CD55 Regulates Bone Mass in Mice by Modulating RANKL-Mediated Rac Signaling and Osteoclast Function
ABSTRACT
CD55 is a glycosylphosphatidylinositol (GPI)-anchored protein that regulates complement-mediated and innate and adaptive immune responses. Although CD55 is expressed in various cell types in the bone marrow, its role in bone has not been investigated. In the current study, trabecular bone volume measured by μCT in the femurs of CD55KO female mice was increased compared to wild type (WT). Paradoxically, osteoclast number was increased in CD55KO with no differences in osteoblast parameters. Osteoclasts from CD55KO mice exhibited abnormal actin-ring formation and reduced bone-resorbing activity. Moreover, macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) treatment failed to activate Rac guanosine triphosphatase (GTPase) in CD55KO bone marrow macrophage (BMM) cells. In addition, apoptotic caspases activity was enhanced in CD55KO, which led to the poor survival of mature osteoclasts. Our results imply that CD55KO mice have increased bone mass due to defective osteoclast resorbing activity resulting from reduced Rac activity in osteoclasts. We conclude that CD55 plays an important role in the survival and bone-resorption activity of osteoclasts through regulation of Rac activity. © 2019 American Society for Bone and Mineral Research.
Introduction
Bone homeostasis and remodeling are tightly maintained by the coupled action of bone-resorbing osteoclasts and bone-forming osteoblasts.1, 2 Osteoclasts originate from bone marrow-derived myeloid lineage cells and are differentiated by cytokines, macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL).3-5 Together, M-CSF and RANKL promote proliferation and survival of osteoclast precursors and their subsequent differentiation into multinucleated and active cells.1 RANKL induces activation of NF-κB and mitogen-activated protein kinases (MAPKs) including JNK, ERK, and p38 via receptor oligomerization and recruitment of signaling adaptor molecules such as the TNF receptor–associated factor (TRAF) family of proteins, which consequently leads to activation of transcription factors, such as c-Fos and nuclear factor of activated T cells, cytoplasmic 1 (NFATc1).6-10 During maturation, osteoclasts reorganize their membrane and have numerous dot-like adhesive structures called podosomes that are mainly made of F-actin and aggregate clusters which later form a ring around the cell periphery.11-13
Morphological changes of osteoclasts are dependent on the activity of Rho guanosine triphosphatases (GTPases), which include RhoA, Rac, and Cdc42. Rho GTPases, members of the Ras superfamily, are well known as molecular switches, which control a wide variety of signal transduction pathways.14 In osteoclasts, Rho GTPases play a critical role in polarization and resorptive function.15-18 The biological properties of Rho GTPases in osteoclasts are both overlapping and distinct. Rac and Cdc42 are activated by both M-CSF and RANKL, whereas RhoA is only activated by M-CSF through the cycling of its conformational state between the inactive guanosine diphosphate (GDP)-bound and the active guanosine triphosphate (GTP)-bound form.18-20 Cdc42 controls osteoclast differentiation and survival by modulating, selectively, M-CSF/RANKL–induced osteoclastogenic signals, but Rac does not affect osteoclastogenic markers.18, 21 In addition, Cdc42 knockout (KO) and Rac double knockout (Rac1 and Rac2: RacDKO) mice exhibit an osteopetrotic bone phenotype because of dysfunctional osteoclasts. Paradoxically, osteoclasts are abundant in RacDKO mice.18, 21
Activated RANK prompts two distinct signaling pathways. One promotes osteoclast formation, and the other organizes the cell's cytoskeleton by tyrosine kinase c-Src-mediated signaling activation. Linkage between activated RANK and αvβ3 integrin by c-Src phosphorylates a second tyrosine kinase, Syk, which leads to phosphorylation of osteoclast-specific guanine nucleotide exchange factor (GEF), Vav3, targeting Rac.22 Interestingly, the knockout of c-Src, Syk, or Vav3 in mice showed an osteopetrotic phenotype with either abundant or normal osteoclasts in vivo.23-25
CD55, also known as decay-accelerating factor (DAF), is a glycosylphosphatidylinositol (GPI)-anchored membrane protein that is ubiquitously found in mouse tissue and expressed in immune, neuronal, endothelial, and epithelial cells.26-28 CD55 has been mostly studied in innate and adaptive immune responses. It is a well-known negative regulator of the complement system that works by inhibiting C3- and C5-convertases, and preventing the terminal polymerization of the membrane attack complex in cooperation with CD59. In bone, the effect of complement system is controversial. Ignatius and colleagues29 reported that complement C3a and C5a modulate osteoclast formation without affecting osteogenic differentiation in human cells whereas C3a derived from osteoclasts can stimulate osteoblast differentiation in mouse in vitro culture system.30 In addition, deficiency of both C3 and C5 complement proteins caused thicker epiphyseal growth plates due to delayed endochondral ossification without exhibiting major trabecular differences31 and bone marrow cells from C3 KO mice formed fewer number of osteoclasts in vitro.32 Further, deficiency in CD59, which is also known as a negative regulator of complement system, resulted in increased femur length and cortical bone volume with reduced bone mineral density.33 These authors also reported that there were increased in osteoclastogenesis in vitro, an increased bone formation and this phenotype was only observed in males not females.33 In humans, the absence of two GPI anchored proteins CD55 and CD59 leads to a rare bone marrow disorder, Paroxysmal nocturnal hemoglobinuria (PNH), which causes hemolytic anemia, thrombosis, and peripheral blood cytopenias due to uncontrolled complement activation.34 In addition the expression level of CD55 is significantly lower in rheumatoid arthritis patients compared with controls.35 CD55 KO leads to disease exacerbation in autoimmune disease mouse models because of enhanced T cell response and the complement system.36, 37 Although CD55 is expressed on macrophages, which may include osteoclast precursors, and on other bone immune cells, the role of CD55 in bone homeostasis and development has not been previously studied.38, 39
We found that CD55 is expressed transiently during osteoclastogenesis and CD55 deletion leads to dysfunctional osteoclasts and increased bone mass. Furthermore, CD55 associates with RANK in osteoclast precursor cells leading to Src-mediated downstream signaling pathways required for organizing the osteoclast cytoskeleton and resorption activity. Taken together, these genetic and biochemical results indicate that CD55 regulates osteoclast function.
