Sexual dimorphism of growth plate prehypertrophic and hypertrophic chondrocytes in response to testosterone requires metabolism to dihydrotestosterone (DHT) by steroid 5-alpha reductase type 1
Abstract
Rat costochondral growth plate chondrocytes exhibit sex-specific and cell maturation dependent responses to testosterone. Only male cells respond to testosterone, although testosterone receptors are present in both male and female cells, suggesting other mechanisms are involved. We examined the hypothesis that the sex-specific response of rat costochondral cartilage cells to testosterone requires further metabolism of the hormone to dihydrotestosterone (DHT). Resting zone (RC) and growth zone (GC, prehypertrophic and upper hypertrophic zones) chondrocytes from male and female Sabra strain rats exhibited sex-specific responses to testosterone and DHT: only male cells were responsive. Testosterone and DHT treatment for 24 h caused a comparable dose-dependent increase in [3H]-thymidine incorporation in quiescent preconfluent cultures of male GC cells, and a comparable increase in alkaline phosphatase specific activity in confluent cultures. RC cells responded in a differential manner to testosterone and DHT. Testosterone decreased DNA synthesis in male RC cells but DHT had no effect and alkaline phosphatase specific activity of male RC cells was unaffected by either hormone. Inhibition of steroid 5α-reductase activity with finasteride (1, 5, or 10 μg/ml), reduced the response of male GC cells to testosterone in a dose-dependent manner, indicating that metabolism to DHT was required. RT-PCR showed that both male and female cells expressed mRNAs for steroid 5α-reductase type 1 but lacked mRNAs for the type 2 form of the enzyme. Male cells also exhibited 5α-reductase activity but activity of this enzyme was undetectable in female cells. These observations show that sex-specific responses of rat growth zone chondrocytes to testosterone requires the further metabolism of the hormone to DHT and that the effect of DHT in the male growth plate is maturation-state dependent. Failure of female chondrocytes to respond to testosterone may reflect differences in testosterone metabolism, since these cells possess greater ability to aromatize the hormone to estradiol. © 2004 Wiley-Liss, Inc.
Recent studies indicate that testosterone exerts sex-specific effects on growth plate chondrocytes. Treatment of neonatal mouse bone organ cultures with testosterone shows that effects of the hormone are limited to bones from male donors [Schwartz et al., 1991]. The sex-specific response seen in bone organ cultures is due to fundamental differences at the cellular level. Experiments examining response of rat costochondral cartilage growth plate chondrocyte cultures to testosterone confirm that only cells from male donors respond [Schwartz et al., 1994]. Moreover, the response is dependent on the maturation state of the cells in the endochondral lineage [Schwartz et al., 1994, 1995]. Other cells also exhibit sex-specific responses to androgens [Weisman et al., 1993]. However, little is known concerning the mechanisms by which testosterone exerts its sex-specific effects.
In many tissues, testosterone is further metabolized suggesting that sexual dimorphism at the cellular level is due to its metabolites and not to the parent hormone per se. Chondrocytes from male and female rats both possess the ability to aromatize testosterone to estrogen, but female cells do so to a greater extent [Sylvia et al., 2002], thereby reducing the testosterone concentration while increasing local levels of estradiol. Testosterone is also metabolized to dihydrotestosterone (DHT) and 5-alpha-androstenedione in epiphyseal cartilage [Jaffe, 1969; Ackerman and Hamilton, 1976; Audi et al., 1984; Takahashi et al., 1984; Blanchard et al., 1991], and in many testosterone-sensitive tissues, DHT is the active metabolite of the hormone [Mauvais-Jarvis, 1986; Horton, 1992; Occhiato et al., 2004].
Receptors for DHT and testosterone are present in both male and female chondrocytes [Carrascosa et al., 1981; Nasatzky et al., 1994a; Ben Hur et al., 1997]. Sexual dimorphism in the response of chondrocytes to testosterone may also be due to differences in receptor binding. DHT has greater binding affinity to the androgen receptor (AR) than testosterone and the DHT–AR complex is more stable than the testosterone–AR complex [Chan and O'Malley, 1976]. In addition, DHT levels are likely to be higher in male cells, in part because of the larger pool of systemic testosterone in vivo, and the lower capacity to produce estradiol locally [Vermeulen, 1976; Brodie, 1979]. Sex-dependent differences in the rate of DHT production may also play a role.
