involve ion transportation, such as cytochrome c oxidase subunit VIII-H and Via, ATPase Ca++, Na+, and K+ transporting, and calcium channel alpha 1S subunit; (iii) energy derivation and shuttle (11%) including enolase 3 beta, sarcomeric mitochondrial creatine kinase, phosphofructokinase, lactate dehydrogenase B, and aldolase A; (iv) catalytic activity (30%); and (v) muscle development/cytoskeleton (25%). The number of genes included in each of the functional categories is statistically significantly enriched within this group of genes (P < 0.01). Early-response genes seem unevenly distributed on chromosomes. Over 20% of known genes in this group are localized on Chromosome 1q, which is significantly higher than a random event (P < 0.05).
2. Continuously increased genes (Group B)
This group contains 77 genes/transcribed sequences (Fig. 2B). The most obvious characteristic of this group is the bone repair activity related genes. Thirty-five of 60 known genes (58%) are well-known bone formation- and matrix-related genes including IGF-I, PGDFR, FGFR, fibronectin, glypican, biglycan, osteomodulin, osteonectin, tenascin C, procollagens, collagens, and matrix metalloproteinases. Binding and catalytic activities are drastically diminished, accounting for 13% and 15% of known genes, respectively, compared with 52% and 30% in the early-response gene cluster. Many others have no reported functions in bone, such as angiotensin receptor, sushi-repeat-containing protein, and anti-quitin or no defined function including 17 transcribed sequences.
3. Constantly upregulated genes (Group C)
This group includes 47 genes/transcribed sequences (Fig. 2C) falling into several functional groups (binding activity, transporter activity, skeletal muscle and bone matrix gene activity, and catalytic activity). Different from the early-response and continuously increased gene clusters, none of the listed functional groups dominates this cluster, while many of those genes regulate general cell growth and maintenance, including cholinergic receptor, urinary plasminogen activator, prostaglandin-endoperoxide synthase 2, outer mitochondrial membrane receptor rTOM20, and calcium channel alpha2/delta subunit 1. Noticeably, there are 42% of known genes in this group that are also upregulated in various cancer tissues. This percentage is significantly higher than that in other gene clusters.
4. Constantly suppressed genes (Group D)
Fifty-three genes/transcribed sequences belong to this category (Fig. 2D), 50% of which are immune responsive genes with particular enrichment of immunoglobulin genes, such as kappa and lambda light chains, and alpha and mu heavy chains. Genes with binding, transporter, and catalytic activities account for 11%, 11%, and 15%, respectively.
Gene Family Analyses
To complement the above generalized analysis approach, we further examined differential gene expression after fracture across members of five gene families (IGF, TGFβ, PDGF, FGF, and BMP) that are known to be involved in bone healing (Table I). Given the fact that twofold changes of many of these signaling molecules can have substantial effects on bone healing, we relaxed our selection criteria in this analysis to ≥twofold difference and P < 0.05 at least at one time point. Consistent with literatures, many members of these families are upregulated after fracture including IGF-I, IGF-II, BMP2, FGFR1, and PDGF A chain. Notably, almost all members of IGF family are elevated (some of those are not statistically significant, thus not listed in Table I, such as IGF-IR, IGFBP2, and IGFBP5). TGFβ3 is continuously upregulated while TGFβ1 is significantly downregulated at day1 and returned to baseline at day 4.
| Family | Gene name | Accession | D1/Ba | P-value | D2/B | P-value | D4/B | P-value |
|---|---|---|---|---|---|---|---|---|
| IGF | IGF-I | X06107 | 6.9 | ns | 16.8 | 0.014 | 89.65 | 0.010 |
| IGF-II | X17012 | 3.1 | 0.001 | 2.0 | 0.012 | 2.68 | 0.015 | |
| IGF-IIR | U59809 | 2.2 | 0.02 | 4.0 | 0.001 | 4.21 | 0.005 | |
| IGFBP1 | M58634 | 3.3 | ns | 2.2 | ns | 3.6 | 0.043 | |
| IGFBP3 | M31837 | 6.3 | 0.037 | 7.9 | ns | 3.82 | 0.018 | |
| IGFBP6 | M69055 | 5 | 0.032 | 4.1 | 0.002 | 8.78 | 0.031 | |
| TGF | TGFb1 | X52498 | −2.5 | 0.002 | −1.6 | 0.022 | 1 | ns |
| TGFb3 | U03491 | 2.1 | 0.05 | 3.5 | 0.005 | 9 | 0.028 | |
| PDGF | PDGFR alpha | AI232379 | 3.8 | 0.001 | 5.1 | 0.003 | 8.2 | 0.000 |
| PDGF A chain | D10106 | 1.9 | 0.009 | 1.8 | 0.017 | 2 | 0.030 | |
| FGF | FGFR1 | S54008 | 5 | 0.014 | 5.8 | 0.003 | 10.7 | 0.000 |
| FGF14 | AB008908 | 1.8 | 0.05 | 3.0 | 0.001 | 1.6 | 0.018 | |
| FGF5 | D64085 | −1.8 | 0.028 | −2.1 | 0.02 | −1.1 | ns | |
| BMP | BMP3 | D63860 | 1.1 | ns | −1.2 | ns | −2.7 | 0.049 |
| BMP2 | L20678 | 4.8 | 0.008 | 1.8 | ns | 1.6 | ns | |
| BMPR type 1A | S75359 | 2 | ns | 3.1 | 0.01 | 2.8 | 0.006 |
- a Post-fracture day 1/no fractured control (baseline).