Subjects and Methods
Animals
CD55 KO mice were previously described and provided.40 Heterozygous CD55 KO mice were crossed with each other to develop homozygous CD55 KO mice and wild type (WT) control littermates that were then maintained independently. All WT and CD55 KO mice used in the experiments were 7 to 9 weeks old in a C57BL/6 background. Mice were maintained and handled in accordance with the guidelines of the Animal Care Committee of the University of Connecticut Health Center.
μCT and histomorphometric analyses
For μCT analysis, femurs from WT and CD55 KO mice were removed and fixed in 70% ethanol at 4°C. Cortical morphometry of the femoral mid-diaphysis, and trabecular morphometry within the metaphyseal region of distal femurs, were quantified using X-ray μCT (μCT40; Scanco Medical AG, Bassersdorf, Switzerland). Femurs were imaged in 70% ETOH at 55 kV (145 μA) within a 16.4-mm-diameter field of view, employing 2000 cone beam projections per revolution and an integration time of 300 ms. A total of 100 slices at midshaft and 160 slices at the distal metaphysis were acquired at an isotropic voxel size of 216 μm3 and a slice thickness of 8 μm, and chosen for analysis. Three-dimensional images were reconstructed at 8-μm resolution using standard convolution back projection algorithms with Shepp and Logan filtering, and rendered at a discrete voxel density of 1,953,125 voxels/mm3 (isometric 8 μm voxels). Bone mineral was calibrated to a discrete-step hydroxyapatite phantom, and segmented from marrow and soft tissue in conjunction with a constrained Gaussian filter to reduce noise, applying mineral density thresholds of 740 and 285 mg hydroxyapatite (HA)/cm3 for cortical and trabecular bone, respectively. Volumetric regions for trabecular bone analysis were selected within the endosteal borders to include the secondary spongiosa of femoral metaphyses located ~0.9 mm (~6% of length) from the growth plate and extending ~1.6 mm proximally, scaling for bone length. Trabecular morphometric parameters were measured without imposing a presumed structural model to obtain direct measures of trabecular volume fraction (BV/TV), thickness (Tb.Th), number (Tb.N), and spacing (Tb.Sp). Cortical morphometry was averaged within a mid-diaphyseal span of ~0.6 mm to obtain measures of total area (Tt.Ar), cortical area (Ct.Ar), marrow area (Ma.Ar), and cortical thickness (Ct.Th). The measurement terminology and units used for μCT analysis were those recommended by the Nomenclature Committee of the American Society for Bone and Mineral Research (ASBMR).41 For bone histomorphometric analysis, femurs of WT and CD55 KO mice at 8 weeks age were fixed in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) in PBS, decalcified in 14% EDTA, and embedded in paraffin block. Samples were sectioned at 7 μm thickness, followed by tartrate-resistant acid phosphatase (TRAP) staining and counterstaining with hematoxylin. For dynamic histomorphometry, WT and CD55 KO female mice were injected with 10 mg/kg calcein (Sigma-Aldrich) and 30 mg/kg Alizarin-3-methyliminodiacetic acid (Sigma-Aldrich) at 7 and 2 days prior to euthanasia. Femurs were fixed in 4% paraformaldehyde, incubated in 30% sucrose overnight at 4°C and embedded in OCT compound (Thermo Fisher Scientific, Waltham, MA, USA). Sections of 7 μm were prepared using a cryostat (Leica, Wetzlar, Germany) and tape transfer system (Section-lab, Hiroshima, Japan). Histomorphometric analyses were quantified 0.2 mm underneath growth plates and cortical bones based on recommended by the Nomenclature Committee of the ASBMR42 using Osteomeasure (OsteoMetrics, Decatur, GA, USA).
ELISA of osteoclast and bone resorption marker
Serum was harvested at time of euthanasia from animals at 8 weeks of age and subjected to ELISA as per the manufacturer's protocol. Bone resorption was quantitated with c-terminal telopeptide type I collagen (CTX) (Mouse CTX ELISA; MyBioSource, San Diego, CA, USA), and osteoclast number was quantitated with TRAP (Mouse TRAP ELISA; MyBioSource) ELISAs.
Flow cytometry
Isolated bone marrow cells were incubated in ACK lysing buffer (Thermo Fisher Scientific, Waltham, MA, USA) for 5 min and washed with Hank's balanced salt solution containing 10mM HEPES (pH 7.4) and 2% FBS. Cells were incubated with proper fluorochrome-conjugated antibodies, including anti-CD3, anti-CD45R (B220), anti-CD11b (Mac-1), anti-CD11c, anti-CD117 (c-kit), anti-CD115 (c-Fms), anti-Ly6G, anti-Ly6C, and anti-F4/80 for 30 min on ice. All antibodies were purchased from eBioscience (Santa Clara, CA, USA) or BD Biosciences (San Jose, CA, USA). After wash, cells were analyzed using an LSR flow cytometer (BD Biosciences) and FlowJo software (FlowJo, LLC, Ashland, OR, USA).