The enzyme responsible for DHT synthesis from testosterone is steroid 5-alpha reductase (5α-reductase). There are two isoforms of human 5α-reductase, named type 1 and type 2 [Chen et al., 1996]. Expression of 5α-reductase isoenzymes is considered to be tissue specific and species specific. Generally, it is accepted that type 2 isozyme is localized primarily in male reproductive tissues while type 1 is mainly expressed in peripheral tissues [Thigpen et al., 1993; Stoffel-Wagner et al., 1998]. The tissue distribution differences may suggest that type 1 and type 2 have a catabolic and anabolic role, respectively [Thigpen et al., 1993; Chen et al., 1996; Stoffel-Wagner et al., 1998]. Recently, it was found that human osteoblast-like cells express predominantly steroid 5α-reductase type 1 [Issa et al., 2002], but it is unknown whether this is the case for growth plate chondrocytes or if 5α-reductase type 1 activity is related to the sex-specific effects of testosterone.
The present study was based on the hypothesis that sex-specific responses of growth plate chondrocytes to testosterone are mediated by its metabolite DHT. To test this hypothesis we: (I) compared the effects of DHT and testosterone on DNA synthesis and alkaline phosphatase specific activity in costochondral chondrocytes and determined whether DHT effects are sex-specific and maturation dependent; (II) examined the effect of 5α-reductase inhibition on the activity of testosterone in costochondral chondrocytes; and (III) determined the distribution of 5α-reductase isoenzymes in costochondral growth zone and resting zone chondrocytes from female and male rats.
MATERIALS AND METHODS
Chondrocyte Cultures
The culture system used in this study has been described in detail previously [Boyan et al., 1988] and is outlined below. Chondrocytes were derived from the costochondral cartilage of young adult 150 g male or female Sabra strain rats (Jerusalem, Israel). The Sabra strain of rat was originally derived from the Sprague–Dawley rat strain, and chondrocytes isolated from the costochondral cartilages of 150 g Sabra rats behave in a comparable manner to growth plate chondrocytes from 125 g Sprague–Dawley rats with respect to responsiveness to 1α,25(OH)2D3, 17β-estradiol and testosterone. The resting zone and adjacent growth zone cartilage were separated and care was taken to dissect intervening tissue to limit cross contamination of the different cell zones. Perichondrium tissue and calcified cartilage were discarded to limit contamination by fibroblasts and osteoblasts. After the dissection, the cartilage was sliced and then incubated overnight in Dulbecco's modified Eagle's medium (DMEM) in a 5% CO2 atmosphere at 37°C. Chondrocytes were released from the tissue by sequential incubations in 1% trypsin (Beit Haemek Industries, Beit Haemek, Israel) for 1 h and in 0.02% Type II collagenase for 3 h in Hanks' balanced salt solution. Chondrocytes were plated at an initial density of 10,000 cells/cm2 for resting zone cells and 25,000 cells/cm2 for growth zone chondrocytes. Cells were incubated in DMEM containing 10% fetal bovine serum (FBS) and 50 μg/ml vitamin C in an atmosphere of 5% CO2 at 37°C and 100% humidity. The culture media were replaced at 24 h and then at 72 h intervals until cells reached confluence. Passaged cells were plated at the same seeding densities. Fourth passage cells were used for all experiments, because previous studies have shown that they retain their chondrogenic phenotype, including the ability to form cartilage nodules when implanted in nude mice thigh muscle. They also retain their differential responsiveness to vitamin D metabolites at this passage, as well as differential responsiveness to a number of other factors [Schwartz et al., 1989, 1999; Sylvia et al., 2000].
Physiological Responses to Testosterone and DHT
For the experiments described below, resting zone and growth zone chondrocytes were cultured in media containing is 10−11–10−7 M testosterone or DHT. Testosterone and (Sigma Chemical Co., St. Louis, MO; Sigma Aldrich Chemie, GmbH, Germany) were dissolved in absolute ethanol and diluted at least 1,000 fold with DMEM to the required concentration in the final medium. Control cultures contained ethanol at the highest concentration used in the experimental cultures. Normal serum testosterone concentration is 10−10–10−9 M. The level of testosterone in FBS used to supplement the medium was measured using a commercially available radioimmunoassay (RIA) for testosterone (Diagnostic Products Corp., Los Angeles, CA) and was found to be 3 × 10−10 M, resulting in a basal concentration of 3 × 10−11 M testosterone in the FBS-supplemented media.