Gene Ontology Analyses
To group genes into functional categories, we performed GO analyses using each of the three gene lists differentially expressed at post-fracture day 1, 2, and 4, respectively. As shown in Table II, 6 of 27 significant GO terms (P < 0.001) are unique for day 1. Genes associated with these unique GO terms are dominated by ion transporter activity. The remaining 21 are common between day 1 and day 2 including cytoskeleton organization and biogenesis, inflammatory response, muscle development, and actin cytoskeleton. Nine out of the 17 GO terms are unique for day 4. Genes representing those unique GO terms are largely involved in calcium binding, cell adhesion, and bone structural proteins. Morphogenesis and organogenesis represent two large GO terms and are common across three-time points. To relate the differential expression to functional significance at protein level, we also identified and compared significant protein domains (P < 0.001) from each of the three gene lists (Table III). Consistent with the GO analysis, bone matrix-related protein domains and calcium binding domain were abundantly identified at day 4.
| Gene ontology | Number of genes (P-Value) | ||
|---|---|---|---|
| PFa day 1 | PF day 2 | PF day 4 | |
| Ossification | 3 (0.0007) | ||
| Cytochrome c oxidase activity | 4 (0.0009) | ||
| Cation transporter activity | 9 (0.0002) | ||
| Heme-copper terminal oxidase activity | 4 (0.0009) | ||
| Ion transporter activity | 10 (0.0003) | ||
| Bone remodeling | 3 (0.0008) | ||
| Cytoplasm | 21 (0.0001) | 24 (0.000003) | |
| Cytoskeleton | 10 (0.00002) | 10 (0.00002) | |
| Non-muscle myosin | 4 (0.0002) | 5 (0.000007) | |
| Chemotaxis | 4 (0.00001) | 4 (0.00001) | |
| Muscle contraction | 5 (0.0006) | 5 (0.0006) | |
| Inflammatory response | 5 (0.0002) | 5 (0.0001) | |
| Organelle organization and biogenesis | 8 (0.0002) | 8 (0.0002) | |
| Cytoskeleton organization and biogenesis | 7 (0.00003) | 7 (0.00003) | |
| Development | 13 (0.00001) | 11 (0.0003) | |
| Muscle development | 7 (0.000002) | 6 (0.00002) | |
| Chemokine activity | 4 (0.0007) | 4 (0.0007) | |
| Response to wounding | 5 (0.0006) | 5 (0.0006) | |
| Actin cytoskeleton | 7 (0.000003) | 8 (0.00000) | |
| Myosin | 4 (0.0002) | 5 (0.000007) | |
| Chemoattractant activity | 4 (0.0007) | 4 (0.0007) | |
| Chemokine receptor binding | 4 (0.0007) | 4 (0.0007) | |
| Innate immune response | 5 (0.0002) | 5 (0.0002) | |
| Response to chemical substance | 4 (0.00004) | 4 (0.00004) | |
| Taxis | 4 (0.00001) | 4 (0.00001) | 3 (0.0007) |
| Morphogenesis | 12 (0.000004) | 10 (0.0001) | 10 (0.0004) |
| Organogenesis | 12 (0.000004) | 10 (0.0001) | 10 (0.0004) |
| Motor activity | 6 (0.0001) | ||
| Endopeptidase inhibitor activity | 6 (0.0009) | ||
| Intracellular | 29 (0.0005) | ||
| Protease inhibitor activity | 6 (0.0009) | ||
| Extracellular matrix structural constituent | 6 (0.00003) | 9 (0.000000) | |
| Extracellular | 8 (0.0004) | 16 (0.000000) | |
| Extracellular matrix | 6 (0.0002) | 13 (0.000000) | |
| Metalloendopeptidase inhibitor activity | 3 (0.0002) | 4 (0.000006) | |
| Metal ion binding | 13 (0.0009) | 19 (0.000002) | |
| Globin | 4 (0.000006) | ||
| Structural molecule activity | 15 (0.00002) | ||
| Calcium ion binding | 15 (0.00003) | ||
| Collagen | 4 (0.00004) | ||
| Basement membrane | 4 (0.0001) | ||
| Chemotaxis | 3 (0.0007) | ||
| Cell adhesion | 9 (0.0001) | ||
| Muscle development | 5 (0.0006) | ||
| Oxidoreductase activity | 3 (0.0007) | ||
- a Post-fracture.
