Bone Matrix Mineralization and Response to Burosumab in Adult Patients With X-Linked Hypophosphatemia: Results From the Phase 3, Single-Arm International Trial
Public clinical trial registration: http://clinicaltrials.gov/show/NCT02537431. An Open-Label, Single-Arm, Phase 3 Study to Evaluate the Effects of KRN23 on Osteomalacia in Adults With X-linked Hypophosphatemia (XLH).
ABSTRACT
X-linked hypophosphatemia (XLH) is characterized by excess fibroblast growth factor 23 (FGF23) secretion, renal phosphate wasting, and low 1,25(OH)2D3. Adult patients present with osteomalacia, hypomineralized periosteocytic lesions, bone fragility, and pain. Burosumab is a fully human monoclonal FGF23 antibody approved for XLH treatment. UX023-CL304 was an open-label, phase 3 study investigating the effects of burosumab on osteomalacia in adults with XLH, who remained untreated at least 2 years prior enrollment. Here, we present the effect of burosumab on bone material properties. We analyzed transiliac bone biopsy samples from 11 individuals before and after 48 weeks of subcutaneous burosumab treatment (1.0 mg/kg administered every 4 weeks). We used quantitative backscattered electron imaging (qBEI) and Fourier transform infrared imaging (FTIRI) to assess bone mineralization density distribution (BMDD), mineralized bone volume, properties of the organic matrix, and size of periosteocytic lesions. The outcomes were compared with reference values from healthy adults and with four XLH patients either untreated or treated by conventional therapy. Prior to burosumab, the average mineralization in cancellous bone was lower than in healthy reference. CaLow, the fraction of lowly mineralized matrix, and CaHigh, the fraction of highly mineralized matrix, were both elevated resulting in a broad heterogeneity in mineralization (CaWidth). Burosumab resulted in a decrease of CaHigh toward normal range, whereas CaLow and CaWidth remained elevated. The mineralized bone volume was notably increased (+35.9%). The size of the periosteocytic lesions was variable but lower than in untreated XLH patients. FTIRI indicated decreased enzymatic collagen crosslink ratio heterogeneity. In summary, matrix mineralization in XLH is very heterogeneous. Highly mineralized regions represent old bone packets, probably protected from osteoclastic resorption by osteoid seams. The concomitant decrease of highly mineralized matrix, persistence of lowly mineralized matrix, and increase in mineralized bone volume after burosumab suggest a boost in mineralization of preexisting unmineralized or very lowly mineralized matrix, providing a potential explanation for previously observed improved osteomalacia. © 2022 American Society for Bone and Mineral Research (ASBMR).
Introduction
Loss-of-function mutations in the phosphate regulating endopeptidase homolog X-linked (PHEX) gene results in X-linked hypophosphatemia (XLH, OMIM #307800), a lifelong disorder with a prevalence of one in 20,000 births and the most common form of inherited rickets and osteomalacia.(1) PHEX encodes a neutral Zn-metalloprotease expressed predominantly in bone and teeth that degrades osteopontin (OPN), a non-collagenous matrix protein containing highly anionic acidic serine- and aspartate rich motif (ASARM) peptide along with multiple phosphorylated residues and potent inhibitor of mineralization.(2-4) Most importantly, through a still not elucidated mechanism, loss or decreased enzymatic activity of PHEX causes excessive secretion of the phosphaturic hormone, fibroblast growth factor 23 (FGF23) by osteoblasts and osteocytes.(5-7) Elevated serum levels of FGF23 cause chronic hypophosphatemia due to impaired phosphate reabsorption in the kidney and inappropriate low 1,25-dihydroxyvitamin D production.(5) At the tissue level, XLH bone show excessive osteoid accumulation not only on trabecular and osteonal surfaces but also focally within the mineralized tissue in the perilacunar matrix surrounding osteocytes.(8, 9)
Children with XLH typically present with rickets, leg bowing, undermineralization of bones and teeth, predisposition to dental abscesses, periodontitis, and growth retardation.(10, 11) Osteomalacia often persists after completion of skeletal growth impacting bone material quality and, thus, contributing to the classical disturbance in adulthood such as elevated fractures risk often with impaired healing, musculoskeletal pain, and joint stiffness.(1, 12)
The conventional therapy for XLH consists of oral phosphate repletion to compensate hypophosphatemia and supplementation of vitamin D analogs to compensate the low 1,25-dihydroxyvitamin D formation.(13) In children, the treatment improves rickets and dentinomalacia and partially prevents leg deformities.(1, 14-16) However, in the long term, the standard therapy that requires multiple daily intakes of medication is burdensome and patients are at increasing risk to develop nephrocalcinosis, hypercalciuria, and hyperparathyroidism.(13, 17) Moreover, the effects on bone turnover are uncertain since skeletal and dental mineralization defects persist beyond adolescence.(10, 17, 18) Hence, currently there is no general consensus whether therapy should be continued during adulthood and therapy is often disrupted after completing skeletal growth.
Burosumab is a fully human monoclonal FGF23 antibody recently approved for XLH treatment in children and adults.(10, 11, 19-23) In phase 3 studies, burosumab significantly improved fracture healing and pain, increased serum phosphorus levels and circulating markers of bone turnover, and markedly reduced osteomalacia in adults with XLH.(24-27) Here, we present the effect of burosumab on the mineralized bone volume and the degree of mineralization, the properties of the organic matrix, and the size of the periosteocytic lesions.
Subjects and Methods
UX023-CL304 (clinicaltrials.gov identifier NCT02537431) was an open-label, phase 3 study investigating the effects of burosumab on osteomalacia in adults with XLH.
Study cohort
Clinical data from the 11 study participants (mean age at the first biopsy: 38.4 ± 8.4 years; four males, seven females) have been reported previously.(26)
Briefly, all probands had confirmed XLH with documentation of a PHEX mutation or a serum intact FGF23 level greater than 30 pg/mL, an age between 24.6 and 49.6 years, a fasting serum phosphorus and renal tubular maximum reabsorption of phosphate per glomerular filtration rate (TmP/GFR) lower than 2.5 mg/dL and severe skeletal pain. Most patients were on conventional therapy (supplementation of phosphate and calcitriol) before the age of 18 years but none of them was treated by conventional therapy within 2 years prior to burosumab intervention.
