Bcl-2 prevents hypoxia/reoxygenation-induced cell death through suppressed generation of reactive oxygen species and upregulation of Bcl-2 proteins
Abstract
The function of bcl-2 in preventing cell death is well known, but the mechanisms whereby bcl-2 functions are not well characterized. One mechanism whereby bcl-2 is thought to function is by alleviating the effects of oxidative stress upon the cell. To examine whether Bcl-2 can protect cells against oxidative injury resulting from post-hypoxic reoxygenation (H/R), we subjected rat fibroblasts Rat-1 and their bcl-2 transfectants b5 to hypoxia (5% CO2, 95% N2) followed by reoxygenation (5% CO2, 95% air). The bcl-2 transfectants exhibited the cell viability superior to that of their parent non-transfectants upon treatment with reoxygenation after 24-, 48-, or 72-h hypoxia, but not upon normoxic serum-deprivation or upon serum-supplied hypoxic treatment alone. Thus bcl-2 transfection can prevent cell death of some types, which occurred during H/R but yet not appreciably until termination of hypoxia. The time-sequential events of H/R-induced cell death were shown to be executed via (1) reactive oxygen species (ROS) production at 1–12 h after H/R, (2) activation of caspases-1 and -3, at 1–3 h and 3–6 h after H/R, respectively, and (3) loss of mitochondrial membrane potential (ΔΨ) at 3–12 h after H/R. These cell death-associated events were prevented entirely except caspase-1 activation by bcl-2 transfection, and were preceded by Bcl-2 upregulation which was executed as early as at 0–1 h after H/R for the bcl-2 transfectants but not their non-transfected counterpart cells. Thus upregulation of Bcl-2 proteins may play a role in prevention of H/R-induced diminishment of cell viability, but may be executed not yet during hypoxia itself and be actually operated as promptly as ready to go immediately after beginning of H/R, resulting in cytoproteciton through blockage of either ROS generation, caspase-3 activation, or ΔΨ decline. © 2003 Wiley-Liss, Inc.
Bcl-2, a mammalian homologue of the anti-apoptotic gene ced-9 in C. elegans, is localized mainly to the mitochondrial membrane [Hockenbery et al., 1990; Akao et al., 1994] and is known to be a key regulator to apoptosis, functioning as an anti-apoptotic protein with the ability to protect against a variety of physiologic or pathologic insults and environmental stimuli [Tsujimoto and Croce, 1986; Vaux et al., 1988; Korsmeyer, 1992; Hawkins and Vaux, 1994; Reed, 1994]. A number of mechanisms have been proposed to explain the ability of Bcl-2 to suppress apoptosis [Oltvai et al., 1993; Kluck et al., 1997; Yang et al., 1997; Shimizu et al., 1998]. One of the proposed mechanisms is a function of Bcl-2 as an apparent antioxidant [Hockenbery et al., 1993; Kane et al., 1993], which seems to be important because there are growing evidences that reactive oxygen species (ROS) plays a key role in the regulation of apoptosis [Buttke et al., 1994; Bonfoco et al., 1995; Salgo et al., 1995; Lin et al., 1997]. On the other hand, an antioxidant role for Bcl-2 was questioned in other reports [Jacobson and Raff, 1995; Shimizu et al., 1995] because Bcl-2 expression blocked apoptosis under anaerobic conditions in which ROS is not generated. Thus, it is not yet clear about the anti-apoptotic function of Bcl-2 in spite of extensive studies.
Post-hypoxic reoxygenation (H/R) is important in human pathophysiology because it occurs in a wide variety of vital clinical conditions such as circulatory shock, myocardial ischemia, stroke, and transplantation of organs [Robin and Theodore, 1982; McCord, 1985; Levinson et al., 1986]. Numerous studies have implicated ROS as playing an important role in the pathogenesis of H/R injury, and ROS has been implicated in apoptosis after H/R [Huang et al., 2002; Li et al., 2002a]. Cellular models of H/R have provided useful tools for the study of ROS-mediated mechanisms of cellular dysfunction [Watkins et al., 1995].
In the present study, we used a cell culture model of H/R to investigate the effects of Bcl-2 on ROS-induced cell death and to elucidate the practical mechanisms involved in anti-apoptotic function of Bcl-2.