| Protein domains | Number of genes (P-Value) | ||
|---|---|---|---|
| PF day 1 | PF day 2 | PF day 4 | |
| Myogenic basic muscle-specific protein | 2 (0.0009) | ||
| Crystallin, N-terminal | 3 (0.0008) | ||
| Small chemokine, C-C subfamily | 3 (0.0008) | 3 (0.0008) | |
| Small chemokine, interleukin-8 like | 4 (0.0008) | 4 (0.0006) | |
| Myosin tail | 6 (0.00002) | 6 (0.00001) | |
| Myosin N-terminal SH3-like domain | 4 (0.00008) | 5 (0.000002) | |
| Alpha crystallin | 6 (0.000000) | 5 (0.00001) | 4 (0.0006) |
| Heat shock protein Hsp20 | 6 (0.000001) | 5 (0.00002) | 4 (0.0009) |
| IQ calmodulin-binding region | 6 (0.0006) | ||
| Myosin head motor domain | 6 (0.000009) | ||
| Tissue inhibitor of metalloproteinase | 3 (0.0002) | ||
| Cysteine-rich flanking region, N-terminal | 5 (0.0007) | 9 (0.000000) | |
| Leucine-rich repeat, typical subtype | 5 (0.00006) | 8 (0.000000) | |
| Leucine-rich repeat | 9 (0.00001) | ||
| Type II fibronectin collagen-binding domain | 4 (0.0006) | ||
| Hemopexin repeat | 4 (0.0004) | ||
| Fibrillar collagen, C-terminal | 8 (0.000000) | ||
| Globin | 4 (0.000002) | ||
| von Willebrand factor, type C | 5 (0.0007) | ||
| Matrixin | 5 (0.0002) | ||
| Tissue inhibitor of metalloproteinase | 4 (0.000008) | ||
| Osteonectin-like | 3 (0.0008) | ||
| Calcium-binding EF-hand | 14 (0.000003) | ||
| Follistatin-like, N-terminal | 4 (0.0006) | ||
| Actin/actin-like | 4 (0.0003) | ||
| Neutral zinc metallopeptidase | 5 (0.00006) | ||
| Collagen triple helix repeat | 9 (0.000000) | ||
Common Signals Between Bone Regeneration And Tumorigenesis
A fundamental common characteristic between bone regeneration and tumorigenesis is the rapid tissue growth. Thus, we hypothesized that two processes might share some common molecular signals regulating cell migration, invasion, adhesion, and proliferation. We used “differential expression in cancer” as a term to search PubMed and identified 61 upregulated and 3 downregulated genes in both bone healing and tumor tissues (Table IV. This Table does not represent a comprehensive list, only represents a sample of the common signals). Over 50% of those commonly upregulated genes are ECM metabolism-related genes, including collagen type V, fibronectin 1, osteonectin, procollagen type XI, matrix metalloproteinase 2, fibromodulin, matrix Gla protein, and some growth factors/cytokines. Many of those genes have become targets for anti-cancer drugs (such as FGFR, PDGFR, Cyclin B1, interleukin 18, thrombospondin 4, and matrix metalloproteinases). Protein kinase C beta 1, topoisomerase DNA 2 alpha, matrix metalloproteinase 9, cyclin B1, rhoB, and decorin were differentially expressed in an opposite direction between tumor and healing, suggesting a difference between the controlled growth in bone healing and uncontrolled growth in cancer. To make the comparisons more biologically meaningful, we further examined differentially expressed genes in both healing bone and bone tumors (Table V). Among the limited number of relevant publications in bone tumors, we identified 17 common genes that are differentially expressed in both processes. Of those, 13 genes are commonly upregulated; fibronectin 1 and biglycan are upregulated in healing bone but downregulated in osteosarcoma; in contrast, stathmin 1 and matrix metalloproteinase 9 are downregulated in healing bone but upregulated in bone tumors.
