Microstructured scaffolds for liver tissue cultures of high cell density: Morphological and biochemical characterization of tissue aggregates
Abstract
Very high cell densities and optimal vascularization characterize among others organs and tissues in vivo. In order to study organ-specific functions in vitro or to make use of them in medical devices/treatments in the future, this natural architecture should be rebuilt. An important aspect in this context is the appropriate ratio of medium to cell volume being so far not optimally reestablished in most of the currently available in vitro systems. To improve such culture conditions, we constructed a microstructure to culture hepatocytes and (without any addition of extracellular matrix material) characterized liver tissue in the form of evenly sized aggregates. The liver-specific differentiation status of such aggregates was monitored by their ability to perform CYP450 dependent xenobiotic metabolism along with the measurement of albumin secretion. Freshly isolated adult rat hepatocytes show an initial loss of total CYP450 content and of associated activities (mixed function oxidases). However, in the aggregate system, this level did not decrease further but remained stable or even increased throughout the culture period of 10–13 days. The CYP450 dependent metabolism of the hepatocytes is able to respond to classic inducing agents. The described culture efficiently supports liver-specific functions of adult rat hepatocytes and seems to be suited not only for use in an extracorporeal liver device but also for the formation of evenly sized small aggregates to be of use in transplantation of differentiated liver tissue. Moreover, after design variations, the microstructure can be applied for functional analysis of metabolically active hepatocytes as well as for toxicological and pharmacological validation. © 2005 Wiley-Liss, Inc.
Abbreviations used:
PB, phenobarbital; MC, 3-methylcholanthrene; CYP450, cytochrome P450; DMSO, dimethyl sulfoxide; BROD, 7-benzyloxyresorufin O-debenzylase; ECOD, 7-ethoxycoumarin O-deethylase; EROD, 7-ethoxyresorufin O-deethylase; PROD, 7-pentoxyresorufin O-depenthylase; 3D, three-dimensional; MFO, mixed function oxidase.
Isolated hepatocytes cultured in vitro have been used as a tool for the analysis of liver functions under defined experimental conditions [Gomez-Lechon et al., 1988; Guguen-Guillouzo et al., 1988]. For the analysis of long-term toxicity or the cancerogenicity of certain substances, it is necessary to establish culture conditions that allow the stable maintenance of hepatocyte functions. This also holds for the application of in vitro cultures as extracorporeal devices to temporarily support patients with acute liver failure.
In the past, many in vitro culture systems suffered from the disadvantage that the isolated hepatocytes had a relatively short life-span and lost most of their liver-specific functions very rapidly [Acosta et al., 1979; Enat et al., 1984; Dich et al., 1988]. Therefore, many different modifications and adaptations related to the composition of the culture medium [Jefferson et al., 1984; Flaim et al., 1985; Nawa et al., 1986; Fujita et al., 1987; Hutson et al., 1987] and the coating of culture vessels, in which the cells predominantly form monolayers, have been undertaken in order to reproduce better conditions for the hepatocytes in vitro [Michalopoulos and Pitot, 1975; Koebe et al., 1994; Bader et al., 1996]. Promising approaches include, for example, collagen sandwich configurations [Koebe et al., 1994; Bader et al., 1996] and/or co-culture of hepatocytes with other cell types [Morin and Normand, 1986; Goulet et al., 1988; Corlu et al., 1991; Akrawi et al., 1993; Donato et al., 1994] indicating the importance of cell-matrix interaction as well as interspecific cell communication.
Another important aspect seems to be the establishment of cell specific contacts, which are realized, for example, by gap junctional complexes as are a common characteristic of hepatocytes in vivo [Fraslin et al., 1985]. In conventional monolayer cultures, gap junctional communication is rapidly lost [Spray et al., 1987].
In vitro it is possible to re-establish to some extent a tissue-like arrangement of hepatocytes using the spheroid technique. Even adult rat hepatocytes are able to form spheroids and to remain viable for at least several weeks [Landry et al., 1985; Koide et al., 1990; Tong et al., 1992; Ma et al., 2003; Xu et al., 2003]. In these aggregates, hepatocytes stay in close contact with each other, promoting cellular communication resulting in the stabilization of hepatocyte-specific functions. But also this conventional 3D-culture technique has several drawbacks with respect to critical aggregate size (inducing necrosis for large aggregate diameters [Groebe and Mueller-Klieser, 1996]) as well as inappropriate cell to medium ratios.
Organs and tissues of living organisms are, among others, characterized by very high cell densities, while being fully vascularized. Also directed transport of substances is an important characteristic.
Artificial organs and realistic organotypic in vitro tissue models should most closely rebuild this natural architecture, especially for use as extracorporeal assist systems. To date most devices are working far from optimal conditions. An essential determinant for the limitation of such systems is the inappropriate ratio of medium to cell volume. To overcome this problem, we designed a suitable cell housing shaped as a precisely microstructured polymer scaffold, offering evenly sized containers for the three-dimensional culture of cell aggregates.