Antibodies
The following antibodies were used for Western blotting. Rat anti-CD55 antibody was purchased from R&D Systems (Minneapolis, MN, USA). Mouse anti-NFATc1 antibody was purchased from BD Biosciences. Rabbit anti-c-Fos, rabbit anti-Src, mouse anti-Src, rabbit anti-Rac1, rabbit anti-Cdc42, rabbit anti-RhoA, rabbit anti-cleaved Caspase-3, rabbit anti-cleaved Caspase-8, rabbit anti-cleaved Caspase-9, Bim, rabbit anti-p-Src, rabbit anti-p-Syk, rabbit anti-p-PLCγ rabbit anti-Syk, rabbit anti-PLCγ, rabbit anti-RANK and rabbit anti-β-actin antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Rabbit anti-p-Vav3 and rabbit anti-Vav3 antibodies were purchased from Abcam (Cambridge, MA, USA).
In vitro osteoclast formation and survival assay
Bone marrow cells were isolated from tibias and femurs of female mice and incubated overnight to remove stromal cells. Nonadherent cells were collected and BMM cells were prepared using Ficoll–Hypaque (GE Healthcare, Piscataway, NJ, USA) density gradient. Red blood cell–free buffy coat cells (2 × 104 cells/well in 96-well plate) were cultured with M-CSF (30 ng/mL) and RANKL (30 ng/mL). For coculture assay, primary osteoblast were isolated from calvaria of 2-day-old to 3-day-old mice with 0.06% Collagenase P (Roche Diagnostics, Mannheim, Germany). Primary osteoblasts (5 × 103 cells) either from WT or CD55 KO mice and BMM (1 × 105 cells) from WT or CD55 KO mice were cocultured in the presence of 1,25 dihydroxyvitamin D3 (Enzo Life Sciences, Inc., Farmington, NY, USA) in a 96-well plate for 5 days. To verify osteoclast survival rate, culture medium was replaced without cytokines after osteoclast differentiation, and osteoclasts were cultured for indicated time. Osteoclasts were identified as more than three nuclei per cell, visualized by TRAP staining, and counted.
RNA extraction and RT-PCR
Total RNA was extracted from either WT or CD55 KO femurs and calvaria with TRI reagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer's recommendation. Total RNA was converted to cDNA by reverse transcriptase (High Capacity cDNA Reverse Transcription Kit; Applied Biosystems, Foster City, CA, USA) using random hexamer and aliquots of Reverse Transcription (RT) mixtures were used for PCR amplification. PCR amplification was done using gene-specific real-time PCR primers (Applied Biosystems, Foster City, CA, USA) and expressed as fold differences compared to WT controls.
Resorption pit formation assay, TRAP, and CTX ELISA
Osteoclast differentiation was induced on collagen-coated plates. After 6 days, mature osteoclasts were collected by digestion using 0.1% collagenase type l. An equal number (100 cells/bone slice) of osteoclasts was placed on bovine cortical bone slices and cultured with M-CSF and RANKL (both at 30 ng/mL) for 24 hours. Before sonication to remove cells, TRAP staining was performed to confirm cells on bone slices. Resorption pits were visualized by staining with 1% toluidine in 1% borax buffer. To determine the number of TRAP+ osteoclasts and bone resorbing activity, BMM cells from WT and CD55 KO mice were cultured on bone slices for 5 days in the presence of M-CSF and RANKL. CTX was measured from conditioned medium and TRAP activity was measured from osteoclasts that were present on bone slices.
F-actin staining
Isolated BMM cells from WT or CD55 KO mice were cultured in the presence of M-CSF and RANKL (both at 30 ng/mL) for 5 days on culture dishes or for 6 days on bone slices. Cells were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. Cells were then incubated with rhodamine phalloidin (Molecular Probes, Grand Island, NY, USA) for 30 min at 4°C, washed three times, and visualized using fluorescent microscope. 4′,6-Diamidino-2-phenylindole (DAPI) is a fluorescent stain to visualize nuclei. Sealing zone perimeter per osteoclast was measured using a light microscope (BX53; Olympus Scientific, Waltham, MA, USA) and image analysis software (Olympus CellSens) as described.43-45
Apoptosis assay
BMMs were cultured with M-CSF and RANKL (both at 30 ng/mL) for 3 days (for osteoclast precursors) and 5 days (for multinucleated osteoclasts). Apoptotic cells were visualized by the indirect TUNEL assay using ApopTag Plus Fluorescein In Situ Apoptosis Fluorescein Detection kit (Millipore, Billerica, MA, USA) according to the manufacturer's instructions and nuclei were stained with DAPI. Fluorescence-positive cells of mononuclear preosteoclasts cultured for 3 days were counted and expressed as a percentage of DAPI-positive cells. Fluorescence-positive osteoclast cultured for 5 days were counted and expressed as a percentage of total osteoclasts in the well.
Rho GTPase activity assay
BMMs were cultured with M-CSF and RANKL (both at 30 ng/mL) for 3 days and serum-starved for 2 hours. M-CSF and RANKL were added for the indicated time and whole-cell extracts were incubated with glutathione S-transferase (GST)-fused p21-activated protein kinase (PAK1) p21 binding domain (PBD) and GST-fused Rhotekin Rho binding domain (RBD) overnight. GTP-bound Rac1, Cdc42, and RhoA were pulled down by GST pull-down assay.