| Gene name | Accession | D1/B | P-value | D2/B | P-value | D4/B | P-value | In cancer | References |
|---|---|---|---|---|---|---|---|---|---|
| Serine (or cysteine) proteinase inhibitor | M69246 | 3.47 | 0.039 | 5.59 | 0.013 | 7.21 | 0.002 | Up |
Tackels-Horne et al., 2001 |
| Collagen, type V, alpha 2 | AI179399 | 4.34 | 0.007 | 6.37 | 0.000 | 8.3 | 0.000 | Up |
Tackels-Horne et al., 2001 |
| Caveolin | Z46614 | 3.28 | 0.125 | 3.94 | 0.037 | 7.68 | 0.041 | Up |
Tahir et al., 2001 |
| Plasminogen activator inhibitor-1 (PAI-1) | M24067 | 16.56 | 0.006 | 10 | 0.011 | 5.5 | 0.026 | Up |
Kataoka et al., 2002 |
| Tissue factor pathway inhibitor | D10926 | 1.38 | 0.056 | 1.6 | 0.008 | 2.51 | 0.006 | Up |
Kataoka et al., 2002 |
| Fibroblast growth factor receptor 1 | S54008 | 2.79 | 0.015 | 3.01 | 0.003 | 5.27 | 0.000 | Up |
Cronauer et al., 2003 |
| Urinary plasminogen activator, urokinase | X63434 | 4.54 | 0.002 | 4.25 | 0.023 | 6.23 | 0.003 | Up |
Steinmetzer, 2003 |
| Plasminogen activator, tissue | M23697 | 3.93 | 0.001 | 5.96 | 0.003 | 7.64 | 0.007 | Up |
Steinmetzer, 2003 |
| Prostaglandin-endoperoxide synthase 2 | S67722 | 4.42 | 0.009 | 5.07 | 0.010 | 3.51 | 0.003 | Up |
Lee et al., 2003 |
| Insulin-like growth factor 1 | D00698 | 1.19 | 0.216 | 2.1 | 0.037 | 7.85 | 0.046 | Up |
van der Poel, 2004 |
| PDGF receptor alpha | AI232379 | 2.85 | 0.002 | 3.85 | 0.005 | 6.04 | 0.000 | Up |
van der Poel, 2004 |
| Tissue inhibitor of metalloproteinase 1 | AI169327 | 8.29 | 0.000 | 7.33 | 0.000 | 6.59 | 0.004 | Up |
Gerritsen et al., 2002 |
| Laminin chain beta 2 | AI104225 | 4.39 | 0.007 | 6.95 | 0.005 | 7.07 | 0.024 | Up |
Gerritsen et al., 2002 |
| Fibronectin 1 | X05834 | 2.89 | 0.000 | 3.21 | 0.000 | 3.44 | 0.002 | Up |
Gerritsen et al., 2002 |
| Thrombospondin 4 | X89963 | 10.89 | 0.026 | 8.5 | 0.005 | 7.5 | 0.000 | Up |
Gerritsen et al., 2002 |
| Osteonectin | U75929 | 1.46 | 0.298 | 2.5 | 0.074 | 4.1 | 0.012 | Up |
Gerritsen et al., 2002 |
| Procollagen, type XI, alpha 1 | AJ005396 | 1.84 | 0.118 | 4.5 | 0.050 | 9.64 | 0.005 | Up |
Iyengar et al., 2003 |
| Matrix metalloproteinase 2 | U65656 | 2.25 | 0.009 | 2.72 | 0.001 | 4.1 | 0.003 | Up |
Iyengar et al., 2003 |
| Tenascin C | U09401 | 4.48 | 0.205 | 6.31 | 0.094 | 13.64 | 0.037 | Up |
Watanabe et al., 2003 |
| Integrin alpha 7 | X65036 | 6.55 | 0.009 | 8.06 | 0.007 | 5.66 | 0.007 | Up |
Kramer et al., 1991 |
| Lumican | X84039 | 1.94 | 0.036 | 3.79 | 0.020 | 3.53 | 0.000 | Up |
Leygue et al., 2000 |
| Fibromodulin | X82152 | 8.78 | 0.042 | 9.48 | 0.007 | 11.94 | 0.017 | Up |
Jelinek et al., 2003 |
| Matrix Gla protein | AI012030 | 5.73 | 0.010 | 5.52 | 0.008 | 4.34 | 0.000 | Up |
Hough et al., 2001 |
| Cathepsin L | AI176595 | 4.09 | 0.000 | 4.62 | 0.000 | 3.9 | 0.006 | UP |
Zajc et al., 2002 |
| Cyclin D1 | D14014 | 1.7 | 0.022 | 2.91 | 0.006 | 2.73 | 0.001 | Up |
Rowlands et al., 2004 |
| Inhibitor of DNA binding 1 | L23148 | 2.99 | 0.001 | 2.91 | 0.002 | 2.13 | 0.002 | Up |
Sikder et al., 2003 |
| Inhibitor of DNA binding 3 | AI171268 | 3.15 | 0.009 | 3.98 | 0.033 | 3.56 | 0.000 | Up |
Vandeputte et al., 2002 |
| Smooth muscle alpha-actin | AA900769 | 2.31 | 0.002 | 4.7 | 0.000 | 4.79 | 0.008 | Up |
Sharma et al., 2003 |
| Crystallin, alpha B | M55534 | 29.05 | 0.019 | 26.63 | 0.003 | 14.66 | 0.021 | Up |
Andley et al., 2001 |
| Cystatin B | AI008888 | 2.98 | 0.008 | 3.54 | 0.000 | 3.19 | 0.021 | Up |
Strojan et al., 2001 |
| Early growth response 1 | AF023087 | 3.05 | 0.094 | 3.85 | 0.016 | 3.78 | 0.000 | Up |