MATERIALS AND METHODS
Cell Culture
Rat fibroblastic cells Rat-1 [Topp, 1981] and their bcl-2 transfectants b5 [Tsujimoto, 1989] were kindly provided by Dr. Shoji Yamaoka of Tokyo Med Dent Univ Rat-1 cells (non-transfectants) were cultured in Dulbecco's modified Eagle's medium (DMEM, Nissui Pharmaceutical CO., LTD, Tokyo, Japan) containing 10% heat-inactivated fetal bovine serum (FBS) (GIBCO BRL, Grand Island, NY), 4 mM l-glutamine, 50 μg/ml penicillin, and 50 μg/ml streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2. The bcl-2 transfected b5 cells (transfectants) were cultured in DMEM containing 10% heat-inactivated FBS, 4 mM l-glutamine, 50 μg/ml penicillin, and 50 μg/ml streptomycin and 600 μg/ml Geneticin disulfate (Wako Pure Chemical Industries, Osaka, Japan) at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Hypoxia/Reoxygenation Experiments
Hypoxia was created by incubating cells in a modular-incubator with a diameter of 35 cm (Billups-Rothenberg, Inc., Del-Mar, CA) containing a deoxidizer Anaerocult A mini (Merck, Tokyo, Japan) at 37°C in a humidified atmosphere of 95% N2 and 5% CO2 for 24, 48, or 72 h. By replacing under the standard cell culture conditions (37°C, 95% air and 5% CO2), the cells were exposed to normoxic atmosphere (reoxygenation) for indicated times.
Cell Viability (Mitochondrial Dehydrogenase Activity) Assay
Cell viability, closely correlated with mitochondrial dehydrogenase activity, was evaluated by WST-1 assay. Briefly, the cell layer in a dish was incubated with WST-1 (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) (Dojin Laboratories Co., Kumamoto, Japan) solution at 1:10 volume of phenol red-free culture medium for 3 h at 37°C. Viable cells with activity of mitochondrial dehydrogenases such as succinate dehydrogenage are capable of reducing the WST-1 dye to generate the yellowish formazan. At the end of incubation period, the absorbance of each sample was measured at 450 nm with a Bio-Rad absorbance plate reader (Bio-Rad, Hercules, CA), and the absorbance detected has been demonstrated to be proportional to viable cell number. Since there was no difference in the basal viability (mitochondrial dehydrogenase activity) between non-transfectants and transfectants, the values obtained from control cultures (non-treated non-transfectants and transfectants) are represented as 100% viability. The values of treated cultures are expressed as a percentage of those versus the corresponding control cells.
Measurement of ROS Production
The ROS production was assessed using the fluorescent probe 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (CDCFH-DA) (Molecular Probes, Eugene, OR). This dye is a stable compound that readily diffuses into cells and is hydrolyzed by intracellular esterase to yield CDCFH, which is trapped within cells. Hydrogen peroxide or low-molecular-weight peroxides produced by cells oxidizes CDCFH to the highly fluorescent compound 6-carboxy-2′,7′-dichlorofluorescin (CDCF). Thus, the fluorescence intensity is proportional to the amount of intracellular peroxide produced by the cells. Cells were rinsed twice with phosphate-buffered saline (PBS) and replaced by phenol red-free DMEM containing 20 μM CDCFH-DA. After 60-min incubation, the fluorescence intensity was measured with a fluorescence microplate reader CytoFluor 2350 (Millipore, Bedford, MA) with excitation and emission wavelengths of 510 and 534 nm, respectively. Fluorescence of the CDCF increased in a manner dependent on cell numbers and incubation times for viable cells, but scarcely for methanol-killed cells that were similarly treated as the blank. ROS production is expressed as a fluorescence intensity per 104 cells. The total number of cells was determined using a Coulter electric particle counter ZBII (Beckman Coulter, Tokyo, Japan) after trypsinization of the cells.
Assay of Caspase-1 and -3 Activities
Caspase-1 and -3 activities were assessed by fluorometric assay quantifying the extent of cleavages of the fluorometric peptide substrate using a CaspACE™ Assay System kit (Promega, Madison, WI). Cells were collected by trypsinization and suspended in an ice-cold buffer containing 10 mM Tris-HCl (pH 7.5), 2 mM MgCl2, 5 mM EDTA, 2 mM DTT, 2 mM PMSF, 4 μM leupeptin, and 3 μM pepstatin A. After three-times freeze-thawing steps, the lysates were obtained by centrifugation at 16,000g for 20 min at 4°C and the supernatant was collected, and the amount of protein was measured using DC Protein Assay kit (Bio-Rad). The lysates were mixed with ICE-Like Assay Buffer containing 312.5 mM HEPES (pH 7.5), 31.25% sucrose, 0.3125% CHAPS, 2% DMSO, 10 mM DTT, and 50 μM inhibitors of caspase-1 and -3, respectively. After 30-min incubation at 30°C, 50 μM of tetrapeptide substrate, acetyl-tyrosyl-valyl-alanyl-aspartic acid 7-amino-4-methyl coumarin; (Ac-YVAD-AMC) for caspase-1 or acetyl-aspartyl-glutamyl-valyl-aspartic acid 7-amino-4-methyl coumarin; (Ac-DEVD-AMC) for caspase-3 were added to the lysates and incubated for 60 min at 30°C. The fluorescence which was derived from free aminomethylcoumarin (AMC) released by substrate cleavage was measured with a fluorescence plate reader CytoFluor 2350 (Millipore) with excitation and emission wavelengths of 360 and 460 nm, respectively. Fluorescent units were converted to picomoles of AMC using a standard calibration curve generated with free AMC per microgram protein.