Subjects were treated with burosumab 1.0 mg/kg administrated subcutaneously every 4 weeks for at least 96 weeks. The transiliac bone biopsy samples analyzed here and previously by bone histomorphometry were obtained from 11 study participants, before initiating burosumab treatment and after 48 weeks of treatment.(26) Double labeling was performed prior to biopsy by oral doses of tetracycline-HCl (or demeclocycline-HCl) for two 3-day periods separated by a 12-day free interval. Undecalcified biopsy samples were embedded in polymethylmethacrylate using standard procedures.(28)
Fourier transformed infrared spectroscopy imaging
Fourier transform infrared imaging (FTIRI) analyses were performed in transmission mode, in 5-μm thin sections obtained from the biopsy blocks. Details on the methodology used have been published.(29, 30) We used a Bruker Tensor 27 FTIR spectrometer (Bruker Optics, Ettlingen, Germany) interfaced to a mercury cadmium telluride focal plane array detector (64 × 64 array) imaged onto the focal plane of an infrared microscope (Bruker Hyperion 3000; Bruker Optics). The instrument was continuously powered to avoid instrumental instability and purged with dry air to minimize water vapor spectral interference. Spectra were recorded with 8 cm−1 spectral resolution. The thin sections were placed between two BaF2 windows. Background spectral images were collected under identical conditions from the same BaF2 windows at the beginning and end of each experiment.
The following parameters were determined for each sample, in the areas of cancellous bone with evident osteoid as well as adjacent mineralized tissue:
(i)Mineral to matrix ratio: This parameter has been validated against ash weight measurements.(31, 32) It is a form of bone density that, unlike other measures such as BMD (bone mineral density) by dual-energy X-ray absorptiometry (DXA), directly measures and accounts for the amount of the organic matrix in the sample volume analyzed.
(ii)Mineral maturity/crystallinity: FTIR is particularly useful in establishing the chemical makeup of the poorly crystalline apatitic crystals in bone (ie, the presence of impurities, and, based on comparison to X-ray line broadening analysis, on their shape and size).(33, 34) Healthy bone consists of crystallites whose size and shape fall within a certain range.
(iii)Enzymatic collagen cross-links ratio (pyridinoline/divalent). This parameter has been validated against biochemically characterized collagen crosslinked peptides, as well as biochemically analyzed model tissues.(35-37)
Following the calculation of these parameters, the spectroscopic images were converted to population histograms, and the mean and standard deviation (SD) values were recorded for each biopsy, as well as the coefficient of variation (CV) as a surrogate of tissue heterogeneity for the specific parameter considered.
Quantitative backscattered electron imaging
The residual sample blocks were further prepared for quantitative backscattered electron imaging (qBEI) by trimming with a low-speed diamond saw (Isomet-R; Buehler Ltd. Lake Buff, IL, USA) and subsequently grinding and polishing to obtain plane parallel surfaces. These were carbon coated by vacuum evaporation (Agar SEM Carbon coater; Agar Scientific, Stansted, UK) and analyzed using a field emission scanning electron microscope (Zeiss Supra 40; Zeiss, Oberkochen, Germany) equipped with a four-quadrant semiconductor backscatter electron detector. The accelerating voltage was 20 kV. The gray levels reflecting the mineral/calcium content were calibrated by the material contrast of pure carbon and aluminum as described.(38-41)
The calibration allows to relate the measured gray value of each pixel to the local mineralization, measured in weight percent calcium (wt% Ca). Counting the number of pixels with a certain calcium content and normalizing to the bone area, results in a frequency distribution, the so-called bone mineralization density distribution (BMDD). The entire cross-sectional area of the transiliac bone sample was imaged with a spatial resolution of 1.8 μm per pixel.
- CaMean: weighted average calcium concentration of the mineralized tissue area.
- CaPeak: indicating the most frequently occurring calcium concentration in the sample.
- CaWidth: width at half maximum reflecting the heterogeneity in matrix mineralization.
- CaLow: fraction of lowly mineralized bone tissue, defined as the percentage of bone area that is mineralized below the 5th percentile of the reference BMDD curve (here: % bone area mineralized <18.20 wt% Ca).(41)
- CaHigh: fraction of highly mineralized bone tissue, defined as the percentage of bone area that is mineralized above the 95th percentile of the reference BMDD curve (here: % bone area mineralized >26.86 wt% Ca).(41)
The outcomes were compared between baseline and after burosumab treatment and with reference data from healthy adults.(41)
Structural indices of mineralized bone histomorphometry
- Md.BV/TV (%): mineralized bone volume per tissue volume
- Md.BS/TV (%): mineralized bone surface per tissue volume
- Md.Tb.Th (μm): mineralized trabecular thickness
- Md.Tb.N (1/mm): mineralized trabecular number
Size of hypomineralized periosteocytic lesions
The size of hypomineralized periosteocytic lesions was determined as the area around an unmineralized osteocyte lacunae (OL) core mineralized up to 10 wt% Ca as described.(42) Briefly, qBEI images were captured at a pixel resolution of 0.88 μm/pixel, as done previously to characterize two-dimensional (2D) osteocyte lacunar sections.(43, 44) First, the unmineralized core of osteocyte lacunae (that appear black in the qBEI images) were selected by setting a threshold 1.73 wt% Ca. Then, a second threshold was set to capture areas mineralized below 10 wt% Ca around the core of the osteocyte lacunae. Thus, the hypomineralized periosteocytic lesions are defined as poorly mineralized matrix (between 1.73 and 10 wt% Ca) around unmineralized osteocyte lacunae. The obtained images were analyzed with a custom-made ImageJ macro (version 1.53d; NIH, Bethesda, MD, USA; https://imagej.nih.gov/ij/). Per sample we analyzed the area of hypomineralized periosteocytic lesions of about 766 (median value) single osteocyte lacunae cores having a size comprised between 4.7 and 80 μm2. Subsequently, in each biopsy sample we evaluated the cumulative frequency in percent of total osteocyte lacunae core and associated hypomineralized area and compared the size of hypomineralized periosteocytic lesions at the 90th core percentile, before and after burosumab treatment. In addition, we compared the outcomes with control values obtained previously from two healthy premenopausal women (37 and 42 years old at time of biopsy) with normal bone mineral density (BMD obtained from DXA), normal BMDD, and no history of fractures, as well as with data obtained from four adult XLH patients.(42, 45) These were two males (33 and 37 years old at time of biopsy) with late diagnosis and therefore untreated until adulthood and two patients diagnosed and treated continuously from childhood onward with oral phosphate and the vitamin D analog calcitriol (male and female, 38 and 43 years old at the time of biopsy). Further clinical details of these patients are reported elsewhere.(42)
Statistical analysis
Statistical analysis for all outcomes and calculation of mean ± 1-SD values and median with interquartile range were performed using GraphPad Prism version 9.2.0 for macOS (GraphPad Software, San Diego, CA, USA). For comparison of BMDD outcomes of pre-burosumab and post-burosumab versus reference values, unpaired t tests or Mann-Whitney tests were used as appropriated. Comparison between pre-burosumab and post-burosumab groups was performed by paired t tests or Wilcoxon tests as appropriate. For correlation analyses of qBEI with bone histomorphometry data published previously by Insogna and colleagues(26) we evaluated Pearson correlation coefficients. Normality of FTIRI outcomes was first tested using a Kolmogorov-Smirnov test. Subsequently, results were compared by either paired t tests or Wilcoxon tests. In all instances, significance was assigned to p < 0.05.