Immunoprecipitation
BMMs were cultured with M-CSF or M-CSF and RANKL for 3 days. Cells were washed in cold PBS and lysed on ice. Five-hundred micrograms (500 μg) of proteins were incubated with 0.5 to 1 μg of monoclonal mouse anti-Src antibody or monoclonal rat anti-CD55 antibody and protein G agarose at 4°C overnight. Immunoprecipitates were washed three times in lysis buffer and visualized by Western blot.
Retroviral transduction
Retroviral vectors encoding cDNA for c-Src, ca-Rac1V12, ca-RhoV14, and ca-Cdc42V12 were kindly provided by Dr. Hyunsoo Kim (University of Pennsylvania, Philadelphia, PA, USA).20, 46 Retroviral vectors were transfected into Plat E packaging cells using Lipofectamine 2000 (Thermo Fisher Scientific) and retroviruses were collected 48 hours after transfection. BMM cells were cultured with M-CSF (150 ng/mL) for 2 days, were transduced in the presence 8 μg/mL polybrene (Millipore, Burlington, MA, USA) for 6 hours and cultured overnight in the presence of M-CSF. Cells were subcultured further with 30 ng/mL M-CSF and 2 μg/mL puromycin (Millipore) for 2 days. Puromycin-resistant BMM cells were used for experiments.
Statistics
Statistical analyses were performed using the two-tailed Student's t test to compare differences between groups or one-way analysis of variance (ANOVA) and the Bonferroni post hoc test when ANOVA demonstrated significant differences. p < 0.05 was considered statistically significant. Results represent the means ± SE for three independent replicate experiments and a representative experiment is shown.
Results
CD55 deficiency increases bone mass in female mice
The functional communication between bone cells and immune cells has been extensively studied for immune response and bone homeostasis. Though it has been reported that CD55 is highly expressed in cells in the bone marrow, the function of CD55 in bone has not been previously examined. To characterize the role of CD55 in bone, we first investigated its expression levels in hematopoietic lineage cells by flow cytometric analysis. More than 60% of CD3+ (T lymphocyte lineage), CD45R+ (B lymphocyte lineage), CD11c+ (dendritic cell lineage), and CD11b+ (myeloid lineage) cells express CD55, whereas triple negative (TN, CD45R− CD3− CD11b−/lo) CD115+ and TN CD117+ CD115+ cells, which have been reported to be effective osteoclast precursors,47, 48 express CD55 minimally (Fig. 1 A). We further examined CD55 protein levels during osteoclast differentiation by Western blot using cultured BMM cells that were treated with M-CSF or the combination of M-CSF and RANKL (Fig. 1 B). Both M-CSF and the combination of M-CSF and RANKL transiently induced CD55 expression in these cultures.
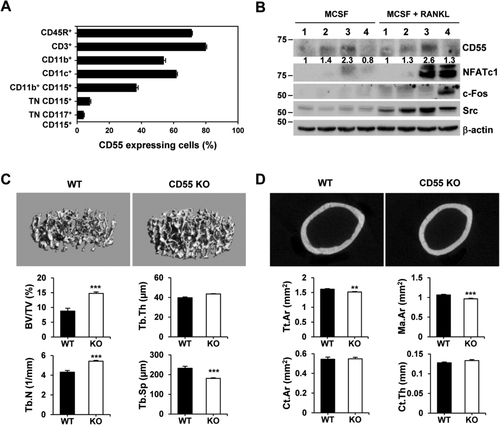
To determine if CD55 functions to alter bone, we examined the bone phenotype of CD55 KO mice at 8 weeks of age. μCT analysis showed significantly enhanced trabecular bone volume (BV/TV, 1.7-fold) and trabecular number (Tb.N, 1.3-fold) and a concomitant decrease in trabecular spacing (Tb.Sp, 30%) in female CD55 KO mice (Fig. 1 C). Trabecular bone volume in male CD55 KO mice was similar to WT controls, with the exception of trabecular thickness (Tb.Th, 10% decrease) and trabecular spacing (Tb.Sp, 20% decrease) (Supplemental Fig. 1A). Furthermore, mid-shaft cortical bone analysis showed female CD55 KO mice had significantly diminished total area (Tt.Ar, 6%) and marrow area (Ma.Ar, 10%) without an altered cortical bone area (Ct.Ar), cortical thickness (Ct.Th) or bone length (Fig. 1 D and Supplemental Table 1), indicating that female CD55 KO bones are more compact compared to WT controls.