Two physiological indicators were used to determine if the effects of DHT were sex-specific and if they differed from those of the parent hormone. Effects of DHT and testosterone on chondrocyte proliferation were assessed as a function of DNA synthesis. Growth zone and resting zone chondrocytes from male and female rats were grown to confluence in 96-well culture plates. Testosterone or DHT (10−11–10−7 M) was added and cultures were incubated for an additional 24 h. [3H]-Thymidine (2 μCi/ml) was added 4 h before harvest. At harvest, the cell layers were washed twice with cold PBS and twice with 5% trichloroacetic acid (TCA), and then treated with saturated TCA for 30 min. TCA precipitable material was dissolved in 0.2% ml 1% sodium dodecyl sulfate and radioactivity was measured by liquid scintillation spectroscopy.
Effects of DHT and testosterone on chondrocyte differentiation were assessed as a function of alkaline phosphatase activity. Alkaline phosphatase (orthophosphoric monoester phosphohydrolase alkaline, EC 3.1.3.1) specific activity was assayed in lysates of the cell layer as described previously [Hale and Wuthier, 1987; Schwartz et al., 1991]. A colorimetric assay based on release of para-nitrophenol from para-nitrophenylphosphate at pH 10.2 was used [Lowry et al., 1951; Bretaudiere and Spillman, 1984]. Protein was measured by the BCA protein assay reagent by Pierce (Rockford, IL).
Sex-Specific Requirement for Testosterone Metabolism
To assess the requirements for metabolism of testosterone, 5α-reductase was inhibited with finasteride (Sigma Chemical Co.), which inhibits the steroid form of the enzyme [Thomas et al., 2003]. Finasteride has high affinity for the type 1 isozyme and moderate affinity for the type 2 isozyme of 5α-reductase. In order to determine, if the physiological responses to testosterone were mediated by 5α-reductase-dependent metabolism of the hormone, cultures were treated with vehicle or 10−9 and 10−8 M testosterone. Finasteride was added to one half of the cultures at concentrations of 1, 5, and 10 μg/ml.
5α-Reductase expression
RT-PCR was used to determine which 5α-reductase isoenzyme is expressed by resting zone and growth zone chondrocytes from male and female rats. Total RNA was extracted from fourth passage cells [Chomczynski and Sacchi, 1987] using TRIzol reagent (Invitrogen, Carlsbad, CA). Optimal primers were constructed from rat 5α-reductase type 1 and type 2 isoenzyme cDNA sequences available in GenBank (accession numbers NM_017070 and M95058, respectively) [Jaffe, 1969; Ackerman and Hamilton, 1976; Somjen et al., 1989]. Primer sequences are shown in Table I. The expected product sizes were 340 base pairs for type 1 isoenzyme and 344 base pairs for type 2 isoenzyme. All primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). Reverse transcription was performed using First strand cDNA synthesis kit (Amersham Bioscience, Piscataway, NJ), according to the manufacturer's instructions. PCR conditions were determined and conducted using a PCR kit (Fisher, Pittsburgh, PA). The PCR steps were: denaturation for 30 s at 94°C, annealing for 60 s at 61°C, and extension for 30 s at 72°C. The reaction was run for 35 cycles. The samples were then loaded on 5% polyacrylamide gels using TBE buffer and run at 90 mA per gel for 30 min. The gels were then stained with 10 μg/ml ethidium bromide in TBE buffer for 30 s, destained in TBE buffer for 10 min and visualized [Normington and Russell, 1992; Berman and Russell, 1993; Lopez-Solache et al., 1996].