The aim of the study presented was to investigate the persistent differentiated functions of adult rat hepatocytes, which have been cultured as multicellular aggregates using these scaffolds without any addition of extracellular matrix material. We used for all experiments the same defined culture medium, which has previously been shown to permit only minimal cell proliferation [Walser et al., 1989, 1991]. Several liver specific key functions have been monitored over the culture period (up to 2 weeks). We examined actin gene activity as an indicator for cytoskeleton reorganization. Electron microscopic examination of ultra thin sections of aggregates was carried out to identify organotypic structures within the multicellular aggregates. Tyrosine aminotransferase as an important function of aminoacid metabolism, and aldolase B, the liverspecific aldolase isoform, an important factor in glycolysis, along with several other mostly liver specific genes have been analyzed qualitatively on gene level. The secretion rate of the multifunctional protein albumin, which is in vivo released by hepatocytes in huge amounts into the blood serum, was studied by ELISA, and compared with the transcriptional activity of the albumin gene. Another predominant function of the liver is the xenobiotic metabolism. We investigated the transcriptional activity of CYP1A1 and CYP2B1, two representatives of the big CYP450 family (reviewed in [Koebe et al., 1994]), after induction with the classical inducers 3-methylcholanthrene (MC) and phenobarbital (PB), respectively. The basal metabolic capacity of hepatocytes in 3D and monolayer culture was measured as the turnover of scoparone. This substance is regioselectively demethylated by members of the abovementioned CYP450 family to its metabolites scopoletin and isoscopoletin [Jefferson et al., 1984, 1985; Muller-Enoch et al., 1985], which were quantified by HPLC analysis.
MATERIALS AND METHODS
Chemicals
PB, MC, CPSR-1 (a serum substitute), insulin, hydrocortisone-21-phosphate, umbelliferone (hydroxycoumarin), ethoxycoumarin, ethoxyresorufin, pentoxyresorufin, benzyloxyresorufin, and δ-aminolevulinic acid were obtained from Sigma (Deisenhofen, Germany). Nicotinamide, transferrin, and collagenase were purchased from Serva (Heidelberg, Germany). Percoll was from Pharmacia (Freiburg, Germany) and resorufin was purchased from Aldrich (Steinheim, Germany).
Isolation of Hepatocytes
Hepatocytes were isolated from livers of 24-h fasted male Sprague–Dawley rats, weighing about 200 g, using a standard perfusion technique as described in [Berry and Friend, 1969; Seglen, 1973]. Hepatocyte preparations with viabilities >90%, as determined by trypan blue exclusion tests, were used in all experiments.
Hepatocyte Culture
The culture medium was optimized to result in minimal cell proliferation, reflected in a genomic ploidy pattern similar to that found in cell populations of freshly isolated hepatocytes (determined by flowcytometry; data not shown). This led to the following medium composition: Waymouth 752/1 medium was supplemented with 10% CPSR-1, 50 µg/ml gentamycin, 2 × 10−3 M glutamine, 1 × 10−8 M insulin, 4 × 10−7 M hydrocortisone, 2 × 10−3 M nicotinamide, 2.5 × 10−7 M Fe(NO3)3, 1.3 × 10−8 M transferrin, and 1 × 10−4 M δ-aminolevulinic acid.
For aggregation, 3 × 106 cells were seeded in microstructures with 0.4 ml of culture medium. This culture medium contained all the components mentioned above except hydrocortisone. Hydrocortisone had been added to the medium only from day 2 on, since aggregation of cells in the early phase had been hindered by presence of this hormone (personal observation); experiential observations, also from other investigators, however, demonstrated positive effect of hydrocortisone on hepatocyte functions (hormone is found in most medium compositions for these cells). After 24 h, medium was changed and aggregation of the hepatocytes was allowed to continue for another 24 h.
For the induction experiments, cells were incubated for 2 days with either 1.5 mM PB or 2 µM MC in DMSO, leading to a final DMSO concentration of 0.1% in the culture medium.
The induction data for total liver were obtained by feeding rats for 3 days with either 100 mg/kg MC or 80 mg/kg PB, respectively. The drugs were dissolved in 0.2% agar and 10 ml/kg administered p.o. Control animals received agar without drugs.
Viability Assay
To examine the viability of the aggregates, they were disaggregated by using an enzyme cocktail of trypsin (0.25%), dispase (2.4 U/ml), and collagenase (10 mg/ml). An aliquot of cells was then incubated in medium with trypan blue. All aggregates hardly contained dead cells; this holds for all time points of cell harvest. Single dead cells without contact to the aggregates, however, were detected in the culture medium.
Albumin Determination
Albumin secretion rates were determined by ELISA from the culture medium, which was completely exchanged prior to a 24-h incubation period. ELISA plates (NUNC Polysorp) were coated overnight at 4°C with 200 μl rat albumin (1 μg/ml, SIGMA), dissolved in coating buffer (NaHCO3 16.8 g/L, Na2CO3 4.2 g/L, pH 9.6). The next day, plates were washed five times with 300 μl washing buffer (NaCl 8.5 g/l, Na2HPO4.2H2O 8.9 g/L, NaH2PO4 6.9 g/L, Tween 20 1 ml/L, pH 7.2). Fifty microliters of standards (1–100 μg/ml rat albumin in culture medium) and samples were pipetted in triplicate, and 50 μl of antibody solution was added (anti-rat albumin HRP conjugate, CAPPEL, diluted 1:10 in washing buffer without Tween 20). After 1-h incubation under gentle agitation in the dark, plates were washed again. Hundred microliter TMB substrate (PIERCE) were added, and plates were incubated for another 15 min. Enzyme reaction was stopped with 100 μl 2M H2SO4. Optical density was determined in a plate reader (SpectraMax, Molecular Devices) at 450 nm within 1 h.