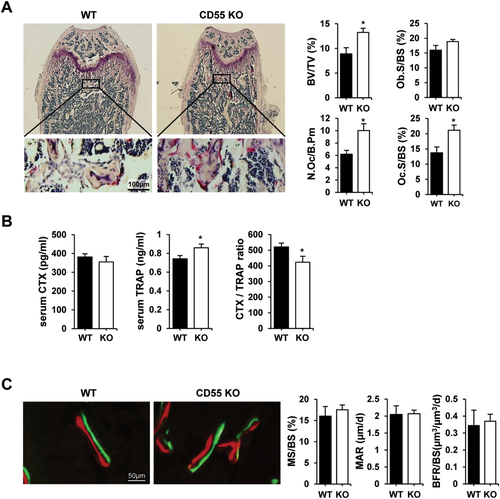
We next examined whether the increased trabecular bone mass was due to the changes in osteoclasts and/or osteoblasts by both static and dynamic histomorphometric analyses. Static histomorphometric analysis revealed that CD55 KO female mice had a significant increase in trabecular bone volume as observed in μCT analysis. In contrast, osteoclast surface (Oc.S) and number (N.Oc) was significantly increased with no difference in osteoblast surface (Ob.S) in CD55 KO female mice when compared to WT controls (Fig. 2 A). However, CTX levels in serum, which measure mature osteoclast activity, were comparable in control and CD55 KO mice (Fig. 2 B). We also measured TRAP activity and calculated CTX/TRAP ratio in serum from CD55 KO mice (Fig. 2 B) to determine if TRAP activity was altered in CD55 KO mice because CD55 KO mice exhibited a mild osteoclast-rich osteopetrosis. TRAP activity in vivo was significantly increased in serum from CD55 KO mice as expected and CTX/TRAP ratio was significantly reduced in serum from CD55 KO, indicating that these osteoclasts in CD55 KO mice may have reduced bone-resorbing activity. Further, we examined myeloid lineage distribution by flow cytometric analysis using various antibodies that represent myeloid lineage to determine if myeloid lineage skewing caused increased osteoclast number in vivo. As shown in Supplemental Fig. 2, there was no significant difference in myeloid lineage populations. In addition their overall cell numbers were not significantly different (data not shown). In males, there was no significant difference in trabecular bone volume as observed in μCT analysis seen in Supplemental Fig. 1A. CD55 deficiency did not affect osteoclast surface and number as well as osteoblast surface in males (Supplemental Fig. 1B). We subsequently examined the in vivo bone formation rate using injection with calcein and alizarin complexone as shown in Fig. 2 C. There were no significant changes in mineralized surface (MS), mineral apposition rate (MAR), and bone formation rate (BFR) in CD55 KO female mice relative to WT, indicating that CD55 may not influence osteoblast differentiation or function. These results suggest that CD55 protein influences bone primarily through osteoclast differentiation and function. Because of the sex-specific impact of CD55 deletion, we focused our subsequent analyses on cells from female mice.
CD55 is essential for survival of osteoclast precursors and osteoclasts
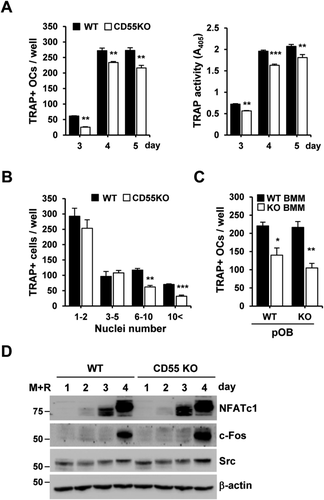
To determine if CD55 is essential during osteoclast differentiation and survival, BMMs were cultured with M-CSF and RANKL for up to 5 days. The number of TRAP(+) osteoclasts was significantly reduced in cells from CD55 KO mice in the presence of M-CSF and RANKL as compared to WT control cells. We also examined the TRAP activity in conditioned medium, which showed a similar decrease in cultures from CD55 KO cells (Fig. 3 A). We next analyzed the frequency distribution of osteoclasts with a different number of nuclei in WT and CD55 KO BMMs, treated with RANKL and M-CSF (30 ng/mL for both) for 5 days (Fig. 3 B). This showed that CD55 KO BMMs formed fewer osteoclasts with more than six nuclei, a finding which further argues that CD55 KO cells have a decreased fusion capacity. Because CD55 KO BMM cells formed fewer osteoclasts in vitro and showed a discrepancy between in vitro and in vivo results, we examined if any osteoclast-specific gene expression was altered in femurs from CD55 KO female mice. As shown in Supplemental Fig. 3A, we found that the majority of osteoclast-specific gene (NFATc1, CtsK, TRAP, β3 integrin, c-Fms, RANK, V-Atpase a3, and Clc7) mRNA expression was comparable in CD55 KO cells when compared to WT controls. Further, the expression of RANKL, OPG, and RANKL/OPG ratio in calvaria (Supplemental Fig. 3B) and femurs (Supplemental Fig. 3C) from CD55 KO mice was not significantly different.