Results
Bone matrix was not uniformly undermineralized prior to burosumab treatment
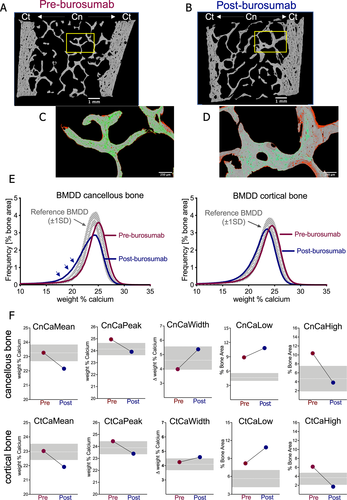
Figure 1 shows an example the bone matrix mineralization in a paired biopsy sample obtained from a 28.3-year-old female at the time of the first biopsy before and after burosumab treatment (Fig. 1A,B). Highly and lowly mineralized bone matrix, are highlighted in green and red, respectively (Fig. 1C,D). The relative amount of highly mineralized matrix decreased after burosumab, whereas the relative amount of lowly mineralized areas clearly increased. Consistently, the BMDD curve of the patient prior to treatment was shifted toward higher matrix mineralization compared to the reference BMDD of healthy adults. After burosumab treatment the curve is shifted toward lower calcium concentration. This is reflected by a marked broadening, hence the appearance of a pronounced “shoulder” at the left side of the BMDD curve, consistent with a marked increase of lowly mineralized matrix (Fig. 1E). The relative increase of lowly mineralized matrix and relative decrease of highly mineralized matrix was also confirmed by the numerical values of the single BMDD parameters (Fig. 1F).
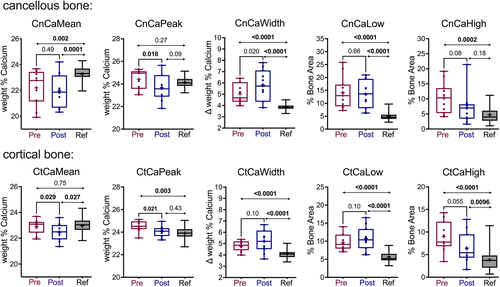
In accordance, the analysis of the entire cohort (n = 11, Fig. 2), prior to burosumab treatment showed that the average mineral density in cancellous bone was significantly lower than reference values (CnCaMean: -4.5%, p = 0.002), whereas there was no statistical difference for cortical bone (CtCaMean: −0.26%, p = 0.75). However, the low CnCaMean was not due to a simple shift of the BMDD curve toward low mineralization since the typical calcium content of the bone matrix reflected by the peak position (mode) of the BMDD curve showed no significant difference compared to the reference population in cancellous bone (CnCaPeak: +1.08%, p = 0.27) and was even slightly elevated in cortical bone (CtCaPeak: +2.55%, p = 0.003). Nevertheless, a marked increase in heterogeneity in mineralization in both bone compartments was found (CnCaWidth: +30.75%, p < 0.0001; CtCaWidth +16.8%, p < 0.0001), leading to a concomitant increase in the portion of lowly mineralized bone matrix (CnCaLow: +205.64%, p < 0.0001; CtCaLow: +61.2%, p < 0.0001) and highly mineralized bone matrix (CnCaHigh: +331.19%, p < 0.0002; CtCaHigh: +110.41%; p < 0.0001).
Burosumab treatment resulted in a decrease of highly mineralized matrix and an increase of lowly mineralized trabecular bone volume
The differences in BMDD in the XLH cohort between baseline (pre-burosumab) and after burosumab intervention (post-burosumab) and versus healthy reference values are compiled in Table 1 and Fig. 2. The 48-week treatment with burosumab resulted in a modest decrease in CnCaPeak, CtCaMean, and CtCaPeak and about 30% decrease in CnCaHigh and CtCaHigh, that did however not reach statistical significance due to a large interindividual variability (Fig. 2). In contrast, CaLow was not significantly altered and CaWidth was increased in cancellous bone, whereas the difference was not significant in cortical bone.