Determination of Mitochondrial Membrane Potential (ΔΨ)
Mitochondrial membrane potential (ΔΨ) was determined by the uptake of Rhodamine 123 (R123) (Molecular Probes). Cells were harvested by trypsinization and centrifuged at 800g for 5 min. Then, cells were incubated with prewarmed phenol red-free DMEM containing 10 μM of R123 for 15 min at 37°C. After being labeled with R123, cells were washed and resuspended in PBS. Then the ΔΨ was determined by analysis with a Coulter flow cytometer Epic Elite (Beckman Coulter). The excitation wavelength was 505 nm. The emission fluorescence for R123 was monitored at 534 nm.
Western Blot Analysis
Cells were washed twice with PBS and lysed with an ice-cold buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM DTT, 1 mM PMSF, 1% IGEPALCA-630, 1% SDS, 4 μM leupeptin, and 3 μM pepstatin A. After being three times freeze-thawed, the lysate was centrifuged at 20,000g for 5 min at 4°C and the supernatant was collected. The amount of protein was measured using DC Protein Assay kit (Bio-Rad). The cell lysates were resuspended in buffer containing 62.5 mM Tris-HCl (pH 6.8), 15% glycerol, 10% β-mercaptoethanol, 0.005% bromophenol blue, and 4% SDS. Then the cell lysates were boiled for 3 min and applied to a 12% SDS–polyacrylamide gel, and the separated proteins were blotted to 0.45-μm polyvinylidene difluoride (PVDF) membranes (Millipore). Nonspecific binding was blocked by incubating the membranes for 2 h at room temperature in a blocking buffer containing 50 mM Tris-HCl (pH 7.5), 3% bovine serum albumin, and 150 mM NaCl. The membranes were then stained with the 1:2,500 diluted mouse monoclonal antibody against human Bcl-2 (product number sc-509; Santa Cruz Biotechnology, Santa Cruz, CA) in blocking buffer overnight at 4°C with agitation. After they were washed three times with washing buffer containing 50 mM Tris (pH 7.9), 100 mM NaCl, and 0.05% Tween-20, the membranes were incubated with the 1:3,000 diluted horseradish peroxidase-conjugated anti-mouse antibody in a blocking buffer for 30 min at room temperature. After they were washed twice with the washing buffer, the membranes were washed with the blocking buffer. The specific bands were detected using an enhanced chemiluminescence (ECL) detection system (Amersham-Pharmacia Biotech, England, UK), and blots were exposed to Hyperfilm MP (Amersham-Pharmacia Biotech) for 0.5–2 min. Laser scanning densitometry was conducted for semiquantitative analysis of the data. Loading of approximately equivalent amount of protein content was confirmed by the densitometric values of a randomly selected band on the Coomassie Brilliant Blue stained gel.
Statistical Analysis
Unpaired Student's t-test was used to evaluate the significance of differences between groups, and the criterion of statistical significance was taken as P < 0.05.
RESULTS
Effects of Serum-Deprivation on Cell Growth
Serum is well-known to act as an ROS scavenger, and is necessary to be removed from cell culture medium in the experiments which might generate ROS upon H/R. So, we firstly examined whether there are some differences in growth rates of non-transfectants Rat-1 and their bcl-2 transfectants b5 under the serum-deprivation condition. Serum-deprivation slightly reduced the rate of cell growth compared with serum-supplied condition (Fig. 1), but it did not markedly induce cell death in both cells. In addition, there were not significant differences in cell growth rates between non-transfectants and transfectants under either condition. Therefore, we decided to thereafter conduct the H/R experiments under the serum-free condition.