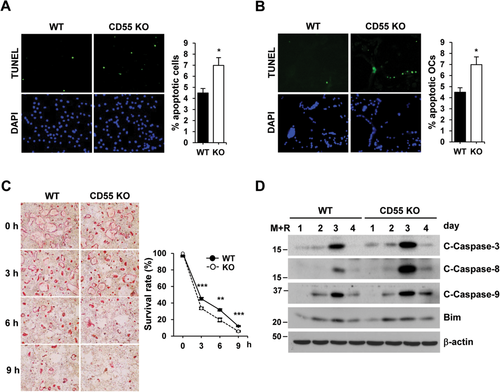
Because CD55 has been known to downregulate C3 and C5 convertase activity in complement system, we first examined if CD55 alters C3 and C5 expression in BMM cultures in the presence of M-CSF and RANKL for 3 or 4 days. As shown in Supplemental Fig. 4A, C3 and C3R (receptor) mRNA expression was significantly enhanced in CD55 KO cells compared to WT cells because CD55 deficiency can lead to increase in C3 convertase activity. We also measured C5 mRNA expression in these cells but they were not detectable (data not shown). Similar to C3 and C3R mRNA expression, C5R mRNA expression was increased in KO cells when compared to WT cells. Additionally, we examined the C3 and C3R mRNA expression in femurs from CD55 KO mice. There were no significant differences in C3 or C3R mRNA expression between WT and CD55 KO femurs (Supplemental Fig. 4B). In addition, we determined whether osteoblasts influence the osteoclast formation in coculture systems although osteoblasts do not express CD55 (Supplemental Fig. 5A). As shown in Fig. 3 C, osteoclast formation was not affected in cultures with primary osteoblasts either from WT or CD55 KO mice when cultured with WT BMM cells. However, when CD55 KO BMM cells were cultured with osteoblasts from either WT or CD55 KO mice, there was a significant decrease in osteoclast formation in vitro, indicating that CD55 influences osteoclast precursor cells. However, RANKL-induced activation of MAP kinases and expression of factors required for osteoclast differentiation such as NFATc1, c-Fos, and Src were similarly induced in cultures from CD55 KO cells when compared to WT cells (Supplemental Fig. 5B and Fig. 3 D). We subsequently measured cell numbers during culture with M-CSF and RANKL. Cell number in cultures from CD55 KO cells was reduced compared to WT cells (Supplemental Fig. 6). Based on these results, we speculated that there might be a difference in apoptosis in CD55 KO cells. To determine if cells are more prone to apoptose in the absence of CD55, we performed an indirect TUNEL assay in both osteoclast precursors and mature osteoclasts, which has been designed to detect apoptotic cells that undergo extensive DNA degradation during the late stages of apoptosis. Cells from CD55 KO mice showed a 1.6-fold increase in apoptosis in the presence of M-CSF and RANKL for 3 days or 5 days (Fig. 4 A,B). In addition, multinucleated osteoclasts from CD55 KO mice appeared to have induced cell death by removing cytokines, and activation of effectors for apoptosis (all three cleaved form of caspases: caspase-3, caspase-8, and caspase-9) were elevated compared to WT controls (Fig. 4 C,D). Caspases are a family of proteases important for apoptosis and Bim is a pro-apoptotic member of the BCL-2 protein family. However, there was no significant change in Bim expression between WT and CD55 KO cells. Thus, these data indicate that the lack of CD55 induces cell death in preosteoclasts and osteoclasts, resulting in impaired osteoclast differentiation in vitro.
CD55 selectively modulates Rac activity regulating osteoclast cytoskeleton and function
As shown in Fig. 2 A, although osteoclast numbers were increased in CD55 KO mice in vivo, serum markers of bone formation were not increased. Therefore, we speculated that osteoclasts from CD55 KO mice may have reduced ability to resorb bone. To determine if cells from CD55 KO mice have altered function, we measured bone resorbing activity in vitro. BMM cells from WT and CD55 KO mice were differentiated to form osteoclasts on a culture dish coated with collagen type I. Osteoclasts were released from the plate by collagenase digestion and equal numbers were cultured on bovine cortical bone slices for 24 hours. Resorption pit area per osteoclast and CTX/TRAP ratio were significantly reduced in cells from CD55 KO mice, indicating that osteoclasts from CD55 KO mice have reduced bone resorbing activity (Fig. 5 A). Subsequently, we examined if actin ring formation was altered in CD55 KO cells and found that CD55 KO cells exhibited irregular actin ring formation when cultured on culture dishes as well as on bone slices (Fig. 5 B). Because cytoskeletal organization and function in the osteoclasts are regulated by Rho GTPases,15-18, 49, 50 we determined whether CD55 regulates Rho GTPase activity. BMMs were cultured with M-CSF and RANKL for 3 days, briefly serum-deprived, then exposed to both M-CSF and RANKL for up to 30 min. Activated Rho-GTPase pull-down assays demonstrated that Rac activation was selectively downregulated in CD55 KO cells compared to WT controls, with no changes in RhoA and Cdc42 activation (Fig. 5 C). To determine if the downregulated Rac activation is RANKL-specific, we performed a similar experiment with M-CSF or RANKL separately, as shown in Fig. 5 D. Rac activation was downregulated in CD55 KO cells in response to RANKL treatment whereas Rac was activated in both WT and CD55 KO cells in response to M-CSF treatment. Thus, these data indicate that lack of CD55 markedly downregulates RANKL-induced Rac activation, likely contributing to impaired osteoclast cytoskeletal organization and decreased osteoclast function.
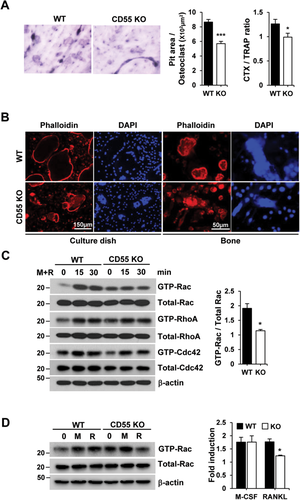
Association of CD55 and RANK modulates Src-downstream signaling pathway
RANK activation by RANKL elicits cytoskeleton reorganization via Src downstream signaling, which includes Vav3, a guanine exchange factor (GEF) targeting Rac. To determine if CD55 participates in this signaling pathway, BMMs were cultured for 3 days, serum-starved, and treated with RANKL for up to 40 min. As expected, RANKL treatment upregulated the phosphorylation and activation of c-Src, Syk, and Vav3 in WT BMM cultures, as shown in Fig. 6 A. In contrast, activation of c-Src, Syk, and Vav3 were decreased in cells from CD55 KO mice relative to WT responses (Fig. 6 A).