| BMDD parameter | Pre-burosumab (n = 11) | Post-burosumab (n = 11) | Effect of burosumab (%) | p | Reference values (n = 25)(41) |
|---|---|---|---|---|---|
| Results for cancellous (Cn) bone | |||||
| CnCaMean [wt% Ca] | 22.20 (1.36) | 21.97 (1.23) | −1.04 | 0.49 | 23.26 (0.58) |
| CnCaPeak [wt% Ca] | 24.40 (0.86) | 23.68 (1.11) | −2.97 | 0.018 | 24.14 (0.51) |
| CnCaWidth [ wt% Ca] | 5.06 (0.85) | 5.81 (1.34) | +14.95 | 0.020 | 3.87 (0.28) |
| CnCaLow [% bone area] | 14.09 (6.14) | 13.50 (5.33) | −4.24 | 0.66 | 4.61 [3.95; 5.57] |
| CnCaHigh [% bone area] | 10.36 [5.29; 13.57] | 6.98 [3.45; 8.20] | −32.63 | 0.08 | 4.70 (2.84) |
| Results for cortical (Ct) bone | |||||
| CtCaMean [wt% Ca] | 22.90 (0.54) | 22.47 (0.65) | −1.88 | 0.029 | 22.96 (0.57) |
| CtCaPeak [wt% Ca] | 24.50 (0.46) | 24.04 (0.48) | −1.86 | 0.021 | 23.89 (0.54) |
| CtCaWidth [] | 4.78 (0.47) | 5.22 (1.01) | +9.23 | 0.10 | 4.09 (0.41) |
| CtCaLow [% bone area] | 9.60 (2.17) | 10.73 (3.23) | +11.70 | 0.102 | 5.62 (1.43) |
| CtCaHigh [% bone area] | 9.01 (3.30) | 6.42 (3.37) | −28.79 | 0.055 | 3.70 [2.12; 4.80] |
- All comparisons: pretreatment versus posttreatment by paired t test or Wilcoxon test as appropriate. Significant values are in bold.
After the 48-week burosumab treatment, the average degree of mineralization was still low in the XLH cohort compared to reference values from healthy individuals: (CnCaMean: −5.55%, p = 0.0001; CtCaMean: −2.13%, p = 0.027), but the typical calcium content of the matrix was shifted toward the normal range (CnCaPeak: −1.91%, p = 0.09; CtCaPeak: +0.63%, p = 0.433). Because the fraction of lowly mineralized matrix remained elevated after burosumab versus healthy controls (CnCaLow: +192.84%, CtCaLow: +91.03%; both, p < 0.0001) the heterogeneity in mineralization was still abnormally high (CnCaWidth: +50.13%; CtCaWidth: +27.53%; both, p < 0.0001). Due to large interindividual variations the differences in CaHigh were again not significant in cancellous bone (CnCaHigh: +74.95%, p = 0.18; CtCaHigh: +39.16%, p = 0.0096). Thus, after burosumab, CaWidth and CaLow were highly elevated in both bone compartments (see Fig. 2).
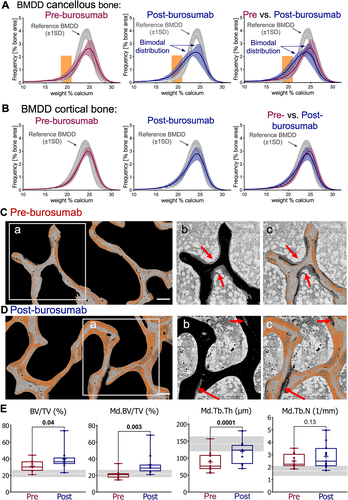
Figure 3A,B shows the mean BMDD curves calculated over all patients pre-burosumab and post-burosumab treatment for cancellous and cortical bone, respectively. In both compartments posttreatment curves show a slight shift to the left, toward lower bone matrix mineralization (Fig. 3A,B). In cancellous bone the posttreatment mean BMDD curve shows a bimodal distribution that is due to a marked increase of lowly mineralized matrix seen as a “shoulder” on the left side of the BMDD curve (Fig. 3A). To localize these specifically lowly mineralized areas, in the qBEI images, an arbitrary range of 18.5 to 21.0 wt% Ca, corresponding to the degree of mineralization of the additional shoulder, was chosen. Fig. 3C shows a representative example with pixels between 18.5 to 21.0 wt% Ca colored in bright red. It can be observed that these specific lowly mineralized areas were localized on trabecular surfaces and were often covered by thick osteoid. After burosumab the overall mineralized trabeculae features appeared thicker with, however, less osteoid and broader seams of lowly mineralized bone matrix (Fig. 3D). The latter observation was confirmed by pairwise comparison of structural indices of bone histomorphometry performed on qBEI images, showing that mineralized (Md.) trabecular thickness was increased by +34.7%, whereas trabecular number, Md.Tb.N was not altered (p = 0.1345). Consistently, a large increase of +37% (n = 11, p = 0.003) in mineralized trabecular bone volume Md.BV/TV was found (Fig. 3E). In comparison, BV/TV obtained by bone histomorphometry (consisting of mineralized matrix and unmineralized osteoid) was increased by +20.9% (n = 9, p = 0.04).(26) Together, these data indicate that burosumab treatment resulted in an increase in unmineralized and mineralized trabecular bone volume.
Relation between bone matrix mineralization and bone histomorphometric indices of osteomalacia and bone remodeling
Because CaLow reflects the fraction of undermineralized bone area, which is expected to be increased in situation of osteomalacia, we further evaluated its relation with previously published histomorphometric indices by Insogna et al., (see also Table S1).(26) Table 2 shows the results of correlation analyses between CnCaLow pre-burosumab, CnCaLow post-burosumab with key histomorphometric outcomes obtained pre-burosumab and post-burosumab. We observed in both groups, ie, before and after burosumab treatment, strong positive correlations between CnCaLow and osteoid thickness (O.Th). Moreover, there was a strong positive correlation between CnCaLow and osteoid volume per bone volume (OV/BV) in the pre-burosumab group. No association was found between CnCaLow and osteoid surface per bone surface (OS/BS) neither pretreatment nor posttreatment (Table 2).