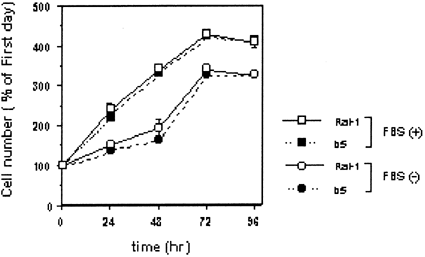
Effects of serum on cell growth of rat fibroblasts Rat-1 and their bcl-2 transfectants b5 cells. Cells were plated at a density of 1.0 × 104 cell/cm2 in 24-well plates. After preincubation for 18–24 h, the medium was replaced by serum-supplied medium or serum-deprived medium. Cells were further incubated under the normoxic condition (95% air and 5% CO2). After incubation for the indicated periods, the cell viability was assessed by WST-1 assay. The data shown are typical of three independent experiments. The bar represents the SD of wells in triplicate. Significantly different from Rat-1 cells: *P < 0.05; **P < 0.01.
Effects of Hypoxia on Cell Growth
We examined the effect of hypoxia on cell growth in non-transfectants and transfectants after graded times of exposure to the hypoxic condition (Fig. 2). Even upon exposure to hypoxia for less than 72 h, cell number gradually increased in time-dependent manner in both cells, but upon 96-h hypoxia, cell number began to be decreased in both cells. Thus, hypoxia by itself without reoxygenation did not markedly affect the cell growth so far as a period shorter than 72 h was concerned. From these results, we decided to subject to hypoxia for 24, 48, or 72 h. Additionally, there were not significant differences in the cell number between non-transfectants and transfectants under the hypoxia condition.
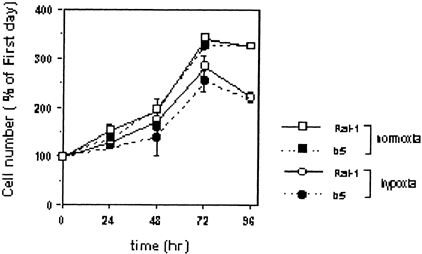
Effects of hypoxic treatment on cell viability of non-transfectants Rat-1 and their bcl-2 transfectants b5. Cells were plated at a density of 1.0 × 104 cell/cm2 in 24-well plates. After preincubation for 18–24 h, the medium was replaced by serum-free medium. Cells were then incubated under the hypoxic condition (95% N2 and 5% CO2). After incubation for the indicated periods, the cell viability was assessed by WST-1 assay. The data shown are typical of three independent experiments. The bar represents the SD of wells in triplicate. Significantly different from non-transfectants: *P < 0.05; **P < 0.01.
Treatment With H/R Induces Cell Death
To examine whether H/R induces cell death in non-transfectants Rat-1 and their bcl-2 transfectants b5, we investigated the effect of reoxygenation on cell viability after hypoxia for 24, 48, and 72 h. When non-transfectants and transfectants were exposed to hypoxia for 24 h, cell viability of non-transfectants was slightly diminished after reoxygenation. Hence, the cell viability of transfectants was not affected by reoxygenation, and was significantly higher than that of the parent non-transfectants (Fig. 3A). In the case of hypoxia for 48 h, the cell viability of non-transfectants was diminished to levels lower than those for 24-h hypoxia. In contrast, transfectants were scarcely affected by reoxygenation, and the viability was markedly superior to that of the parent non-transfectants against H/R-induced cellular injuries (Fig. 3B). Hypoxia for a period as long as 72 h appreciably diminished the cell viability in both cells after reoxygenation (Fig. 3C). After 6 h of reoxygenation, the percentage of the viable cells decreased drastically, reaching levels as low as only 20% in non-transfectants and 43% in transfectants versus that of the normoxic control, respectively. For any periods after reoxygenation, however, the cell viability of transfectants was not so low as that of the parent non-transfectants. Regardless of hypoxia periods of 24, 48, or 72 h, the cell viability of both the cell lines began to be restored after 6 h of reoxygenation, assumedly because of proliferation of the surviving cells that tided over the crisis immediately after beginning of reoxygenation. Thus reoxygenation is a more potent inducer towards cell death. Taken together, the cell viability of non-transfectants was decreased after reoxygenation in a manner dependent on hypoxia periods. In addition, the bcl-2 transfectants could significantly attenuate the reoxygenation-induced cell death than the parent non-transfectants could against H/R-induced injuries, especially after 48-h hypoxia (Fig. 3B). These results showed that transfection with bcl-2 genes exerts a cytoprotective effect against H/R-induced injuries.