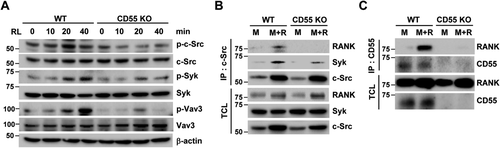
Because c-Src interacts with RANK carboxyl terminus after RANKL stimulation and recruits Syk, we next questioned if CD55 modulates RANK/c-Src/Syk complex formation. WT and CD55 KO cell lysates were immunoprecipitated with c-Src antibody and immunoblotted for RANK and Syk. In WT cells, RANK/c-Src/Syk complex was formed in response to M-CSF and RANKL, whereas CD55 KO cells failed to form the RANK/c-Src/Syk complex (Fig. 6 B). Next, we asked how CD55 influences cytosolic protein complex formation because Shenoy-Scaria and colleagues51 reported that CD55 associates with cytosolic phosphoprotein, and it was speculated that this interaction may be mediated by a transmembrane protein.52 Thus, we tested if CD55 associates with the transmembrane protein, RANK. Interestingly, CD55 is associated with RANK when cells were treated with M-CSF and RANKL for 3 days (Fig. 6 C). Based on these results, CD55 can associate with RANK in response to M-CSF and RANKL treatment to initiate the activation of c-Src downstream signaling for osteoclast cytoskeleton organization.
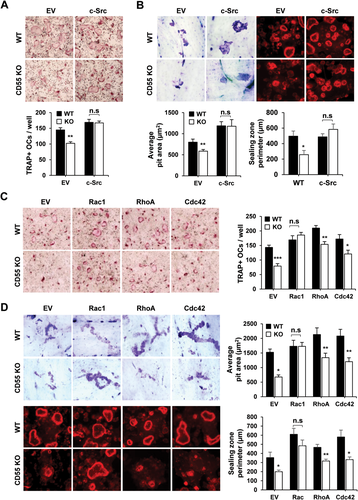
Overexpression of c-Src or Rac1 rescues osteoclast differentiation and function in CD55 KO cells
As shown Figs. 5 C and 6 A, Rac, as well as c-Src activation, was downregulated in CD55 KO cells. To determine if overexpression of c-Src can rescue the reduced osteoclast formation in CD55 KO cells, we transduced c-Src cDNA into WT and CD55 KO BMMs using a retroviral gene transfer system. Transduced BMMs were selected by puromycin and induced to differentiate into osteoclasts with M-CSF and RANKL. Overexpression of c-Src in CD55 KO BMMs completely rescued the number of TRAP+ multinucleated cells (MNCs) formed compared WT controls (Fig. 7 A). In addition to osteoclast formation assay, we also examined the actin ring formation and bone resorption activity after Src overexpression in WT and CD55 KO BMM cells. Src overexpression in CD55 KO BMMs completely rescued actin ring formation as well as bone resorbing activity when compared to WT cells (Fig. 7 B). Further, we examined the effect of constitutively active Rac1 in WT and CD55 KO BMMs. As shown in Fig. 7 C, BMM cells from WT and CD55 KO mice transduced with retrovirus expressing constitutively active Rac1(ca-Rac1), RhoA (ca-RhoA), or Cdc42 (ca-Cdc42) cDNA were induced to form TRAP(+) MNCs. Only ca-Rac1 overexpression in CD55 KO BMMs rescued osteoclast differentiation not ca-RhoA or ca-Cdc42 (Fig. 7 C). In similar experiments, we cultured WT or CD55 KO cells containing ca-Rac1, ca-RhoA, or ca-Cdc42 cDNA on bone slices to examine the actin ring formation and bone resorbing activity. We found only overexpression of Rac1 rescued the reduced actin ring formation and bone resorbing activity in CD55 KO cells (Fig. 7 D). These results argue that Rac1 is an essential element in the defective osteoclast function of CD55 KO cells.
Discussion
In the current study we found a novel role of the cell autonomous effect of CD55 in bone. Loss of CD55 caused the enhancement in trabecular bone volume and this was due to the decrease in osteoclast bone resorbing activity by regulation of the osteoclast cytoskeleton. Mechanistically CD55 deficiency blocked RANK/c-Src/Syk complex formation and reduced final maturation and activity.
Because CD55 has been known to regulate the C3 and C5 convertase activity in the complement system, we initially examined the possibility that the complement system is involved in the alteration in trabecular bone volume in CD55 KO mice. As expected, we also found that C3 mRNA expression was increased in CD55 KO cells and speculated that osteoclast formation as well as bone formation rate would increase in CD55 KO mice. The number of osteoclast per bone surface was increased in CD55 KO mice possibly due to increase in C3 expression caused by CD55 deficiency. However, our bone formation rate, which was expected to increase if the increase in C3 causes the increase in bone formation, was not altered in the femurs from CD55KO mice (Fig. 2 C) and we concluded that this phenomenon was due to the cell autonomous effect of CD55 in osteoclasts rather than the influence of alteration in the complement system. We set out to investigate the cell autonomous effect of the mechanism by which CD55 regulates osteoclasts and bone mass.