Kobayashi et al., 2002 |
| Transgelin Smooth muscle 22 protein | M83107 | 2.5 | 0.021 | 5.85 | 0.004 | 9.3 | 0.004 | Up |
Ryu et al., 2003 |
| Cytochrome P450, 1B1 | AI176856 | 2.73 | 0.006 | 3.4 | 0.000 | 2.88 | 0.002 | Up |
Chun and Kim, 2003 |
| Interleukin 18 | U77777 | 2.59 | 0.047 | 4.13 | 0.001 | 3.4 | 0.044 | Up |
Riedel et al., 2004 |
| Interferon gamma receptor | U68272 | 2.32 | 0.011 | 2.42 | 0.023 | 2.76 | 0.000 | Up |
Royuela et al., 2000 |
| Glycoprotein 38 | U92081 | 4.08 | 0.001 | 3.59 | 0.001 | 4.59 | 0.001 | Up |
Li et al., 1996 |
| LPS-induced TNF-alpha factor | U53184 | 2.8 | 0.000 | 2.74 | 0.001 | 2.81 | 0.001 | Up |
Cao et al., 1999 |
| Chemokine C-C motif) ligand 3 | U22414 | 8.41 | 0.008 | 6.26 | 0.009 | 2 | 0.254 | Up |
Terpos et al., 2003 |
| Apolipoprotein E | X04979 | 1.19 | 0.215 | 2.18 | 0.021 | 2.79 | 0.000 | Up |
Hough et al., 2001 |
| Interleukin 6 interferon, beta 2 | M26744 | 21.3 | 0.007 | 20.45 | 0.026 | 8.59 | 0.029 | Up |
Leu et al., 2003 |
| Secreted phosphoprotein 1 | M14656 | 6.21 | 0.000 | 7.73 | 0.012 | 5.93 | 0.005 | Up |
Ariztia et al., 2003 |
| Mitochondrial NADH dehydrogenase | M22756 | 2.52 | 0.003 | 1.81 | 0.028 | 1.56 | 0.042 | Up |
Oien et al., 2003 |
| GADD 45 alpha | AI070295 | 3.89 | 0.029 | 2.1 | 0.027 | 1 | 0.998 | Up |
Chen et al., 2002 |
| Superoxide dismutase 2 | Y00497 | 3.84 | 0.000 | 3.6 | 0.003 | 2.13 | 0.018 | Up |
Plymate et al., 2003 |
| Sarcomeric mitochondrial creatine kinase | X59736 | 93.4 | 0.007 | 36.88 | 0.006 | 14.66 | 0.000 | Up |
Okano et al., 1987 |
| Nuclear protein 1 | AF014503 | 3.22 | 0.037 | 4.01 | 0.030 | 5.74 | 0.000 | Up |
Iovanna, 2002 |
| Amyloid beta A4 precursor protein | X07648 | 1.88 | 0.017 | 1.79 | 0.034 | 2.67 | 0.000 | Up |
Kataoka et al., 2002 |
| Coagulation factor 3 | U07619 | 6.99 | 0.006 | 7.13 | 0.002 | 6 | 0.014 | Up |
Akashi et al., 2003 |
| RAB11a, member RAS oncogene family | M75153 | 1.96 | 0.006 | 2.44 | 0.001 | 2.24 | 0.024 | Up |
Adjei, 2001 |
| Heat shock protein 70 | Z75029 | 4.29 | 0.002 | 5.09 | 0.043 | 2.04 | 0.029 | Up |
Kao et al., 2003 |
| Jun B proto-oncogene | AA891041 | 4.3 | 0.001 | 3.68 | 0.005 | 2.58 | 0.001 | Up |
Casas et al., 2003 |
| v-myc homolog | Y00396 | 2.94 | 0.004 | 2.47 | 0.033 | 1.67 | 0.078 | Up |
Tselepis et al., 2003 |
| Diaphorase 1 | D00636 | 1.31 | 0.241 | 1.61 | 0.075 | 2.63 | 0.028 | Up |
Leerkes et al., 2002 |
| Protein phosphatase 1 | J05592 | 2.93 | 0.001 | 1.56 | 0.007 | -1.01 | 0.960 | Up |
Leerkes et al., 2002 |
| Catenin cadherin-associated protein | L24897 | 11.66 | 0.001 | 8.34 | 0.023 | 5.77 | 0.015 | Up |
Leerkes et al., 2002 |
| Heat shock 27kDa protein 1 | AA998683 | 46.16 | 0.008 | 31.02 | 0.008 | 15.82 | 0.043 | Up |
Leerkes et al., 2002 |
| Glutathione peroxidase 3 | D00680 | 1.81 | 0.009 | 2.18 | 0.001 | 2.56 | 0.001 | Up |
Hough et al., 2001 |
| Tumor-associated antigen 1 | L12025 | 15.36 | 0.018 | 6.24 | 0.000 | 4.6 | 0.009 | Up |
Ito et al., 2003 |
| Phosphodiesterase 4B | AA799729 | 7.05 | 0.000 | 5.08 | 0.001 | 4.31 | 0.001 | Up |
Jiang et al., 1998 |
| Creatine kinase | M10140 | 19.57 | 0.000 | 10.23 | 0.000 | 6.81 | 0.004 | Up |
Joshi et al., 2003 |
| CD14 antigen | AF087943 | 2.61 | 0.002 | 3.64 | 0.012 | 3.24 | 0.000 | Up |
Deininger et al., 2003 |
| Immunoglobulin alpha heavy chain | AI234828 | -9.69 | 0.000 | -14 | 0.000 | -22.8 | 0.001 | Down |
Oien et al., 2003 |
| Lipocalin 2 | AA946503 | -3.32 | 0.002 | -7.61 | 0.002 | -9.91 | 0.005 | Down |