| CnCaLow pre-burosumab versus | CnCaLow post-burosumab versus | |||
|---|---|---|---|---|
| Variables of bone histomorphometry reported26 | Pearson r | p | Pearson r | p |
| Variables of osteoid formation | ||||
| O.Th (μm) pre-burosumab | 0.948 | <0.0001 | 0.616 | 0.044 |
| O.Th (μm) post-burosumab | 0.726 | 0.011 | 0.674 | 0.023 |
| OV/BV (%) pre-burosumab | 0.785 | 0.007 | 0.501 | 0.141 |
| OV/BV (%) post-burosumab | 0.120 | 0.725 | 0.443 | 0.173 |
| OS/BS (%) pre-burosumab | −0.053 | 0.876 | −0.184 | 0.587 |
| OS/BS (%) post-burosumab | −0.074 | 0.828 | −0.023 | 0.948 |
| Variables of static bone formation | ||||
| Ob.S/BS (%) pre-burosumab | −0.139 | 0.766 | 0.291 | 0.527 |
| Ob.S/BS (%) post-burosumab | 0.485 | 0.131 | 0.631 | 0.037 |
| Variables of dynamic bone formation | ||||
| MS/BS (%) pre-burosumab | 0.059 | 0.871 | 0.187 | 0.611 |
| MS/BS (%) post-burosumab | 0.640 | 0.046 | 0.410 | 0.240 |
| BFR/BS (μm3/μm2/year) pre-burosumab | −0.038 | 0.944 | 0.498 | 0.314 |
| BFR/BS (μm3/μm2/year) post-burosumab | 0.852 | 0.0017 | 0.394 | 0.260 |
| Mlt (days) pre-burosumab | −0.214 | 0.528 | −0.325 | 0.330 |
| Mlt (days) post-burosumab | −0.747 | 0.013 | −0.456 | 0.181 |
| Variables of bone resorption | ||||
| Nb.Ocl/B.Pm (1/mm) pre-burosumab | 0.310 | 0.455 | 0.483 | 0.226 |
| Nb.Ocl/B.Pm (1/mm) post-burosumab | 0.758 | 0.007 | 0.516 | 0.104 |
| ES/BS (%) pre-burosumab | 0.202 | 0.552 | 0.442 | 0.174 |
| ES/BS (%) post-burosumab | 0.700 | 0.017 | 0.427 | 0.191 |
| Variables of bone volume | ||||
| BV/TV (%) pre-burosumab | 0.042 | 0.915 | 0.022 | 0.956 |
| BV/TV (%) post-burosumab | 0.669 | 0.025 | 0.207 | 0.541 |
| Md.BV/TV (%) pre-burosumab | −0.185 | 0.586 | −0.222 | 0.512 |
| Md.BV/TV (%) post-burosumab | 0.576 | 0.064 | −0.023 | 0.947 |
- Pearson correlations. Significant values are in bold.
- CnCaLow = percentage of bone area in cancellous bone mineralized below 18.20 weight % calcium; BFR/BS = bone formation rate per bone surface; BV/TV = bone volume per tissue volume (obtained by bone histomorphometry); ES/BS = eroded surface per bone surface; Md.BV/TV = mineralized bone volume per tissue volume (obtained by quantitative backscattered electron imaging); Mlt = mineralization lag time; MS/BS = mineralizing surface per bone surface; Nb.Ocl/B.Pm = number of osteoclasts per bone perimeter; Ob.S/BS = osteoblast surface per bone surface, OS/BS = osteoid surface per bone surface, O.Th = osteoid thickness; OV/BV = osteoid volume per bone volume.
Additionally, we observed unexpected associations between CnCaLow obtained in the biopsy samples prior burosumab with a series of histomorphometric parameters obtained in the group post-burosumab. In particular, there were positive associations with dynamic parameters of bone formation: mineralizing surface/bone surface (MS/BS) and bone formation rate/bone surface (BFR/BS), and an inverse relation with mineralization lag time. Moreover, there was a positive association with parameters of bone resorption: number of osteoclasts/bone perimeter (Nb.Ocl/B.Pm) and eroded surface bone surface (ES/BS) as well as BV/TV. In contrast, CnCaLow post-burosumab was only associated with the static parameter of bone formation osteoblast surface per bone surface (Ob.S/BS) post-burosumab (Table 2). These data suggest that the amount of bone formation post-burosumab was dependent on the extent of osteomalacia pre-burosumab. Correlation analysis confirmed that the bone formation rate per bone surface (BFR/BS) post-burosumab was positively associated osteoid thickness (r = 0.905, p = 0.0003) osteoid volume (r = 0.7148, p = 0.022) but not with the surface extent of osteoblasts post-burosumab (r = 0.48, p = 0.153).
The size of the periosteocytic lesions were variable before and after burosumab treatment
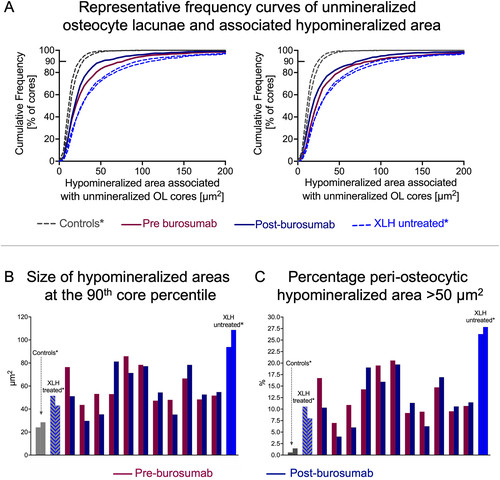
Figure 4A shows the cumulative frequency of the size distribution of the hypomineralized periosteocytic lesions (defined as having up to 10 wt% Ca) around unmineralized osteocyte lacunae (defined as having up to 1.73 wt% Ca) in the biopsy samples of two patients (one from Fig. 3C,D). For comparison, the data from two healthy controls and two untreated XLH patients presented previously are also plotted.(42) In both patients it can be observed that the curves obtained after burosumab treatment were little altered versus pretreatment and, in both cases, clearly separated from the controls and from the untreated XLH subjects. For each patient, the mean size of the hypomineralized lesions at the 90th core percentile is plotted in Fig. 4B. About one-half of the patients show a decrease in the mean size of the hypomineralized periosteocytic lesions (up to −33%) after burosumab treatment and one-half of them an increase (up to +53%). Before burosumab treatment the size of the lesions ranged from 43.6 μm2 to 85.8 μm2 (median value: 53.0 μm2) and post-burosumab between 29.7 μm2 and 81.2 μm2 (median value: 54.4 μm2) and were therefore all larger than in controls (24.1 μm2 and 24.5 μm2) and smaller than in the bone from the two untreated XLH patients (93.8 μm2 and 108.7 μm2). We further evaluated the percentage of hypomineralized periosteocytic lesions larger than 50 μm2 and observed that independently of treatment, all XLH patients had a much higher amount of such large hypomineralized areas around osteocytes than controls (Fig. 4C).