CYP450 Measurement
Microsomal CYP450 content was determined by using the differential spectrophotometry method described by Omura and Sato [1964]. Spectra were taken with a Perkin Elmer spectrophotometer (Lambda 5 UV/VIS).
Metabolic Assays (Mixed Function Oxidases)
The mixed function oxidase enzyme activities were measured fluorimetrically according to the methods described by Lake [1987]. The kinetic endpoints of 7-ethoxycoumarin O-deethylase (ECOD), 7-ethoxyresorufin O-deethylase (EROD), 7-pentoxyresorufin O-depenthylase (PROD), and 7-benzyloxyresorufin O-debenzylase (BROD) reactions were determined with a Perkin Elmer luminescence spectrometer (LS-5B). Wavelength settings for excitation and emission were 380 and 452 nm for umbelliferone and 535 and 582 nm for resorufin.
Northern Analysis
Northern blot analysis has been performed according to Sambrook et al. [1989]. RNA was prepared by using the RNeasy Kit supplied by Qiagen. Fifteen microgram RNA per sample was separated in a 1% agarose gel and MOPS running buffer (0.4 M MOPS, 0.1 M NaOAc, 10 mM EDTA). RNA was blotted onto a positively charged nylon membrane (Boehringer) by the capillary blot procedure for at least 4 h at room temperature. For labeling of the DNA probes, the DIG DNA Labeling Kit (Boehringer) was used.
cDNA probes were kind gifts from the following persons and institutions: albumin cDNA from the Health Science Research Resources Bank, Osaka, Japan, aldolase B cDNA from A. Corlu, Institut National de la Sante et de la Recherche Medicale, Rennes Cedex, France, tyrosine amino transferase cDNA from A. Cato, Forschungszentrum, Karlsruhe, Germany, CYP450 1A1 and 2B1 cDNA from W.A. Schmalix, GSF Research Center of Environment and Health, Oberschleißheim, Germany. Actin was purchased as a DIG-labeled RNA probe from Boehringer. Hybridization of the Northern blots was performed in DIG Easy Hyb Solution (Boehringer) at 50°C overnight by using 25 ng/ml DNA probe and at 68°C overnight by using 100 ng/ml RNA probe. Stringency washes were performed twice for 5 min with 300 mM NaCl, 30 mM Na-citrate, 0.1% SDS, pH 7.0 at room temperature and twice for 15 min with 75 mM NaCl, 7.5 mM Na-citrate, 0.1% SDS, pH 7.0 at 60°C. Detection was performed with the DIG Luminescent Detection Kit (Boehringer). Blots were exposed using X-ray films (Hyperfilm MP, Amersham).
Analysis of Scoparone Metabolism
Scoparone was dissolved in DMSO as a 0.1 M stock solution. Hepatocytes were incubated for 24 h with 1 mM scoparone. Culture media were harvested at day 1–5 from monolayer cultures and at day 1, 2, 3, 6, 10, and 14 from aggregate cultures. After exact determination of the volume, media samples were stored at −20°C until measurement by HPLC. Ten milliliters of culture medium was used for the determination of scoparone metabolites. Samples were incubated at pH 5.5 with 0.5 ml enzyme solution containing 40,000 Roy U/ml arylsulfatase and 5,000 Fishmann U/ml β-glucuronidase for 18 h at 37°C. Enzyme reaction was stopped by 50% TCA resulting in a final concentration of 5%. After centrifugation for 15 min at 5,000g at room temperature, the supernatant was transferred to a new test tube, and pH was adjusted to 5.5 again. Solid phase extraction (SPE) was performed with Sep-Pak Plus cartridges (Waters), containing a C18 reversed phase sorbent. Cartridges were conditioned with 5 ml methanol and equilibrated with 10 ml 0.1 M NaOAc applied with 10 ml syringes and a flow rate of approximately 10 ml/min. Samples loaded onto the cartridge were washed with 2 ml 0.1 M NaOAc and eluted with 3 ml methanol, dried in an evaporator (Speed Vac), and dissolved in 100 µl NH4OAc. Afterwards, DMSO was added yielding a final volume of 500 µl.
Twenty microliters of this mixture were injected into HPLC by an autosampler (Waters). A reversed phase column (4 µm, Novapak C18, Waters) was used at a flow rate of 1.5 ml/min with a linear elution gradient from 5% to 100% (v/v) methanol at 10%/min in a starting solvent of 50 mM NH4OAc buffer pH 4.25. Detection of scoparone and its metabolites was performed at 340 nm, the concentrations were calculated using Maxima software (Waters). Linear response of scopoletin and isoscopoletin was found within the range of 1.0–300.0 µM. Retention times of the standard substances were found to be about 9.1 and 9.2 min for isoscopoletin and scopoletin, respectively. The recovery rates of the metabolites were 64% for isoscopoletin and 47% for scopoletin.