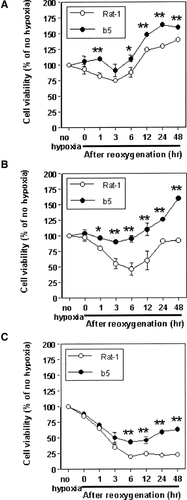
Effects of post-hypoxic reoxygenation (H/R) on cell viability of non-transfectants Rat-1 and their bcl-2 transfectants b5. Cells were subjected to hypoxic treatment for 24 (A), 48 (B), or 72 h (C) and then reoxygenated followed by standing for indicated periods. After the indicated reoxgenation periods, the cell viability was assessed by WST-1 assay. The data shown are typical of three independent experiments. The bar represents the SD of wells in triplicate. Significantly different from non-transfectants: *P < 0.05; **P < 0.01.
Increases in Intracellular ROS After H/R Exposure
We attempted to examine whether the exposure of H/R can cause accumulation of intracellular ROS, and whether bcl-2 gene transfection can influence ROS levels. The intracellular ROS levels were quantified by fluorometry using the fluorescein derivative CDCFH as a redox indicator which primarily detects H2O2, hydroxyl radicals, and diverse peroxides [Sejda et al., 1984]. There was no significant difference in ROS levels of both cell lines within 1 h of reoxygenation after 48- or 72-h hypoxia, when ROS generation was not significantly elevated over levels for the normoxic state in both cell lines (Fig. 4B). However, ROS levels in non-transfectants gradually increased after 1 h of reoxygenation, and reached the maximum level (approximately 2.1- and 6.0-fold increases versus the untreated level, for 48- and 72-h hypoxia, respectively) after 6 h of reoxygenation, whereas the ROS level in transfectants reached the maximal value at the same time, being as low as approximately 1.2- and 2.0-fold increases, respectively. Thus the rise of ROS levels was significantly suppressed in bcl-2 transfectants when compared to non-transfectants. The increase in ROS levels was reversed down to the control level at 24 h after reoxygenation. On the other hand, ROS levels did not change notably by 24-h hypoxia exposure during not only hypoxia but also reoxygenation. In separate experiments, monolayers of cells grown on glass coverslips were exposed to hypoxia or normoxia for 48 h, followed by reoxygenation and standing for 6 h. Intracellular CDCF fluorescence due to ROS was assessed by fluorescence microscopy with digital image analysis. Non-transfectants exposed to H/R showed a marked increase in intracellular fluorescence compared with transfectants (Fig. 4A). Thus the transfection with bcl-2 genes exerts inhibitory effects against reoxygenation-induced ROS production.
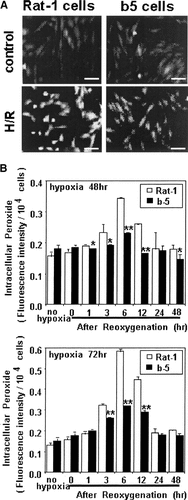
Intracellular reactive oxygen species (ROS) in non-transfectants Rat-1 and their bcl-2 transfectants b5 subjected to hypoxia/reoxygenation. A: Microscopic detection of intracellular 6-carboxy-2′,7′-dichlorofluorescin (CDCF)-attributed fluorescence. Cells grown on glass coverslips were exposed to hypoxia or normoxia for 48 h followed by reoxygenation for 6 h. After the exposure, cells were loaded with the redox indicator dye 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (CDCFH-DA), and the intracellular fluorescence was detected by fluorescence microscopy with digital image analysis (magnification ×200). The scale bar indicates 50 μm. B: Fluorometric analysis of intracellular CDCF fluorescence. Cells were subjected to hypoxia for 24, 48, or 72 h and then reoxygenated for indicated periods. The dye CDCFH-DA was added to the cells, followed by 60-min redox reaction and subjected to fluorometry with excitation at 485 nm and emission at 530 nm. The data shown are typical of three independent experiments. The bar represents the SD of wells in triplicate. Significantly different from non-transfectants: *P < 0.05; **P < 0.01.
Caspase-1 and -3 Activation After H/R Exposure
Caspase-1 and -3, members of the caspase family, play key roles in inflammation and apoptosis in mammalian cells [Thornberry et al., 1992; Nicholson et al., 1995; Tewari et al., 1995; Fernandes-Alnemri et al., 1996]. To investigate the role of bcl-2 genes in H/R-induced cell death in non-transfectants Rat-1 and bcl-2 transfectants b5, the activation of caspase-1 and -3 was monitored as a hallmark of apoptosis (Fig. 5). Caspase-1 activity in both cell lines, at 1–3 h after reoxygenation, was significantly increased about 3-fold increase as compared to before the exposure. The activation and repression of caspase-1 may be involved in the H/R-induced cell death mechanisms, but were not prevented by bcl-2 gene transfection. On the other hand, caspase-3 activity in non-transfectants at 6 h after reoxygenation was increased about 10-fold as compared to the control cells. In contrast, the degree of caspase-3 activation was significantly suppressed in transfectants when compared to parent cells at both 3 and 6 h. Thus activation and repression of caspase-3 may be involved in the mechanisms whereby cell death was induced by H/R and prevented by bcl-2 gene transfection.