Burosumab treatment did not alter the properties of the organic matrix
As shown on Fig. 5A, FTIRI indicated that there were no significant differences in any of the monitored parameters; ie, ratio of mineral to matrix, mineral maturity/crystallinity and enzymatic collagen crosslinks before versus after burosumab. On the other hand, the coefficient of variation of enzymatic collagen crosslink ratio was significantly lower posttreatment (p = 0.012) (Fig. 5B).

Discussion
In the present study we analyzed by qBEI and FTIRI 11 paired transiliac bone biopsy samples obtained from adult XLH patients before and after a 48-week period of burosumab treatment. We demonstrated by qBEI that the bone matrix was not uniformly undermineralized but contains areas with highly mineralized and abnormally lowly mineralized matrix resulting in a broad heterogeneity in mineralization. Burosumab resulted in a decrease in the fraction of highly mineralized matrix toward normal range, in conjunction with an even higher increase of lowly mineralized bone. Furthermore, we observed a high amount of osteocyte lacunae associated with large hypomineralized lesions before and after treatment. FTIRI analyses indicate that burosumab does not alter matrix properties in the mineralized bone, except heterogeneity of the collagen crosslink ratio. Although not shown here, we observed that broad, “osteomalacic” osteoid lack trivalent crosslinks, which is consistent with previous findings in XLH bone.(42)
Despite a large interindividual variability, we noted in the bone biopsy samples of the present study cohort not only elevated values of CaLow indicating a large amount of hypomineralized matrix, but also an above average proportion of highly mineralized matrix, reflected by a concomitant increase in CaHigh in both bone compartments. This finding that the XLH bone matrix is not fully undermineralized is in accordance with previous qBEI findings in patients with XLH.(42, 46) Hence, prior to burosumab treatment, although the average calcium concentration of the mineralized matrix, CaMean was low in trabecular bone, CaPeak, which reflects the most frequently measured calcium concentration of the bone matrix, was in the upper normal range compared to reference values from healthy adults.(41) In cortical bone, CaMean was within and CaPeak even higher than reference range. Thus, and in line with our previous qBEI study on XLH bone, we found also in the present cohort that mineralization was not generally impaired and that normally mineralized trabecular bone volume was similar (or even higher) mineralized than in controls. The largely increased proportion of hypomineralized matrix is in accordance with the severe osteomalacia reported in these biopsy samples by Insogna et al., and our correlation analyses confirmed a strong association between CnCaLow and osteoid thickness (r = 0.948, p < 0.0001) and osteoid volume (r = 0.785, p = 0.007) obtained previously by bone histomorphometry (Table 2).(26) It should be underlined that osteomalacia does not only refer to the state of excessive deposition of unmineralized matrix but also reflects a situation in which the speed of mineralization is decreased. Hence, the highly increased mineralization lag time (Mlt) reported in these subjects leads to excessive accumulation of very lowly mineralized matrix.(26) Normally, when a newly formed bone packet starts to mineralize, the initial increase in mineral content is so fast that the matrix mineralizes to up to about 18 wt% Ca within days.(47, 48) In the healthy population the fraction of matrix mineralized below this calcium concentration, ie, CaLow, represents 5% of total bone area. In the present XLH patients this fraction was found to be about threefold higher in trabecular bone and doubled in cortical bone.
At first glance, the presence of highly mineralized matrix in a bone tissue characterized by severe osteomalacia might be surprising. However, it is well accepted that osteoid seams form an osteoprotective barrier against osteoclasts that do resorb mineralized matrix but can neither attach nor degrade nonmineralized surfaces.(49-51) Prior to burosumab, osteoid was found to cover nearly 90% of the total trabecular bone surface, which is a fourfold increase compared to normal in young adults, and represents a dramatic restriction of the surface accessible for osteoclastic erosion.(26, 28) Because the mineral concentration within the bone matrix increases slowly over years until reaching plateau level, older bone packets “trapped” behind the osteoid surface become gradually more mineralized before being resorbed, mimicking a situation of low bone turnover.(40, 48, 52) In accordance, bone histomorphometry showed that in XLH bone neither osteoclasts nor surface extent of osteoblasts are elevated.(26, 53) Hence, in a long-lasting period of low bone resorption due to osteomalacia, one would expect the BMDD to be shifted toward higher mineral content and concomitantly a broadening of the curve due to the presence of abnormally high amount of lowly mineralized bone. This is exactly what we observed here as well as in the previous studies of XLH bone and, furthermore, also in a patient with tumor-induced osteomalacia.(42, 46, 54)
The mineralized trabecular bone volume in XLH, which is variable but not decreased compared to the healthy population, shows a typical calcium concentration (CaPeak) within normal range.(42, 46, 55) This confirms that XLH bone consists of areas in which mineralization is locally impaired, while in other areas mineralization proceeds normally.(42) Hence, no significant differences were observed in the mean values of the three calculated spectroscopic parameters, ie, mineral/matrix, mineral maturity/crystallinity and collagen cross-links, suggesting that burosumab did not alter the material/compositional properties of bone. Moreover, the lack of differences in the mineral/matrix ratio is in agreement with the qBEI findings showing that the average calcium content of the bone matrix in the cancellous compartment (CnCaMean) was not altered. A previous spectroscopic study of iliac crest biopsy samples from osteomalacia patients, due to vitamin D deficiency, reported that the mineral/matrix ratio was lower in the trabecular bone compared to healthy controls.(56) In the present study, we did not compare the spectroscopy results against healthy controls. Moreover, we did not use spatial restrictions for our analyses. It is, however, interesting to note that vitamin D deficiency leads generally to high bone turnover and insufficient calcium incorporation into the bone matrix and therefore to a decrease in CaMean and CaPeak.(40, 57-59) Thus, it seems likely that the hypophosphatemia in XLH affects matrix properties differently than vitamin D deficiency.