For protein determination, according to the method of Bradford (Biorad), cells were harvested quantitatively.
RESULTS
The developed microstructure is a polymer scaffold (preferably PMMA or PC), consisting of a microstructured planar device offering containers for the three-dimensional culture of cell aggregates, each having a volume of maximal 300 × 300 × 300 µm3 and has been shown to be suitable for 3D cell culture. The special design (Fig. 1a–d) of scaffold and periphery (incubation chamber/bioreactor) allows for different modes of medium supply: superfusion (both sides of tissue are separately supplied with nutritional medium) and perfusion (medium flow is forced to penetrate the tissue layer). The perfusion mode was included in the construction plans in order to create conditions that better mimic the in vivo vascularization (for further description see Weibezahn et al. [1994, 1995]). The specially formed bottom of the microstructure has an array of precisely laser-drilled pores. Pore diameter was chosen to allow medium supply but not the passage of cells, which can be easily washed into the micro containers for seeding. The complete cultivation device is formed by an array of 900 such microcontainers on a microstructured area of 1 cm2. One of these microstructures can be placed in a special incubation chamber (bioreactor) also allowing optical control of the culture (Fig. 1a). It has already been demonstrated that cell lines (e.g., L-929, SV40-3T3, Hep G2) as well as primary cells (rat hepatocytes) can be cultured in the described devices [Weibezahn et al., 1994; Knedlitschek et al., 1999], also in long-term experiments.
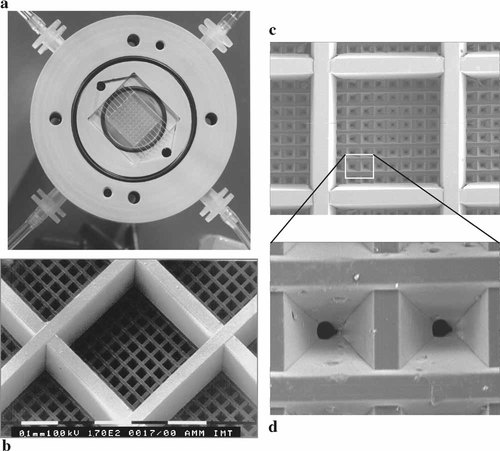
The microstructured scaffold is formed by injection moulding of PMMA. a: Open bioreactor housing with inserted microstructure for application in superfusion or perfusion mode. The 900 microcontainers are arranged in the middle 1-cm2 surface. b: SEM picture of the CellChip's surface, each container has 300 × 300 × 300 µm3. c: The bottom of the microcontainer is treated with an excimer laser resulting in an array of 8 × 8 pores. d: Higher magnification of the bottom. Each pore has a diameter of 2.7 µm.
Here experiments using primary rat hepatocytes were presented offering information with respect to their morphology, viability, and several metabolic functions. In previous studies, we characterized spheroids of adult rat hepatocytes cultured under defined medium conditions (“serum free”), which have been shown to support a ploidy pattern that was similar to the in vivo situation [Walser et al., 1989, 1991] and supported liver-specific enzyme activities (unpublished results). The same medium composition has been used for the 3D culture of rat hepatocyte aggregates in the microstructures. Freshly isolated hepatocytes were inoculated into uncoated microstructures as well as microstructures coated with collagen I (for the influence of surface coating see Knedlitschek et al. [1999]). Cells adhered to the surface of the polymer only in collagen-coated microstructures and formed aggregates completely filling the micro containers (Fig. 2a). In uncoated microstructures, however, the cells formed very stable aggregates, but no adherence was observed (Fig. 2b). The latter procedure can, therefore, be used to produce evenly sized cuboid aggregates of liver cells being very stable as observed, even after continued cultivation in spinner culture (Fig. 2c). To demonstrate that 3D aggregation of the cells alone represents a minimal condition for good tissue viability and long-term differentiated state of the primary rat liver cells, we characterized free aggregates as outlined in the following. Since (at least) during day 1 and 2 cells had not fully recovered from isolation procedure and were in the aggregation phase, we did not analyze cultures during that period. As reported by others [Ma et al., 2003; Xu et al., 2003], specific functions were merely detectable during that period-single experiments in our system (data not shown) supported those findings. Data obtained from monolayer older than 5 days were not available, since most of the cells were dead.
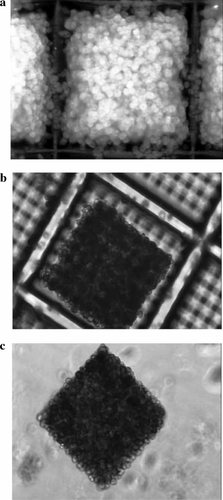
Rat liver cells aggregated in the microcontainers after (a) coating the surface with collagen I, (b) without any coating. c: Aggregates can be flushed out from uncoated containers as freefloating cuboids.
Morphological Observations
The electron micrograph of an ultra-thin-section of a rat hepatocyte-aggregate as achieved in 3D culture is shown in Figure 3. The cell morphology and arrangement exhibited characteristics as seen in the in vivo situation: round nuclei and numerous mitochondria can be observed as well as junctional complexes sealing extracellular lumina, also equipped with microvilli.