Oien et al., 2003 |
| Ig productively rearranged lambda chain | AI234351 | -8.52 | 0.046 | -9.86 | 0.044 | -13 | 0.042 | Down |
Oien et al., 2003 |
| Protein kinase C, beta 1 | X04139 | -4.28 | 0.004 | -3.38 | 0.004 | -1.66 | 0.015 | Up |
Koren et al., 2004 |
| Topoisomerase DNA 2 alpha | AA899854 | -7.92 | 0.005 | -1.36 | 0.067 | -1.5 | 0.038 | Up |
Skotheim et al., 2003 |
| Matrix metalloproteinase 9 | U24441 | -4.76 | 0.011 | -5.5 | 0.004 | -5.83 | 0.008 | Up |
Gerritsen et al., 2002 |
| Cyclin B1 | AA998164 | -8.14 | 0.002 | -2.12 | 0.003 | -1.73 | 0.005 | Up |
Leerkes et al., 2002 |
| rhoB gene | AA900505 | 3.81 | 0.000 | 3.75 | 0.005 | 3.86 | 0.003 | Down |
Wang et al., 2003 |
| Decorin | Z12298 | 5.28 | 0.002 | 5.71 | 0.000 | 6.43 | 0.000 | Down |
Leygue et al., 2000 |
| Gene name | Accession | D1/B | P-value | D2/B | P-value | D4/B | P-value | In cancer | Type of cancer | References |
|---|---|---|---|---|---|---|---|---|---|---|
| HSP 90-beta | AA685903 | 3.3 | 0.19 | 3.7 | 0.09 | 5.5 | 0.04 | Up | Osteosarcoma |
Wolf et al., 2000 |
| Heat shock 27 kDa protein 1 | AA998683 | 46.16 | 0.008 | 31.02 | 0.008 | 15.82 | 0.043 | Up | Osteosarcoma |
Uozaki et al., 1997 |
| Fibronectin 1 | X05834 | 2.89 | 0.000 | 3.21 | 0.000 | 3.44 | 0.002 | Down | Osteosarcoma |
Wolf et al., 2000 |
| Stathmin 1 | AI231821 | −3.57 | 0.010 | −1.33 | 0.100 | −1.3 | 0.100 | Up | Osteosarcoma |
Zhang et al., 2004 |
| Matrix Gla protein | AI012030 | 5.73 | 0.010 | 5.52 | 0.008 | 4.34 | 0.000 | Up | Osteosarcoma |
Leonard et al., 2003 |
| Caldesmon 1 | AI180288 | 1.66 | 0.260 | 3.04 | 0.160 | 4.94 | 0.030 | Up | Osteosarcoma |
Leonard et al., 2003 |
| Collagen, type I, alpha 2 | AF050214 | 3.24 | 0.070 | 5.7 | 0.003 | 11.6 | 0.006 | Up | Osteosarcoma |
Leonard et al., 2003 |
| Biglycan | AA859830 | 1.78 | 0.030 | 3.47 | 0.001 | 4.6 | 0.004 | Down | Osteosarcoma |
Benayahu et al., 2001 |
| Peripheral myelin protein 22 | S55427 | 4.09 | 0.005 | 4.53 | 0.000 | 5.45 | 0.006 | Up | Osteosarcoma |
van Dartel and Hulsebos, 2004 |
| c-myc oncogene | Y00396 | 2.94 | 0.004 | 2.47 | 0.033 | 1.67 | 0.078 | Up | Osteosarcoma |
Gamberi et al., 1998 |
| COX-2 | S67722 | 4.4 | 0.009 | 5.1 | 0.010 | 3.5 | 0.003 | Up | Chondrosarcoma |
Sutton et al., 2004 |
| Decorin | Z12298 | 5.28 | 0.002 | 5.71 | 0.000 | 6.43 | 0.000 | Up | Chondrosarcoma |
Soderstrom et al., 2002 |
| Tissue inhibitor of metalloproteinase 2 | 29543 | 2.23 | 0.003 | 5.03 | 0.000 | 6.7 | 0.001 | Up | Chondrosarcoma |
Soderstrom et al., 2001 |
| Matrix metalloproteinase 13 | M60616 | 3.54 | 0.001 | 4.45 | 0.040 | 2.1 | 0.010 | Up | Chondrosarcoma |
Soderstrom et al., 2001 |
| Matrix metalloproteinase 14 | X83537 | 1.9 | 0.110 | 2.35 | 0.070 | 4.98 | 0.004 | Up | Chondrosarcoma |
Soderstrom et al., 2001 |
| Matrix metalloproteinase 2 | U65656 | 2.25 | 0.009 | 2.72 | 0.001 | 4.1 | 0.003 | Up | Chondrosarcoma |
Soderstrom et al., 2001 |
| Matrix metalloproteinase 9 | U24441 | −4.76 | 0.011 | −5.5 | 0.004 | −5.83 | 0.008 | Up | Giant cell tumor |
Kumta et al., 2003 |
Validation of Microarray Data
Verification of microarray-based differential gene expression was examined on 10 representative genes by using quantitative real time RT-PCR. Seven of those were upregulated and three downregulated based on Microarray results. Figure 3 compared the relative fold changes of these genes with the two methods of measurement. All genes showed highly concordant quantitative measurements with the direction of differential expression.