| Isoenzyme | Nucleotide sequence | Primer |
|---|---|---|
| Type 1 | 5′-TTG-GAT-GAG-CTG-TGC-CTG-CT-3′ | Sense |
| 5′-AAG-GCC-AAG-ACA-AAG-GTG-AC-3′ | Antisense | |
| Type 2 | 5′-CAG-ATT-GTC-TGC-CAT-CAG-GT-3′ | Sense |
| 5′-GTG-GCT-CTC-AAA-AAC-AGC-AC-3′ | Antisense |
5α-Reductase activity
We examined whether active 5α-reductase enzyme was present in male and female growth zone cells. Specific activity of 5α-reductase was assayed by measuring the conversion of [3H]-testosterone to [3H]-DHT. Fourth passage growth zone chondrocytes were grown to confluence in T25 flasks (Nunclon Products, Nalge Nunc International, Rochester, NY). At 24 h prior to the experiment, the cells were washed three times with 3 ml phosphate buffered saline (Sigma Chemical Co.), and then incubated in 5 ml RPMI containing 5% steroid-free fetal calf serum and 5% fetal calf serum (Industerie, Kibbutz Beit Haemek, Israel). Tritiated testosterone (1 μCi/ml) was then added at concentrations of 10−9 or 10−8 M with or without 10 μg/μl finasteride. Twenty-four hours later, media were collected using two volumes of ethylacetate and then the cell layer was incubated with trypsin for 5 min. Full media were added to stop the reaction. The trypsin digest was centrifuged at 500g for 4 min at 4°C, and pelleted cells were counted using a Coulter counter (Coulter Electronics, Buckinghamshire, England). The collected media–ethylacetate mixture was vortexed and centrifuged at 500g for 4 min at 4°C. The organic phase was removed from the aqueous phase and evaporated to dryness under N2. The resulting residue was redissolved in 80 μl ethanol and spotted onto a silica gel TLC plate (Merck 60F254).
Separation of metabolites was carried out by thin layer chromatography using dichloromethane:acetone (1:2 v/v) (Frutarom Laboratory Chemicals, Haifa, Israel). On each plate, reference steroids were used and they were located by exposure of the plate to iodine vapor. Stained areas were scraped off and 2.5 ml liquid scintillation fluid added. Radiolabel was counted in β counter in CPM units. The enzyme activity was determined as conversion percents of labeled testosterone to DHT per cell.
Statistical Analysis
The data presented here are from one of three or more independent experiments. For any given experiment, each data point represents the ±SEM for six individual cultures (cell layers). Treatment control ratios were derived from five or more independent experiments, with controls having a ratio of 1.0. Data were analyzed by analysis of variance and statistical significance was determined by comparing each data point to the control (containing the ethanol vehicle) using Bonferroni's t-test. Treatment/control ratios were compared using the Wilcoxon matched pair rank sum test. P < 0.05 was considered significant.
RESULTS
Growth plate chondrocytes were sensitive to testosterone and DHT, but physiological responses to testosterone and its metabolite were both sex and cell maturation-specific. Testosterone regulated [3H]-thymidine incorporation in male growth zone chondrocytes treated with the hormone for 24 h (Fig. 1A). This effect was dose-dependent, significant from 10−10 to 10−7 M, and maximal at 10−9 M. Comparison of data from five independent experiments confirmed this observation (data not shown). In contrast, testosterone caused a dose dependent inhibitory effect on male resting zone chondrocytes that was significant from 10−8 to 10−7 M and maximal at 10−7 M (Fig. 1B). DHT also caused a dose-dependent increase in [3H]-thymidine incorporation in growth zone cells (Fig. 1C), but there was no effect on male resting zone chondrocytes (Fig. 1D). Neither testosterone nor DHT affected [3H]-thymidine incorporation by growth zone (Fig. 2A,B) or resting zone (data not shown) chondrocytes from female rats.
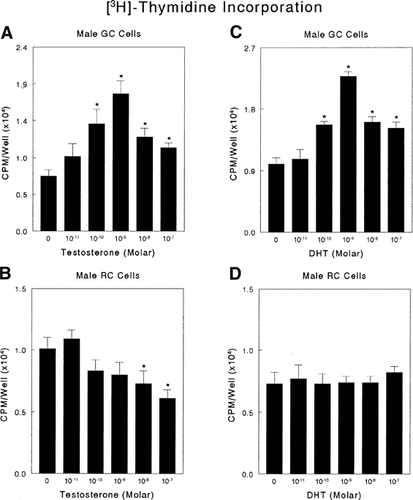
The effect of testosterone and dihydrotestosterone (DHT) on DNA synthesis by growth plate chondrocytes. Quiescent, fourth passage cultures of male rat costochondral growth zone and resting zone chondrocytes were incubated with testosterone (A, B) or DHT (C, D) for 24 h; [3H]-thymidine was added 4 h prior to harvest. Data are from one of five separate experiments, all yielding comparable results. Values are mean ± SEM for six independent cultures. Controls were incubated in medium containing vehicle only. *P < 0.05, treatment versus no exogenous hormone.