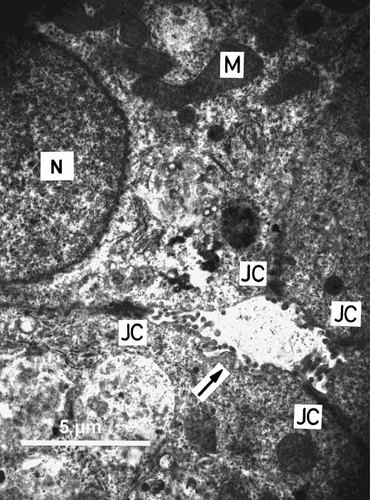
Electron micrograph of the ultrathin section of an aggregate of rat liver cells (day 6). The arrow indicates a bile canaliculi-like cavity lined by protruding microvilli and sealed by junctional complexes (JC). M, mitochondrium; N, nucleus. Bar length = 5 µm.
Albumin Secretion
Hepatocytes in aggregate culture exhibited reduced rates of albumin secretion during the first days when compared with monolayer cells cultivated in collagen I coated Petri dishes. However, aggregate culture enabled hepatocytes to maintain the level reached after around 6 days for at least 14 days of culture (Fig. 4).
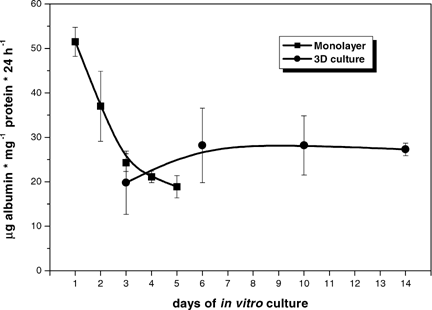
Albumin secretion of hepatocytes cultured as multicellular aggregates determined by ELISA. During these experiments, additional cultures were carried along experiencing the same handling procedures and being sacrificed for determination of protein content at each time point; this was taken as indication for the number of cells present in the system at each time point; the cultures delivering the aliquots for albumin determination were used during the whole investigation period. Each sample was analyzed in triplicate and calculated as microgram albumin secreted per milligram protein per 24 h. Each data point represents the mean value of at least four independent experiments. The error bars indicate the standard error of the mean.
Basal and Induced CYP450 Amounts
Basal and induced CYP450 amounts in aggregates are shown in Figure 5. Since the CYP450 values obtained from freshly isolated hepatocytes were comparable to the values obtained from liver (data not shown), we used the values from freshly isolated hepatocytes as in vivo reference. The basal CYP450 amount in 3-day-old aggregates was decreased to roughly 35%. However, this level proved to be stable throughout at least 10 days of in vitro culture. Moreover, hepatocytes cultured as aggregates were able to respond to the classical inducers PB and MC as the corresponding elevated levels of CYP450 illustrated. Induction values were significant for day 3 and 6, but not for day 10, as judged by the Student's t-test (P < 0.05). Furthermore, despite lower absolute CYP450 amounts, induction of the aggregates (expressed in percent) was in good accordance with the liver values (except with MC at day 10).
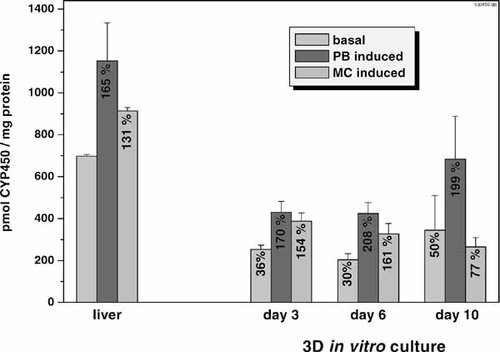
Basal and induced CYP450 amounts (pmol/mg protein) in microsomal preparations from liver and aggregates at different days of in vitro culture. Values represent the mean of at least three independent experiments. Error bars indicate the standard error of the mean. Numbers within the columns represent the induction by phenobarbital (PB) and 3-methylcholanthrene (MC) expressed as percent of the respective basal values.
Basal CYP450 Dependent Mixed Function Oxidase Activities
Activities of mixed function oxidases in microsomes prepared from whole liver and from freshly isolated hepatocytes were also comparable (data not shown) and therefore, the latter were again used as in vivo reference. Basal enzyme activities are shown in Figure 6. During in vitro culture, hepatocytes showed also an initial drop of enzyme activity. The only exception is EROD: this enzyme complex exhibited a higher activity already at day 3 and reached a 4.5-fold elevated level at day 13 as compared to the in vivo situation. BROD activity in aggregates remains at the lower level of day 3 (approximately half of the liver activity). PROD activity on the other hand recovered throughout the culture period yielding enzyme activities comparable to the in vivo situation. ECOD also showed an increase in activity, but remained markedly below the liver reference.