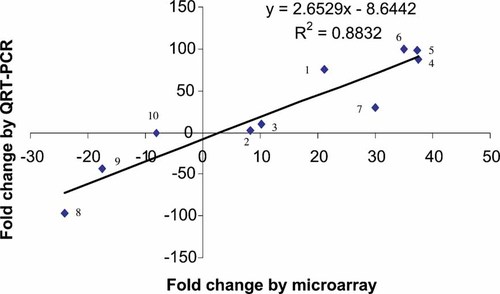
Comparison of differential gene expression determined by quantitative RT-PCR and Affymetrix GeneChip. χ and Y axes represent fold change (post-fracture day 1 vs. baseline control) determined by microarray and QRT-PCR, respectively. (1) Interleukin 6, (2) tissue inhibitor of metalloproteinase 1, (3) myosin, light polypeptide 2, (4) enolase 3, beta, (5) actinin alpha 2 associated LIM protein, (6) cytochrome c oxidase subunit VIII-H, (7) ankyrin-like repeat protein, (8) mast cell protease 10, (9) mast cell protease 8, and (10) Ig non-productively rearranged lambda-chain.
DISCUSSION
Although rapid progress in skeletal cellular and molecular biology has led to the identification of many signaling molecules associated with bone fracture healing, we still lack a global molecular picture underlining early fracture healing. This study was designed to address this issue by investigating global changes in gene expression that occur during the critical phases of early fracture repair. We focused on early molecular events occurring in the first 4 days of fracture healing for two reasons. First, it has not been systematically investigated before, and secondly, early events set the foundation for overall healing, thus representing the most critical stage for therapeutic intervention. Post-fracture day 1 represents an initial inflammatory phase, which is characterized by wound site preparation and clean up. Post-fracture day 4 represents the onset of inflammation and initiation of intramembranous ossification. Post-fracture day 2 is a transitional stage between the two. Although these three time points broadly correspond to the inflammatory stage, cluster analysis revealed dynamic temporal expression changes, which reflect stage-specific functional needs for bone healing.
The first event after fracture is bleeding from the damaged bone end. The accumulated blood forms a clot that fills the space between the fracture surfaces. Then, an acute inflammatory response begins, and inflammatory cells invade the soft tissues surrounding the fracture site, which initiate a full scale preparation for bone repair. Immediate-response genes (Group A), which accounted for 25% of differentially expressed genes at day 1 coincide with the initial wound site preparation, thus, a functionally representative group of genes at this early inflammatory phase. Significant enrichment of genes with energy derivation, transporter, and binding activities suggests that the molecular sense of wound site preparation is primarily a process of energy accumulation and molecular talking by means of ion transport, catalytic reactions, and DNA–DNA, DNA–protein, and protein–protein bindings. This kind of communication marks a major feature for the initial signaling of healing.
Transient activation of this group of genes is also consistent with the concept that wound site preparation is a rapid process. Immediate activation after injury satisfies the wound site preparation's requirement for specific messengers. Quick deactivation after they have completed their function may be as important as the timing of activation because continuous activation of this group of genes could unnecessarily waste energy or may be harmful for the later stage of healing. Although tight regulation of this group of genes appears critical for signaling wound healing. We know little about their precise roles as evidenced by the finding that over 22% of this group of genes are still considered as “transcribed sequences” and lack functional assignment. In addition, the functions of many other known genes in this group have never been described in bone healing.