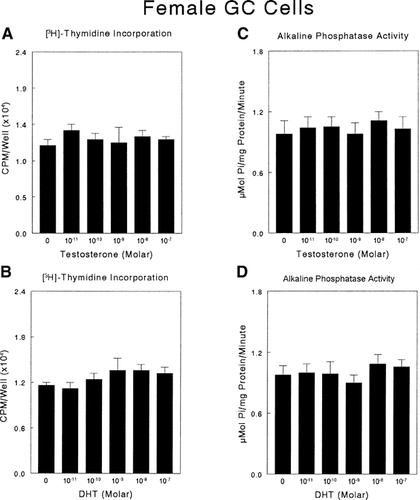
The effect of testosterone and DHT on DNA synthesis and alkaline phosphatase specific activity in female growth zone chondrocytes. Quiescent, fourth passage cultures of female rat costochondral growth zone chondrocytes were incubated with testosterone (A, C) or DHT (B, D) for 24 h. To measure DNA synthesis, [3H]-thymidine was added 4 h prior to harvest (A, B). Alkaline phosphatase specific activity was measured in cell layer lysates (C, D). Data are from one of five separate experiments, all yielding comparable results. Values are mean ± SEM for six independent cultures. *P < 0.05, treatment versus no exogenous hormone.
Testosterone and DHT also regulated alkaline phosphatase in a cell maturation and sex-specific manner. Testosterone caused a dose-dependent stimulation of alkaline phosphatase specific activity in male growth zone chondrocytes, significant at 10−10–10−8 M, with a peak at 10−9 M (Fig. 3A). DHT affected male growth zone chondrocytes in a similar way (Fig. 3C). In contrast to their effects on growth zone cells, neither testosterone (Fig. 3B) nor DHT (Fig. 3D) affected alkaline phosphatase activity in male resting zone chondrocytes. Moreover, neither hormone affected alkaline phosphatase activity in cultures of growth zone cells (Fig. 2C,D) or resting zone cells from female rats (data not shown).
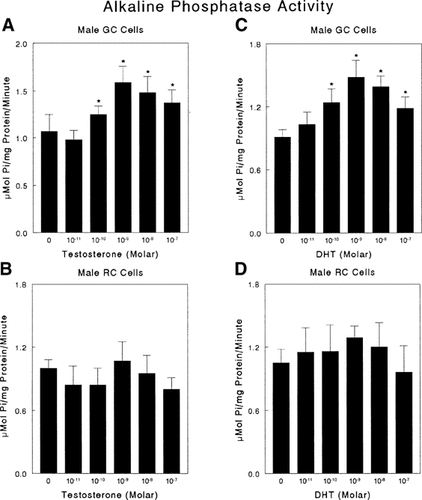
The effect of testosterone and DHT on alkaline phosphatase specific activity in male growth plate chondrocytes. Male rat costochondral growth zone and resting zone chondrocytes were incubated with testosterone (A, B) or DHT (C, D) for 24 h. Alkaline phosphatase specific activity was measured in cell layer lysates. Data are from one of five separate experiments, all yielding comparable results. Values are mean ± SEM for six independent cultures. Controls were incubated in medium containing vehicle. *P < 0.05, treatment versus no exogenous hormone.
The effects of testosterone on male growth zone chondrocytes depended on its metabolism to DHT. The 5α-reductase inhibitor finasteride (1 μg/ml) blocked the effect of testosterone on [3H]-thymidine incorporation in cultures treated with 10−9 M testosterone, but did not impact DNA synthesis in cultures treated with higher concentrations of androgen (Fig. 4A). At higher concentrations, finasteride inhibited DNA synthesis in control cultures as well as in cultures treated with testosterone. Finasteride also reduced the effect of testosterone on alkaline phosphatase in a dose-dependent manner (Fig. 4B). The inhibitor had no effect on alkaline phosphatase in control cultures.