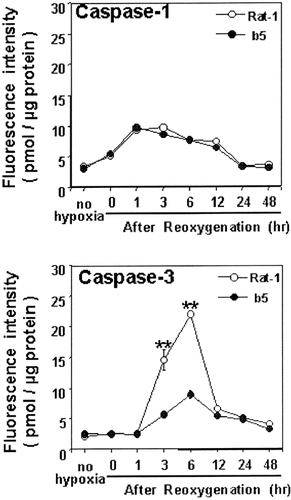
Activation of caspases-1 and -3 in non-transfectants (Rat-1 cells) and bcl-2 transfectants (b5 cells) subjected to hypoxia/reoxygenation. Cells were subjected to hypoxia for 48 h and then reoxygenated for indicated periods. Cytosolic protein was extracted immediately before hypoxia exposure or at indicated periods after H/R, and measured for caspase-1 and -3 activities using synthetic fluorescent substrates. The data shown are typical of three independent experiments. The bar represents the SD of dishes in triplicate. Significantly different from non-transfectants: *P < 0.05; **P < 0.01.
Mitochondrial Membrane Potential (ΔΨ) After H/R Exposure
A number of studies have implicated that the mitochondria plays a central role in apoptosis, and revealed that opening of permeability transition (MPT) pores, which is associated with collapse of the mitochondrial membrane potential (ΔΨ), is a determinative event leading to apoptosis [Green and Reed, 1998; Kroemer and Reed, 2000; Vieira et al., 2000]. To examine whether H/R can induce the collapse of ΔΨ and whether bcl-2 transfection can prevent the ΔΨ collapse, we assessed ΔΨ in H/R-stimulated cells using R123. R123 is a lipophylic, cationic fluorescent dye that is accumulated within the mitochondria according to the ΔΨ, which substantially decides the R123 fluorescence intensity. In non-transfectants that were subjected to reoxygenation after 48-h hypoxia, ΔΨ was reduced at 3–12 h and lowered to 20% of the initial value at 6 h after H/R (Fig. 6). In contrast, ΔΨ of transfectants was retained over 80% versus the initial level, and superior to that of the parent non-transfectants. Thus transfection with bcl-2 genes exerts inhibitory effects against H/R-induced loss of ΔΨ.
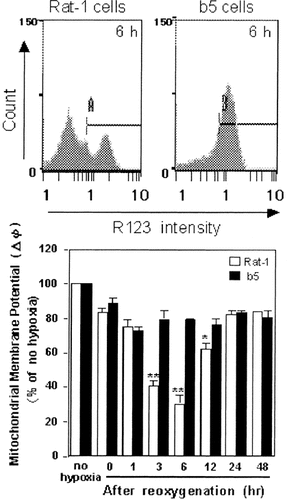
Mitochondrial membrane potential (ΔΨ) in non-transfectants (Rat-1 cells) and bcl-2 transfectants (b5 cells) subjected to hypoxia/reoxygenation. Cells were subjected to hypoxic treatment for 48 h and then reoxygenated for indicated periods. Then the mitochondrial membrane potential was estimated with a flow cytometer using the fluorescent probe Rhodamine 123 (R123) as typically shown by the upper histograms and the time course in the lower graph. The data shown are typical of three independent experiments. The bar represents the SD of dishes in triplicate. Significantly different from non-transfectants: *P < 0.05; **P < 0.01.
Expression of Bcl-2 Protein After H/R Exposure
To obtain further insight into the mechanism of cytoprotection against H/R-induced cell death, we also analyzed Bcl-2 expression after H/R exposure by Western blots and densitometry (Fig. 7). Before hypoxia exposure, Bcl-2 expression in transfectants were markedly overexpressed when compared to their non-transfected counterparts, Rat-1 cells. Bcl-2 expression in transfectants were increased to approximately 208% of the unexposed control immediately after termination of 48-h hypoxia, and the expression was increased to the maximum by 284% at 1 h after reoxygenation. The expression level once returned to the same extent as the control at 6 h after, and rose again even by about 224% at 48 h. In contrast, Bcl-2 expression in non-transfectants was as low as undetectable not only before H/R exposure but also after H/R exposure.