Unexpectedly, CaPeak in cancellous bone, which was not found significantly different than reference values before and after burosumab due to large interindividual variability was significantly decreased after burosumab as revealed by paired t test (Fig. 2; Table 1). In fact, the treatment leads to an unexpected shift of the BMDD curve toward lower mineralization densities in both bone compartments, mirrored not only by the decrease of CaPeak, but also by the appearance of a striking shoulder on the left side of the curve of cancellous bone (Fig. 3). Consistently, there was a further increase in the heterogeneity in mineralization, shown by the increase in CnCaWidth but interestingly not in CnCaLow. This indicates an abnormal increase of rather lowly mineralized matrix although with mineral content higher than 18.20 wt% Ca, the threshold of CaLow.(41)
This very unusual shape of the BMDD curve after burosumab treatment needs further consideration. BMDD is a frequency histogram(40) with a shape that is determined by the interplay between velocity of mineralization and rate of bone turnover.(48, 60) It mirrors the dynamic processes in bone, in which osteoblasts synthesize osteoid that becomes subsequently mineralized and in which osteoclasts resorb the mineralized matrix. Under normal conditions these processes are in a steady state. In particular, the bone volume remains constant. Hence, the shoulder on the left side of the BMDD curve after burosumab treatment could either be due to an unusually slow mineralization kinetics and/or to an abnormal remodeling process. Although we do not know how burosumab affected the mineralization process, we noted that the coefficient of variation of enzymatic collagen cross-link ratio was significantly lower after treatment, implying reduced heterogeneity for this bone quality metric. This finding implies that the kinetics of conversion of divalent to trivalent cross-links were accelerated.(61) In accordance with a possible boost in the kinetic of conversion toward mature trivalent crosslinks, that were never detected in broad osteomalacic osteoid, we observed a +37.1% increase in mineralized bone volume in the group post burosumab. This increase is mostly due to an increase in trabecular thickness, which was associated with a +26% decrease in osteoid surface per bone surface and a +32% decrease in osteoid thickness observed earlier by bone histomorphometry (see also Fig. 3C,D). Because, neither the number of osteoblasts nor the number of osteoclasts was elevated in these patients,(26) it seems likely that part of this newly mineralized trabecular bone preexisted prior to burosumab as osteoid and mineralized after initiation of the treatment.
Of note, the decrease of CaHigh, the fraction of highly mineralized bone matrix (above 26.86 wt% Ca(41)), observed after burosumab can result from a net increase in mineralized bone volume and from the subsequent bone resorption with removal of older, thus, highly mineralized bone matrix. The present data do not allow to distinguish between the proportion of highly mineralized bone areas being removed and lowly mineralized bone being added, a fraction that also might differ in patients depending on the baseline conditions. We found nevertheless remarkable associations between CaLow of cancellous bone in the group pre-burosumab with the earlier presented histomorphometric indices of bone resorption Nb.Ocl/B.Pm (r = 0.758, p = 0.007), ES/BS (r = 0.700, p = 0.017), as well as indices of dynamic bone formation MS/BS (r = 0.640, p = 0.046), and BFR/BS (r = 0.852, p = 0.0017) from the post-burosumab group (Table 2). It should be underlined that MS/BS and BFR/BS are not osteoblast-related indices but reflect mineralization activity based on the quantitative evaluation of tetracycline labels.(28) Conversely, CnCaLow prior burosumab was inversely associated with Mlt (r = −0.747, p = 0.013), after burosumab indicating the higher decrease of Mlt in biopsy samples that had a larger proportion of CaLow at baseline. Moreover, BFR/BS in the post-burosumab group was positively associated with osteoid thickness and osteoid volume in the pre-burosumab group. Under normal circumstances, osteoblasts lay down a collagenous matrix, which is subsequently rapidly mineralized. Thus, the evaluation of the tetracycline labels allows to evaluate the new bone that has been laid down within a given time interval, generally 10–12 days between the two tetracycline labels. Osteomalacia is characterized by deposition of excessive unmineralized matrix, which in the case of XLH mineralizes when the hypophosphatemia is corrected. Thus, mineralization seems here not directly related to osteoblast activity. The relation between CaLow at baseline, dynamic parameters of bone formation post-burosumab and the increase in mineralized bone volume suggest a boost in dynamic bone formation, or more precisely of mineralization, following burosumab treatment. This increase was indeed greater in bone samples having more severe osteomalacia pre-burosumab. Thus, these data strongly suggest the improvement of osteomalacia after burosumab is at least partly due to the mineralization of preexisting osteoid, independent of osteoblast activity. It should be underlined that this finding is not in contradiction with previous findings of increased bone turnover after burosumab.(26) In fact, it seems very likely that two effects occur simultaneously: first, mineralization of a previously unmineralized matrix resulting in a decrease of bone surface covered with osteoid with a concomitant increase of bone surface available for osteoclastic resorption; and, second, new bone formation by osteoblasts resulting in a 20% increase in BV/TV, ie, of mineralized and unmineralized trabecular bone volume observed by histomorphometry.(26) Hence, within the post-burosumab group, CnCaLow was positively associated with the number (surface extent) of osteoblasts, as expected in situations of normal bone formation.(40, 62)
This temporal uncoupling between matrix formation and matrix mineralization in XLH, strongly suggests that the cells within the osteoid are responsible for the mineralization process. Such an assumption is in accordance with the growing body of evidence that mineralization starts around young osteocytes, the osteoid-osteocytes, a distinct cell population descendent from osteoblasts, which become embedded within the unmineralized matrix and are differentiating into mature osteocytes.(63-66) Moreover, because the mineralization process is highly regulated and controlled by this specific cell population it is also dependent on their viability.(64, 66) Under normal circumstances, the majority of differentiated osteoblasts die by apoptosis after secreting the bone matrix, whereas only a small number becomes either quiescent lining cells or transform into osteocytes.(67) It is well accepted that insufficient phosphate levels in hypophosphatemia lead to a decrease in the rate of apoptosis of mature hypertrophic chondrocytes and therefore to an abnormal expansion of the growth plate, the typical radiologic feature of rickets.(5, 68-71) The effect of hypophosphatemia on osteoblast viability has been little explored, but a lower apoptosis rate will increase the amount of bone formed by a given team of matrix forming osteoblasts. Because of the plate-like geometry of trabeculae, a lower apoptosis rate of osteoblasts will not primarily increase the trabecular surface but rather the trabecular thickness.(67) As a consequence, trabecular bone volume will increase, which is indeed, a characteristic feature of XLH bone. Thus, the striking elevated trabecular volume could possibly arise from a transient arrest of differentiation of the osteoid-osteocytes that remain viable and functional in the nonmineralized matrix until serum phosphate levels become sufficiently high to start the mineralization process.(42, 46, 55, 71-74)