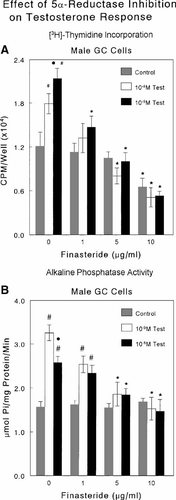
The effect of 5α-reductase inhibition on testosterone-dependent [3H]-thymidine incorporation and alkaline phosphatase specific activity in cultures of male rat costochondral growth zone chondrocytes. Cultures were treated with testosterone ± the 5α-reductase inhibitor finasteride. Effects on DNA synthesis were determined by measuring [3H]-thymidine incorporation in quiescent, preconfluent cultures (A). Effects on differentiation were determined by measuring alkaline phosphatase specific activity in cell layer lysates of confluent cultures treated for 24 h (B). Data are from one of five separate experiments, all yielding comparable results. Values are mean ± SEM for six independent cultures. Controls were incubated in medium containing vehicle only. *P < 0.05, treatment versus no exogenous hormone; #P < 0.05, treatment versus no exogenous hormone for each concentration of finasteride; • < 0.05, 10−9 M testosterone versus 10−8 M testosterone.
5α-Reductase type 1 mRNAs were present in both growth zone and resting zone chondrocytes from male and female rats (Fig. 5A). The identity of the bands was verified by their co-migration with kidney 5α-reductase mRNA at the anticipated size, and the absence of any PCR product from rat epididymis. In addition, the band was sequenced and shown to be the expected PCR product. No mRNA for type 2 was found in either male or female cells (Fig. 5B). Kidney cells were also negative for the type 2 isozyme whereas epididymis cells were positive. Moreover, male growth zone cells possessed active enzyme based on the substrate-dependent conversion of testosterone to DHT and its inhibition by finasteride (Fig. 6). This experiment also showed that the cells secreted DHT into the media. No DHT was found in the conditioned media of female chondrocytes, whether finasteride was present or not (data not shown), indicating that either the cells lacked 5α-reductase activity or that they did not secrete the metabolite.
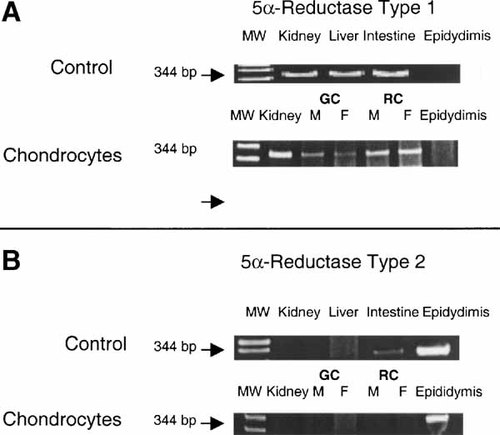
Expression of steroid 5α-reductase isoenzymes in male and female rat costochondral growth zone and resting zone chondrocytes. RNA from rat kidney, liver, intestine, and epididymis as well as male (M) and female (F) growth zone (GC) and resting zone (RC) chondrocytes were examined for the presence of mRNAs encoding type 1 (A) and type 2 (B) isozymes of steroid 5α-reductase. Amplified PCR products were loaded on 5% polyacrylamide gels. Kidney provided the positive control and epididymis provided the negative control for the type 1 isozyme. Kidney was the negative control and epididymis was the positive control for the type 2 isozyme.
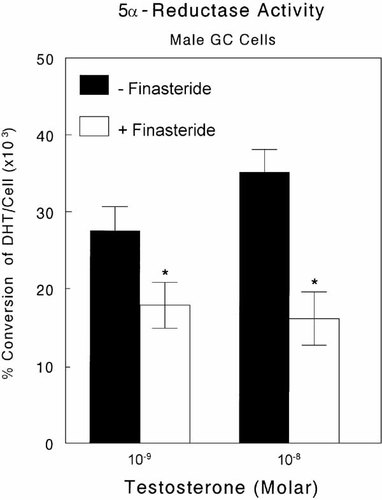
Endogenous steroid 5α-reductase activity in confluent cultures of male rat costochondral growth zone chondrocytes. Cultures were treated for 24 h with 10−9 or 10−8 M testosterone. One half of the cultures were also treated with 1–10 μg/ml finasteride. Values are mean ± SEM for six independent cultures. Data are from one of two separate experiments, both of which yielded comparable results. *P < −0.05, with finasteride versus without finasteride at each testosterone concentration.