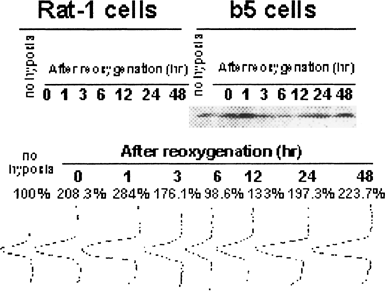
Expression of Bcl-2 proteins in non-transfectants (Rat-1 cells) and bcl-2 transfectants (b5 cells) that were subjected to hypoxia/reoxygenation. Cells were subjected to hypoxia for 48 h and then reoxygenated for indicated periods. Cellular proteins were extracted immediately before hypoxia exposure (control) or at indicated periods of H/R, and subjected to Western blots using anti-Bcl-2 antibody and the resultant densitometry.
DISCUSSION
It has been shown that enforced overexpression of Bcl-2 in cultured cells confers the resistant ability to overcome apoptosis that is induced by diverse cytotoxic triggers [Vaux et al., 1992; Hockenbery et al., 1993; Mah et al., 1993; Zhong et al., 1993; Raffo et al., 1995; Lock and Stribinskiene, 1996; Kirshenbaum and de Moissac, 1997; Mirkovic et al., 1997], but the preventive mechanisms underlying in common with diverse ROS-generators have not been resolved clearly yet. In the present study, to elucidate the anti-apoptotic mechanisms of Bcl-2, we investigated the effects of Bcl-2 on H/R-induced cell death using rat fibroblasts Rat-1 and their bcl-2 transfectants b5, which markedly expressed Bcl-2 proteins (Fig. 7). Although treatment with H/R induced the cellular injury in rat fibroblasts (non-transfectants), Bcl-2 significantly attenuated H/R-induced cell death regardless of hypoxia periods (Fig. 3). Since Bcl-2 did not affect cell proliferation (Figs. 1 and 2), it is thought that Bcl-2 mainly inhibited the cellular injury after H/R.
It is well known that Bcl-2 prevents apoptosis, and many studies suggest that H/R-induced cell death is associated with apoptosis [Huang et al., 2002; Li et al., 2002a]. Then, we investigated the activation of caspases and the loss of ΔΨ in order to prove that apoptosis participates in H/R-induced cell death. Although multiple processes can be involved in apoptosis, extensive investigations indicate that activation of two cysteine proteases, caspase-1 and -3, are participated in the execution of the cell death process [Martins et al., 1997; Yuan, 1997; Thornberry and Lazebnik, 1998]. Especially, caspase-3, when it is once activated, is responsible for many of the downstream events associated with apoptosis. Activation of caspase-3 was prevented in the bcl-2 transfectants after 3- and 6-h of H/R, whereas activation of caspase-1 was not prevented (Fig. 5). Caspase-1 is activated followed by the activation of caspase-3, and may be therefore the upstream regulator of caspase-3 activation as previously indicated [Enari et al., 1996; Cohen, 1997]. Taken together, our results suggest that Bcl-2 participates in control of H/R-induced cell death between caspase-1 and -3. Furthermore, loss of ΔΨ has been defined as an early stage of apoptosis [Green and Reed, 1998; Kroemer and Reed, 2000]. The loss of ΔΨ was detected at 3–12 h after H/R (Fig. 6) suggests that H/R evoked the activation of apoptosis pathway. Additionally, the suppression by overexpressed Bcl-2 (Fig. 7) of the ΔΨ loss after H/R exposure (Fig. 6) suggests an important role of ΔΨ maintenance in the cytoprotective effects of Bcl-2. It is well-known fact that caspase-3 is located at a downstream point of mitochondrial pathway in apoptosis signal transduction. Bcl-2 suppressed the mitochondrial dysfunction due to the loss of ΔΨ, and is therefore thought not to evoke caspase-3 activation. These results were consistent with our previous report that Bcl-2 suppresses a model reagent of lipid hydroperoxide, tert-butyl hydroperoxide (t-BuOOH)-induced apoptosis [Saitoh et al., 2003].
Our results also indicated that H/R induced generation of ROS, which was significantly suppressed by Bcl-2 (Fig. 4), suggesting that cytoproteciton by Bcl-2 against H/R (Fig. 3) may be attributed to a putative ability of Bcl-2 to suppress ROS production. In addition, it was suggested that ROS is an upstream mediator of H/R-induced cell death, because the increase in ROS production was occurred prior to the loss of ΔΨ, activation of caspase-3 and cell death. ROS is considered to be an important trigger to initiate cell death in response to a variety of signals and pathophysiological situations [Voehringer et al., 2000]. Diverse sources of ROS (e.g., enzymes, mitochondria) exist normally within cells, some of which produce excess ROS during H/R. Excess ROS that was generated from endogenous sources induced the cellular injury during reoxygenation [Li et al., 2002a]. The degree of H/R-induced cellular injury was dependent on hypoxia duration time (Fig. 3) and might be correlated with the amount of ROS that was generated during reoxygenation (Fig. 4B). Hypoxia-induced decreases in cellular glutathione and antioxidant enzymes such as Mn-SOD could lead indirectly to cellular reoxygenation injury by exacerbating oxidant stress [Li et al., 2002b; Makarov et al., 2002].