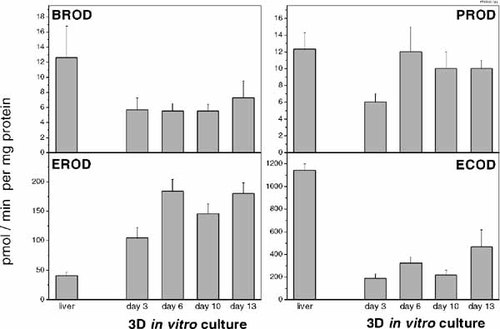
Basal activities (pmol/min*mg protein) of cytochrome P450 dependent mixed function oxidases in microsomal preparations from liver and aggregates at different days of in vitro culture. Values represent the mean of at least three independent experiments. Error bars indicate the standard error of the mean. BROD, 7-benzyloxyresorufin O-debenzylase; PROD, 7-pentoxyresorufin O-depenthylase; EROD, 7-ethoxyresorufin O-deethylase; ECOD, 7-ethoxycoumarin O-deethylase.
Induced CYP450 Dependent Mixed Function Oxidase Activities
Treatment of rats with PB or MC led to typical induction patterns of CYP450s in the liver as reflected by different activities of mixed function oxidases (Fig. 7). A pronounced induction of BROD, for example, was only observed by PB, whereas MC led to an induction of EROD (but not PB), the other MFO tested exhibited intermediate induction by both inducing agents.
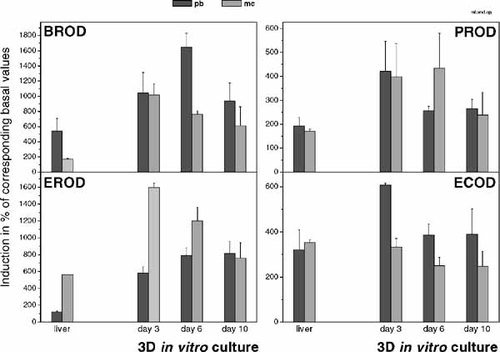
Percentage of induction of cytochrome P450 dependent mixed function oxidases in rat liver and in hepatocytes after three-dimensional in vitro culture at different days of cultivation. Induction by phenobarbital and by methylcholanthrene, respectively. Values represent the mean of at least three independent experiments. Error bars indicate the standard error of the mean. BROD, 7-benzyloxyresorufin O-debenzylase; PROD, 7-pentoxyresorufin O-depenthylase; EROD, 7-ethoxyresorufin O-deethylase; ECOD, 7-ethoxycoumarin O-deethylase.
Figure 7 shows the MFO activities of hepatocytes cultured as aggregates until day 10. Induction values-expressed in percent of the respective basal values-were highly significant as judged by the two-tailed Student's t-test.
BROD was strongly induced by both agents at day 3. At day 6 and 10, induction by PB was stronger than by MC, resembling more the in vivo induction pattern. The overall induction was higher than in liver.
PROD showed the highest induction values at day 3, which were similar for both inducers and higher than in liver. At day 6, induction by PB has declined and at day 10 enzyme activities induced by both, PB and MC, were reduced. The extent and the pattern of induction, however, were then comparable to the in vivo situation.
EROD showed a very pronounced MC induction, which declined throughout time in culture but remained at least at a level comparable to the liver. PB induction, on the other hand, was higher than in liver and showed a slight increase during the in vitro culture period. Percental induction of EROD by both inducers was stronger in aggregates than in liver. Induction patterns until day 6 showed the best accordance with in vivo values.
The percental induction of ECOD activity induced by PB at day 3 was much higher than in the liver, but was comparable for longer culture periods. MC induction at day 3 was comparable to the liver value and showed a slight decrease throughout the culture period. MC induction was less pronounced than PB induction, thereby differing from the in vivo induction pattern.
Induction of Different Liver Specific mRNAs
Actin
Actin as a component of the cell cytoskeleton showed hardly any transcriptional activity in differentiated hepatocytes. Therefore, it is a useful marker to monitor events like cell proliferation and cell spreading. In samples from whole liver and primary hepatocytes, no actin mRNA was detectable by Northern analysis as expected. Hepatocytes cultured as monolayers, however, showed considerable intracellular actin mRNA concentrations until day 3 of in vitro culture. mRNA amounts were also increased in hepatocytes cultured as aggregates at day 3, but signal intensity was markedly reduced during 3D culture time (Fig. 8a).
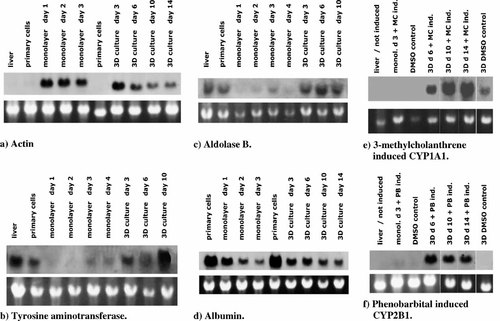
Expression of different liver specific mRNAs in aggregate culture in comparison to monolayer as well as liver of rat hepatocytes. The upper half of each picture represents gene expression detected by Northern analysis. The lower half is a photograph of the gel showing 18S rRNA bands as visual references.
Tyrosine aminotransferase and aldolase B
The mRNA concentrations of these two genes responsible for reactions taking place in the liver were high in liver and primary hepatocytes (Fig. 8b,c). When hepatocytes were cultured as monolayers, their specific gene expression ceased already after 24 h of culture. Both functions were better conserved in aggregate culture, since mRNA was present in considerable amounts at least until day 10 of in vitro culture. Note the increase in the tyrosine aminotransferase RNA amount during the culture period, especially in aggregates.