DISCUSSION
The results of this study show that Sabra strain rat costochondral growth plate chondrocytes respond to testosterone and DHT and that the response is both sex-specific and maturation state dependent. Only male cells were sensitive to testosterone and DHT treatment and their effects on DNA synthesis and alkaline phosphatase activity varied with the zone of origin of the target cell population. Whereas testosterone and DHT stimulated both proliferation and differentiation of growth zone cells, DHT had no effect on resting zone cells and testosterone inhibited DNA synthesis. These observations support our previous studies using chondrocytes from Sprague–Dawley rats, showing that extracellular matrix production is regulated by testosterone and DHT in a sex-specific and cell maturation dependent manner [Nasatzky et al., 1994b].
The present study shows that the growth plate chondrocytes respond differentially to testosterone and DHT. In male growth zone cells, testosterone-dependent DNA synthesis and alkaline phosphatase activity require metabolism to DHT. In contrast, proliferation of male resting zone cells is decreased by testosterone but is insensitive to DHT, and neither testosterone nor DHT regulate alkaline phosphatase activity. We previously found using chondrocytes from Sprague–Dawley rats that in the absence of FBS, testosterone inhibits DNA synthesis in male resting zone cells [Nasatzky et al., 1994b], suggesting that factors present in serum may modulate the response. Our observations also raise the possibility that more than one form of AR may exist, as is the case for other steroid hormones [Razandi et al., 2004]. Alternatively, the signaling pathways that mediate the effects of testosterone and DHT may be differentially expressed or functional at the two states of endochondral differentiation as has been shown for the vitamin D metabolites [Boyan and Schwartz, 2004].
Our observations on the sex-specificity of the rat chondrocyte response to DHT differ from those reported for fetal human epiphyseal chondrocytes [Audi et al., 1984]. In that report, testosterone only affected cells from male donors, whereas DHT affected cells from both male and female donors. The sex-specific differences in response in the human epiphyseal chondrocytes were not due to differences in receptor expression since cells from both male and female donors exhibited receptors for DHT. Similarly, receptors for testosterone have been identified in male and female human fetal cartilage via immunohistochemistry [Ben Hur et al., 1997]. High affinity testosterone receptors with low binding capacity are also present in costochondral cartilage cells from both male and female rats [Nasatzky et al., 1994a], indicating that receptor availability was not a major factor in our study as well.
Although several laboratories have observed that chondrocytes are regulated by testosterone in a sex-specific manner [Schwartz et al., 1991; Knowles and Bonfield, 1993; Nasatzky et al., 1994a,b; Schwartz et al., 1994; Ben Hur et al., 1997], the mechanisms involved were not understood. The sex-steroid 17β-estradiol regulates cells from female donors via a rapid membrane-mediated pathways [Boyan et al., 2003; Qiu et al., 2003; Stoica et al., 2003], and a similar pathway operating in male cells in response to testosterone and DHT has also been identified [Shakil et al., 2002; Estrada et al., 2003; Lutz et al., 2003]. Whether this pathway contributed to the sex-specific effects of testosterone in the present study was not examined. However, our results show that male cells secrete DHT into their media, suggesting the possibility that it may act back on the cells via autocrine regulatory pathways.
Our results indicate that sex-specific responses of rat costochondral growth zone cartilage cells to testosterone require metabolism of the hormone to DHT via a 5α-reductase dependent mechanism. This novel observation is based on inhibition of testosterone metabolism using finasteride, which inhibits steroid 5α-reductase [Thomas et al., 2003]. Finasteride blocked the effects of testosterone on [3H]-thymidine incorporation and alkaline phosphatase specific activity, indicating that both proliferation and differentiation of the growth zone cells are modulated by steroid 5α-reductase. Both parameters were regulated in a dose-dependent manner, demonstrating that the concentrations of the inhibitor that were used in this study were within the appropriate range. In addition to reducing the response of the growth zone chondrocytes to testosterone, finasteride reduced DNA synthesis in control cultures. This may have been due to the low levels of testosterone present in FBS used to supplement the culture media for normal growth of the rat chondrocyte cultures [Brodie, 1979].