A number of mechanisms have been proposed to explain the ability of Bcl-2 to suppress apoptosis [Oltvai et al., 1993; Kluck et al., 1997; Yang et al., 1997; Shimizu et al., 1998]. One of the proposed mechanisms is a function of Bcl-2 as an apparent antioxidant [Hockenbery et al., 1993; Kane et al., 1993], which seems to be important because there are growing evidences that ROS plays a key role in the regulation of apoptosis [Buttke et al., 1994; Bonfoco et al., 1995; Salgo et al., 1995; Lin et al., 1997]. Recently, it was suggested that Bcl-2 overexpression allows cells to adapt to ROS overproduction [Esposti et al., 1999], and Bcl-2 expressing cells elevate their intracellular redox potential through high-concentrated GSH and NAD(P)H and through the enhanced level of fatty acid binding proteins (FABP), which might act as molecular scavengers through binding the cytotoxic oxidized forms of fatty acids that are generated by oxidative stress at the early stages of apoptosis [Mei et al., 1997; Voehringer et al., 2000]; Bcl-2 expressing cells promote intracellular uptake of the exogenously added ascorbic acid and its accumulation [Saitoh et al., 2003]. Thus it is reasonably thought that Bcl-2 proteins may control ROS generation indirectly by reinforcing the intracellular redox potential rather than by eliminating ROS directly. Additionally, the increased expression of Bcl-2 immediately after beginning of H/R (Fig. 7) indicates that upregulation of Bcl-2 may lead without missing of a favorite opportunity to the resolute realization of not only an increase in intracellular antioxidative potentials to suppress ROS production which is steeply generated within 1–30 min after post-hypoxic regeneration [Hashimoto et al., 1994; Eguchi et al., 2003], but also the suppressions of both loss of ΔΨ and caspase-3 activation. Thus, our present study provides an evidence for a close relationship between the suppression of cell death and upregulation of Bcl-2. From these reasons, we suggest that the H/R-induced oxidative stress may trigger the upregulation of Bcl-2, which is one of the important physiological reactions to protect the cells from oxidative stress-induced apoptosis. The upregulation of Bcl-2 proteins by oxidization stress (Figs. 4 and 7) is also consistent with a peroxylipid model reagent-induced upregulation of Bcl-2 as shown by our previous report [Saitoh et al., 2003]. Bcl-2 belong to a member-growing family of apoptosis-regulating gene products, which may either be antagonists (Bcl-2, Bcl-XL) or death agonists (Bax, Bak) [Kroemer, 1997]. Bcl-2 can inhibit the activation of pro-apoptotic family members such as Bax and Bak, through heterodimerization, both of which were known as mediators that are operated by multiple apoptotic stimuli such as the mitochondrial disfunction and the endoplasmic reticulum stress [Wei et al., 2001]. In typical cells that are exempted from apoptosis, both pro-apoptotic and anti-apoptotic family members (such as Bax and Bcl-2, respectively) seem to be in equilibrium. Apoptosis is regulated by the balance of the action of pro- and anti-apoptotic Bcl-2 proteins [Oltvai et al., 1993]. The secondary increase in expression of Bcl-2 protein after 24–48 h of H/R (Fig. 7) may be the cellular defensive reaction for corresponding promptly to a possible oxidative stress that may next occurs.
Our results demonstrated that the cell death-associated events such as ROS production, activation of caspases-1 and -3, and loss of ΔΨ were preceded by upregulation of Bcl-2 that plays a critical role for their control. Moreover, some studies demonstrate that Bcl-2 appears to fortify cellular anti-oxidant defenses [Voehringer et al., 2000]. In summary, we demonstrated that Bcl-2 prevents the H/R-caused cellular injury through the suppression of ROS production concomitantly with upregulation of Bcl-2 protein. The present study raises the possibility for an antioxidant action of Bcl-2 although still more work is necessary to understand the molecular mechanisms that are implicated in the antioxidative role of Bcl-2.
Acknowledgements
The authors thank Ms. Toshimi Kanatate, Mr. Masashi Misumi, and Dr. Norio Nagao for their technical assistance and encouragement.