As the inflammatory phase comes to a close, macrophages, fibroblasts, and endothelial cells move into the fracture space where fracture callus forms. Expression of the group of genes that were continuously increased (Group B) reached a peak at this stage. Functional analysis suggests that the protein products derived from this group of genes are engaged in repair. Sixty-percent of known genes in this group are functionally related to growth, cytoskeleton, and matrix-related gene activity. Many of those genes correlate well with the biological events of callus formation including cartilage collagen types VI and XI, fibronectin, growth factor/receptors IGF-1, FGFR-1, PDGF-α, while some others appears related to bone formation, such as bone collagen types I, V, VI, and XII, glypican, tenascin, and osteomodulin. These results are highly consistent with those reported by Hadjiargyrou et al. [2002]. Various members of the metalloproteinases are also upregulated, including metalloproteinase 2, 14, and 23, each of which cleaves a specific subset of matrix proteins. Surprisingly, metalloproteinase 9 was consistently downregulated over fourfold post-fracture days 1, 2, and 4. Metalloproteinase 9 can cleave basal lamina collagen (type IV) and anchoring fibril collagen (type VII), and was reported to be upregulated in the previous studies [Martin, 1997; Hadjiargyrou et al., 2002]. All these activities suggest that full-scale bone repair processes have been launched at post-fracture day 4. This may include two simultaneous cellular cascades, one involving the formation of cartilage at the site of the hematoma, and the other involving new bone formation at the fracture surface. Consistent with the above, structural molecular activity was identified as one of the largest unique GO groups at day 4. we have noticed that observations made by GO analyses only partially agree with the functionally characterized gene groups in Figure 2. This could be because the genome is incompletely annotated and/or that GO terms are biased in favor of the more historically popular molecules and processes.
Comparing the gene classifications between immediate-response genes and those that continuously increased, there appears to be a major functional shift. The function of immediate-response genes is largely centered on binding activities in coordination with characteristic energy derivation and ion transporter activities. We view these activities as a unique form of communication to inform neighboring cells of the trauma and to initiate healing. In contrast, continuously increased genes are primarily focused on repair, where energy derivation and transporter activities almost disappeared, binding activities were significantly reduced and bone repair activities become dominant.
Focused analysis on five gene families that are known to play a vital role in bone healing revealed elevated expression of many members. Particular attention points to the IGF family, in which almost all members included on the Rat U34A array showed an upregulation after fracture. This systematic reaction to fracture suggests that IGF represent one of the most active families during the early stage of bone healing. Three isoforms of TGFβ exhibited a different expression profile: TGFβ3 continuously increased after fracture, TGFβ2 had no significant change (though slightly upregulated), but TGFβ1 showed a 2.5-fold decrease (P < 0.05) at post-fracture day 1 and returned to baseline at day 4. These differential expression profiles indicate specificity of their functions in bone healing. TGFβ 1 has been showed to promote bone healing in numerous publications. The suppression of TGFβ 1 expression immediately after fracture is a novel finding and its functional significance remains investigation. It is interesting to see if TGFβ 1 is upregulated at the later stage of healing. Increased expression of osteogenic BMP2 and decreased expression of inhibitory-osteogenic BMP3 agree with their biological roles during the early stage of bone healing.
The early stage of bone healing involves complex cellular processes including cell migration, invasion, adhesion, and proliferation. These processes closely resemble cellular events in malignant tissues. Of the 61 commonly upregulated genes between tumor and bone healing, ECM metabolism-related genes represent a dominant group. This extensive similarity in the expression of ECM metabolism-related genes between two processes opens a possibility to use the gene expression profile during early healing as a tool to advance our understanding of carcinogenesis. Distribution of those commonly upregulated genes across gene clusters is biased. The constantly increased gene cluster (Group C) contains over 42% of the known genes also upregulated in various cancer tissues in contrast to 11% in the immediate-response gene cluster (Group A) and 22% in the continuously increased gene cluster (Group B). Furthermore, a significant number of commonly upregulated genes in the constantly increased gene cluster are ECM metabolism-related. This group of genes mimics the ECM metabolism-related activities in cancer tissues. The ECM dominance is also reflected in the direct comparisons between healing bone and bone tumors. It is important to remember that the ECM represents a well-studied group of genes in the literature. Given this literature bias, the ECM dominance in this comparison dose not imply that the ECM will shed more light on carcinogenesis than some other less studied molecules. Nevertheless, this initial observation necessitates further characterization of this potentially important relationship using expression data derived from one particular cancer tissue.
It is a surprising finding that a large number of immune response genes were constantly downregulated after fracture, including immunoglobulin kappa and lambda light chains, and alpha and mu heavy chains. One possible explanation is that decreased expression of immunoglobulin genes was a reflection of the reduction of B-cell population at the fracture site, which was most likely caused by a different amount of marrow included in the compared tissues, given that control bone contained all marrow while the fractured bone only contained partial marrow because of necessary manipulations during the fractural procedures. Of note is that we initially attempted to use controls without bone marrow, but failed to extract enough RNA. However, this cannot be an only explanation because some of the downregulated genes appear unrelated to B cells or bone marrow. It is still an open question whether the constant suppression of this group of genes has a functional impact on early stage of fracture healing.
Acknowledgements
We thank Dr. David Baylink for critically reading the manuscript, OrthoLogic Corp. for providing partial financial support, Jeremiah Convery and Tammy Bigelow for performing animal surgery. This work was also supported in part by the Division of Biological Sciences, the Cancer Research Center, and the NIDDK Biotechnology Center at the University of Chicago (U24D55370).