Finasteride is an example of a steroid 5α-reductase inhibitor that has low affinity for the type 1 form of the enzyme and only moderate affinity for the type 2 isozyme [Liang et al., 1983; Peters and Sorkin, 1993; Foley and Kirby, 2003]. Our results show that both resting zone and growth zone chondrocytes express predominantly mRNAs for 5α-reductase type 1, and this isozyme was recently found to be the predominant form present in osteoblasts [Issa et al., 2002] as well as in other human tissues such as brain, kidney, and skin [Thigpen et al., 1993; Stoffel-Wagner et al., 1998]. Finasteride acts more slowly on 5α-reductase type 1, but it is still an effective inhibitor of this enzyme [Tian et al., 1994; Chen et al., 1996]. The time course of the experiments reported here, 24 h, provided ample time for inhibition of the more abundant type 1 isozyme to occur. Moreover, no mRNAs for the type 2 form of the enzyme were identified by RT-PCR, suggesting that if the type 2 isozyme was present in the cells, it was at very low levels.
Expression of steroid 5α-reductase isozymes does not in itself explain the sex-specificity of the response to testosterone. Male and female chondrocytes both expressed mRNAs for steroid 5α-reductase type 1, indicating that potential for testosterone metabolism was present in both. In vivo, circulating hormone would limit the availability of substrate in female animals, but under the in vitro conditions used here, male and female cells were exposed to identical hormone treatment. However, only male cells had detectable 5α-reductase activity based on the release of radiolabeled DHT into the conditioned media. This suggests that the sex-specificity seen in growth zone cells was conferred via differences in testosterone uptake, differences in the rate or extent of testosterone metabolism to estradiol [Vermeulen, 1976; Brodie, 1979] or DHT, changes in regulators of 5α-reductase activation or deactivation, differences in the release of DHT into the media, or differences in DHT receptors and subsequent downstream pathways.
As noted above, costochondral cartilage cells from male rats respond to DHT and testosterone in distinct ways that depend on the maturation state of the cell. DHT caused a dose-dependent increase in DNA synthesis and alkaline phosphatase specific activity in growth zone cells that was maximal at 10−9 M, which was comparable to the effect of testosterone. These experiments used primary chondrocytes and not cell lines, so variation in control levels were seen between experiments. However, each experiment was conducted multiple times and treatment/control ratios confirmed the dose-dependence, sex-specificity, and cell-maturation-state specificity of the observed effects.
It is likely that testosterone was metabolized to DHT over the 24 h incubation period. DHT elicits effects at 10−10 M and maximal effects of testosterone were seen in cultures treated with 10−9 M hormone. A 30–50% conversion rate of testosterone to DHT accounts for the congruity of the two datasets. Moreover, finasteride treatment reduced the effects of testosterone, supporting this interpretation.
In contrast to growth zone cells, DHT had no effect on [3H]-thymidine incorporation by resting zone cells, whereas testosterone inhibited DNA synthesis, and neither DHT nor testosterone affected alkaline phosphatase activity. These results confirm our previous observations concerning testosterone [Nasatzky et al., 1994b; Schwartz et al., 1994], as well as those of other laboratories [Corvol et al., 1987; Blanchard et al., 1991; Schwartz et al., 1994], and support earlier reports that DHT affects chondrocytes in a maturation-dependent manner [Corvol et al., 1987]. Growth plate chondrocytes respond to other steroid hormones in a cell maturation dependent manner [Schwartz et al., 1988; Swain et al., 1992]. As chondrocytes progress through the endochondral differentiation pathway, there are marked changes in the biochemical composition of the cells and in their physiology. Differences in cell response to testosterone and DHT may reflect shifts in steroid hormone signaling in addition to differences in steroid hormone metabolism as a function of cell maturation state.
In summary, the results of this study demonstrate that 5α-reductase type 1 is the predominant form expressed by rat costochondral cartilage cells. In order to exert its effect on growth zone chondrocytes, testosterone must first, as in most androgen target tissues, be reduced to its more active metabolite, DHT. Moreover, DHT regulation of chondrocyte proliferation and differentiation is sex-specific and maturation-dependent.
Acknowledgements
The authors thank Ms. Wanda Whitfield for her help in the preparation of the manuscript.