A further histopathologic hallmark of XLH bone are the periosteocytic hypomineralized lesions or “halos” reported originally by the group of Francis Glorieux.(8) The origin of these lesions is still unclear but might be related to abnormal periosteocytic matrix remodeling similar as in situations of calcium deficiency.(49, 75) It has also been suggested that osteopontin, which accumulates in the XLH bone matrix, particularly within such lesions, impairs mineralization around osteocytes and canaliculi.(9) Recently, similar hypomineralized lesions have also been described in the Hyp mouse, the murine homolog of XLH. However, Hyp/osteopontin double knockout mice retain these lesions.(76, 77) Results from another study indicate FGF23 also suppresses alkaline phosphatase expression in osteocytes leading to accumulation of its substrate, pyrophosphate, a further potent inhibitor of mineralization.(78) In accordance, it was also reported that Hyp mice treated with a FGF23 antibody, have reduced osteocyte lacunae volume, which in turn suggests burosumab treatment might also improve such lesions in affected patients.(77) We have previously shown the hypomineralized periosteocytic lesions in XLH bone consist of unmineralized and very lowly mineralized matrix and developed a method to quantify the size of such lesions using qBEI.(42) Here, we demonstrate all XLH individuals from the present cohort have enlarged hypomineralized areas associated with unmineralized osteocyte lacunae cores before and after burosumab treatment. The size distribution of the lesions was found to lay in-between healthy controls and nontreated XLH patients as presented previously.(42) Moreover, the current results show a rather large variation in size distribution before treatment. About one-half of the patients showed a similar size distribution as observed in the two XLH patients that were continuously supplemented with phosphate and active vitamin D since childhood. The effects of treatment were not consistent: some individuals showed a decrease in the size of periosteocytic lesions and some patients an increase. Nevertheless, in none of the patients, neither pre-burosumab nor post-burosumab, we observed values similar with those of untreated patients, such as, for example 100 μm2, at the 90th core percentile. These observations suggest a persisting beneficial effect arising from earlier conventional therapy that all patients were subjected to, although none of them was treated for 2 years before initiating burosumab.(26) The lack of consistency in the response of periosteocytic lesions to burosumab could indicate the prevalence of a different mechanism of mineralization as compared to what is observed on bone surfaces. To verify such a hypothesis, expression profiles of cathepsin K, tartrate-resistant acid phosphatase, and alkaline phosphatase in bone cells would be desirable, but such histochemical and immunohistochemical analyses would require a special specimen preparation that was not available in this study.(77, 79, 80) In addition, it is not clear whether a longer treatment with burosumab might further decrease the extent of the periosteocytic lesions.
It is noteworthy that we found no association between serum phosphorus and the degree of osteomalacia and/or the extent of periosteocytic lesions. At baseline, patients demonstrated a wide range in phosphorus levels (1.9 mg/dL to 2.8 mg/dL) and all of them showed an appropriate increase to values within the normal range, when measured at the 4-week dosing interval.(26) After about week 20 of the study, phosphorus levels tended to decrease in all individuals, with trough levels below the normal range. Hence, consistent improvements in osteomalacia were demonstrated despite this variability at the beginning of the study and these lower phosphorus levels at the end of the study.(26) Calcium intake was not monitored. However, evaluation of serum calcium and urine calcium excretion showed that none of the patient had calcium deficiency.(26) Further studies of burosumab dosing should be considered to monitor additional benefits upon osteomalacia and periosteocytic lesions.
In summary, the presented data show mineralization in XLH bone is very heterogeneous and burosumab treatment increases the volume of mineralized matrix, rather than increasing the overall mineralization. This increase of mineralized trabecular bone volume seems to be dependent on the extent of unmineralized matrix present prior to burosumab. Thus, the observed improvement of osteomalacia is partly due to the mineralization of preexisting matrix by osteoid-osteocytes and partly by bone remodeling. In contrast, burosumab did not consistently improve the periosteocytic lesions in the present cohort. The relation between the extent of periosteocytic lesions, osteomalacia, hypophosphatemia, and FGF23 expression in XLH clearly requires further investigations.
Acknowledgments
The Ludwig Boltzmann Institute of Osteology received research support from Ultragenyx Pharmaceutical Inc. The authors thank Petra Keplinger, Sonja Lueger, and Phaedra Messmer for careful qBEI measurements. We are grateful to J Lawrence Merritt, II, MD, from Ultragenyx Pharmaceutical for the discussion of patient's serum phosphorus and calcium data, for comments and critical reading of the manuscript.
This work was supported by Ultragenyx Pharmaceutical Inc., in partnership with Kyowa Kirin International plc., by the Austrian Social Health Insurance Fund (OEGK) and the Austrian Social Health Compensation Board (AUVA).
Author contributions
Nadja Fratzl-Zelman: Conceptualization; data curation; investigation; methodology; project administration; visualization; writing – original draft; writing – review and editing. Markus A. Hartmann: Conceptualization; methodology; validation; writing – review and editing. Sonja Gamsjaeger: Formal analysis; writing – review and editing. Stamatia Rokidi: Formal analysis; writing – review and editing. Eleftherios P. Paschalis: Conceptualization; data curation; formal analysis; investigation; supervision; writing – review and editing. Stéphane Blouin: Data curation; formal analysis; supervision; validation; writing – original draft. Jochen Zwerina: Conceptualization; project administration; resources; writing – review and editing. All authors approved the final version of the manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Open Research
Data Availability Statement
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.