Albumin gene expression
The highest albumin mRNA concentration was found in freshly isolated primary hepatocytes (Fig. 8d). In monolayer culture, hepatocytes still showed relatively high mRNA levels after 24 h, which were rapidly diminished at day 2 and 3 of in vitro culture. Hepatocytes after 3 days of aggregate culture also showed reduced levels of albumin mRNA compared to primary hepatocytes, but maintained relatively high levels at least until day 14 of in vitro culture. These results were supported by the protein secretion data (Fig. 4).
Xenobiotic metabolism
CYP1A1/2 and CYP2B1/2 belong to the group of CYP450 genes involved in the degradation of a variety of endogenous (e.g., hormones) and xenobiotic substrates (e.g., drugs). Basal gene expression of both genes was not detectable in uninduced rat livers (Fig. 8e,f). CYP1A1 and CYP2B1 gene expression was also not detectable in methylcholanthrene or PB treated 3 day hepatocyte monolayer cultures.
Hepatocytes cultured as aggregates, however, responded very strongly after treatment with these classical inducers (as to be judged from the huge mRNA amounts that were detected until day 14 of in vitro culture). As a control, DMSO treated aggregates were analyzed to ensure that gene induction was not due to the solvent used for the application of the inducers. There was, indeed, a slight induction of the CYP1A1 gene by DMSO, whereas CYP2B1 gene expression was not affected.
Metabolism of Scoparone as a Model Substrate
Both abovementioned CYP450 genes are involved in the metabolism of scoparone, which is regioselectively demethylated either to isoscopoletin by CYP1A1/2 or to scopoletin by CYP2B1/2 [Jefferson et al., 1984]. Using this substrate, we analyzed the basal metabolic capacity of rat hepatocytes. Hepatocytes in monolayer culture showed a very active scoparone metabolism during the first 3 days of in vitro culture, which was especially reflected in the formation of isoscopoletin (Fig. 9). Thereafter, the formation of both metabolites declined rapidly.

Metabolism of the model substrate scoparone into scopoletin and isoscopoletin in aggregate culture in comparison to monolayer determined by HPLC. Each sample was prepared twice and injected twice for HPLC analysis. Calculation of scoparone metabolism was done in nanomole metabolites per milligram protein per 24 h. At least three independent experiments were performed to calculate mean values for each single day of in vitro culture. Error bars indicate the standard error of the mean.
Aggregates, on the other hand, showed a lower metabolic activity already from the beginning of in vitro culture, which was maintained until day 14. But this seemed to be rather increasing throughout culture period. Formation of scopoletin was kept at a more or less constant level, whereas isoscopoletin showed even increasing values.
DISCUSSION
In vivo-like culture of hepatocytes from various sources like mouse, rat, pig, and man in in vitro systems is still a challenge. Many attempts have been made to keep especially adult hepatocytes viable and in their liver-specific differentiation status for longer periods [Flaim et al., 1985; Nawa et al., 1986; Hutson et al., 1987].
Among the most important parameters to be varied for improving the functionality of primary hepatocytes were the composition of the nutritional medium and the architecture of the culture system including the extracellular matrix [as summary see Bhatia et al., 1999]. We also gave special attention to these parameters during development of our system as described in this presentation [see also Knedlitschek et al., 1999]. We have optimized the medium composition for minimal cell proliferation and ploidy pattern similar to that observed for freshly isolated rat hepatocytes [Walser et al., 1989] regarding those parameters as a first evidence for the 3D cell culture being in a differentiation state comparable to that in vivo. In addition, we tried to minimize uncontrollable variations (in co-factor and hormone composition and concentrations) as expected when different serum preparations were used. Therefore, we replaced normal fetal calf serum by CPSR as having a more constant composition even though the components were not specified by the distributor (SIGMA). This might have resulted in a lack of specific co-factors necessary for optimal liver-specific functionality and might become of importance during further experiments aimed at optimizing the medium composition for specific differentiated functions. The medium composition as used in this presentation allows long-term culture of hepatocytes in vitro. Nevertheless, we are aware of the problems associated with serum preparations and, as other investigators, would prefer a completely defined medium composition [Jefferson et al., 1985; Saad et al., 1993].
The microstructured scaffold has been used in the presented experiments mostly as a tool appropriate to provide researchers (or in the future: even clinicians) with evenly sized aggregates by placing the respective amount of cells in the uncoated microstructured devices as described. Since aggregates can be further cultured as non-adherent “tissue aggregates” in spinner flasks, there is no obligatory need to add extracellular matrix material or even coat the surface of the culture vessel with extracellular matrix material(s).
For comparison with other in vitro systems described for prolonged culture of primary hepatocytes, their functionality was assessed by determination of albumin secretion [Hutson et al., 1987; Ma et al., 2003]. From the fact that the secretion rate reaches a stable level after about 6 days, one can assume that at least at this time hepatocyte functions have been re-established in the aggregate system and can be maintained at least over the culture period. This is in good agreement with data as presented by the group of Wendy Purcell who also reported a morphological maturity of rat liver hepatocytes after 5 days accompanied by several functional and biochemical changes [Ma et al., 2003; Xu et al., 2003]. Since hardly any dead cell could be detected even after the complete culture period (followed by disaggregation procedure), this observation could be interpreted as an indication for the good efficiency of the system.
The study of actin mRNA expression as compared in vivo and in vitro as monolayer and aggregate cultures demonstrates that the cytoskeleton architecture in the in vitro systems differs from the in vivo situation: there is considerable actin mRNA expression in vitro but not in vivo. The disappearence (fading away) of the actin mRNA signals with increasing culture times may have different reasons in monolayer and aggregate culture. In the monolayer, the rate of cell death increases with culture time (limited lifespan of monolayer cells). On the other hand, reorganization of cells in aggregates to restore a 3D tissue architecture seems to go along with a more differentiated state of the hepatocytes as reflected in the reduction of actin mRNA expression.
In the present study, we focused on the analysis of the xenobiotic metabolism as one of the main functions of differentiated hepatocytes. It serves to detoxify the organism and is carried out by CYP450 dependent mixed function oxidases. The activity of many of the involved enzymes is rapidly lost in conventional monolayer cultures and represents therefore a useful marker for the analysis of the differentiation state of hepatocytes cultured in vitro. We found a decrease to about 35% of the total amount of CYP450 proteins as compared to the liver. This level, however, remains constant throughout the culture period. The initial drop might be due to the dramatic change of environment the hepatocytes have to cope with after disaggregation of the liver tissue. The fact that CYP450 is stabilized after this critical step demonstrates that the culture conditions chosen are supportive to liver cell function(s) as tested. This is further confirmed by the induction data of CYP450 obtained with the classical inducers PB and MC. It should be noted that at least the values expressed as percent of induction (referring to the corresponding not-induced culture of the same preparation) are comparable to the liver values. Although the absolute values describing the extent of induction were in most cases not comparable to the in vivo observations, the experiments clearly demonstrate that the aggregate system was still able to respond to external stimuli. The pronounced differences may be partly due to medium composition not optimized for this liver-specific function(s) and/or the fact, that although the aggregate culture allows for intercellular contacts, other features of the liver lobule might not yet been remodeled in a satisfactory manner: the oxygen gradient, for example, as an important differentiation factor has not yet been remodelled in a controlled way in this system. This will be done in future experiments.
Using different alkyl ether substrates, it is possible to distinguish between classes of CYP450 dependent mixed function oxidases. The complex pattern of metabolism of these substrates can be seen as a fingerprint of liver-specific functions still operative in the respective in vitro system to be compared to the in vivo situation. There were still discrepancies observable between in vivo and aggregate data. They might be due to various reasons, not only those mentioned already, but perhaps also specific demands of the respective enzyme systems to, for example, co-factors necessary for optimal enzyme function(s). Since we did not optimize the medium composition for specific enzyme activities but only for prevention of cell proliferation and ploidy pattern, there might be a considerable shortage in co-factors, resulting in sub-optimal enzyme activities.
Nevertheless, the presented data illustrate the adaptability and efficiency of adult rat hepatocytes cultured as aggregates under defined conditions for prolonged periods of time. The hepatocytes in these aggregates exhibited a differentiated status, which is necessary to perform xenobiotic metabolism, and were still able to respond to external stimuli (inducers). This finding is also supported by the corresponding mRNA data, reflecting an inducible expression profile of at least some liver-specific genes.
The presented data do not provide a complete functional characterization of the hepatocyte aggregate system but only emphasize some aspects of the liver-specific differentiation status as exhibited by the hepatocyte aggregates. They underline, however, the particular properties “expressed” specifically in three-dimensional cellular aggregates that enable cells in this configuration to perform functions that are not observable in conventional monolayer systems. The aggregate system seems therefore to be a tool applicable to remodel a complex situation sufficient to allow induction of xenobiotic metabolism in primary hepatocytes.
The described characterization has been made using aggregates formed without any addition of collagen. Morphological observations, however, suggest that the cells cultured in aggregates produced their own matrix material (data not shown).
The same type of microstructured device used in this presentation as some kind of “moulding tool” for aggregate formation and culture can also be used in a surface modified version (i.e., coated with collagen to encourage cell adhesion) to house primary hepatocytes for long-term culture (single-unit system suitable for microscopic observation during cultivation period). Even better results may be expected from our data obtained so far under culture conditions where the surface of the microstructures have been coated with collagen I to support cell adherence allowing to expect even better results; these observations are still to be confirmed at the level of liver-specific functions.
The culture system is also suitable for upscaling as a bioreactor housing up to 100 CellChips as a multi-unit system. This device can be realized by stacking many microstructures. Devices can be upscaled to result in a cell density of up to half of that found in the human liver. Even in this configuration, the maximal length of supply channels will not exceed 5 mm under perfusion conditions. On this latter account, no additional oxygen supply systems (using membranes or hollow fibres) will be necessary.
Starting from our experiences so far (as described in parts in this publication), the microstructured polymer scaffold and its use will be further developed and optimized. But taken together, the described microstructure device has already given proof of its versatility still to be expanded.
Acknowledgements
The skilfull technical assistance of C. Häge, K. Enright, S. Weigel, and M. Herschbach is highly appreciated. The authors thank N. Howells, Institute of Genetics, Research Center Karlsruhe, Germany, making the rats available for hepatocyte preparation.