Optimal osteoclastic bone resorption depends on an osteoclast's cytoskeletal structure, due to the intense trafficking of lysosomal and endosomal components. Once osteoclasts are matured on the bone surface, protons and enzymes such as TRAP, cathepsin K, and matrix metalloproteinase-9 (MMP-9) are secreted from the ruffled border into resorption lacuna enclosed by actin rings.13, 53 c-Src is the dominant Src family of kinases expressed in osteoclasts and its transient activation by RANKL is required for downstream activation.22 Further, c-Src kinase activity regulates the osteoclast's function.54-56 Thus, RANKL can directly influence organization of the cytoskeleton needed to resorb bone. As shown in Fig. 6 A, BMMs from CD55 KO failed to activate c-Src and downstream signaling proteins (Syk and Vav3), likely due to a block in the formation of CD55/RANK/c-Src complex. Therefore we conclude that CD55 must interact with RANK to initiate a c-Src downstream signaling pathway.
In many cases, osteoclast number in vivo reflects bone phenotype. However, CD55 KO mice showed a high bone mass phenotype despite displaying an increased osteoclast number compared to WT mice. As previously reported, several knockout mouse model showed a high bone mass phenotype with either abundant or normal osteoclast number in vivo.21, 25, 57 In contrast, we found that osteoclast formation was decreased in BMM cells from CD55 KO mice as shown in Fig. 3 A when compared to WT cells. However, the capacity of osteoclast precursors to respond to RANKL and osteoclast-expressing markers, such as NFATc1, c-Fos, and c-Src, were indistinguishable in WT and CD55 KO cells, as shown in Fig. 3 D, and additional osteoclast markers in Supplemental Fig. 3. Further, there were no significant changes in different myeloid lineage populations in the bone marrow as shown in Supplemental Fig. 1. This decrease in osteoclast formation may not be due to a defect in differentiation but the difference in survival of osteoclasts in CD55 KO cells in vitro as shown in Fig. 4. These results suggest that osteoclast precursors from CD55 KO mice have the potential to fuse to become mature TRAP-positive cells with diminished survival. However, our data indicate that these mature osteoclasts have diminished bone resorption. Indeed, CD55-deficient osteoclasts displayed irregular actin ring formation in both a culture dish and on bone. Consistently, mice deficient for the CD55 downstream regulators, c-Src, Syk, Vav3, or Rac, display attenuated bone resorption, resulting in severely increased bone mass despite an abundant or normal osteoclast number in vivo.21-25 Moreover, bone resorption marker levels, CTX or deoxypyridinoline (Pyd), in serum from c-Src, Syk, or Vav3 KO mice, were similar to WT.24, 25, 57 Collectively, these results provide strong evidence that CD55 plays a role in regulating bone mass by organizing the osteoclast's cytoskeleton and resorption.
In the current studies, we showed increased trabecular bone mass in femurs from CD55 KO female mice. Although femurs from CD55 KO mice are narrower than WT, there was no significant difference in cortical area, thickness, bone length, or body weight. Bone elongation during growth is primarily accomplished by chondrocytes in the growth plate and supported through periosteal apposition by osteoblasts.58 Although the lack of CD55 generates osteoclast dysfunction, the bone formation rate was not altered. Therefore, we conclude that the dysfunctional osteoclast in CD55 KO mice do not affect bone elongation and body growth. We also show that stromal/osteoblast lineage cells do not express CD55, as presented in Supplemental Figure 5, and osteoblasts from either WT or CD55 KO mice did not affect osteoclast differentiation in the presence of vitamin D3 as shown in Fig. 4 C.
We found a bone phenotype in CD55 KO mice only in females at 8 weeks of age. Many investigators have reported sex-restricted phenotypes in genetic mouse models.33, 59-63 Skeletal sexual dimorphism depends not only on androgen action in males and estrogen action in females, but also on complex sex- and time-specific interactions between sex hormones, the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis, and mechanical loading. Thus, it is difficult to explain or determine the mechanism of the sex-specific bone phenotype observed in CD55 KO mice at the current time. However, when we induced osteoclast formation in vitro and analyzed proteins by Western blot using BMMs from male CD55 KO mice, those results were identical to BMMs from female CD55 KO mice (data not shown). We also examined the histomorphometric analysis of male femurs as shown in Supplemental Fig. 2B. It appears that male mice respond differently in CD55 deficiency, such as was previously reported that female CD55 KO mice were more susceptible to disease related to inflammation and/or autoimmune diseases.38 CD55 KO females develop severe dermatitis around 5 to 6 months of age whereas males do not (personal observation), indicating that females have a more severe response to CD55 deficiency. Further, male CD55 KO mice had reduced trabecular spacing and trabecular thickness with no change in overall trabecular volume, indicating that there may be delayed bone phenotype in males compared to females CD55 KO mice. These data suggest a cell-autonomous effect of CD55 on osteoclast differentiation. The impact of this was not apparent in the complex in vivo environment at 8 weeks of age.
As shown in Fig. 1 A, lineage-committed populations highly expressed CD55, whereas lineage-negative populations minimally expressed this antigen. Consistently, in BMM cultures CD55 protein levels increased and peaked on day 3 during differentiation into macrophages and osteoclasts by treatment with M-CSF or the combination of M-CSF plus RANKL, respectively. Although CD55 protein levels were reduced at day 4 after a medium change on day 3, it was required for protection against cell death of preosteoclasts or osteoclasts.
Taken together, we found that a novel role of a cell autonomous effect of CD55 in bone was due to regulation of the osteoclast cytoskeleton. Loss of CD55 blocked RANK/c-Src/Syk complex formation in spite of presence of the RANKL. Eventually, although CD55-deficient cells displayed similar expression levels in key osteoclast differentiation marker proteins compared to WT controls, they have reduced final maturation and activity. Hence, CD55, on the outer plasma membrane is essential for initiation of c-Src downstream signaling, suggesting it could be an accessible target for therapeutic intervention in bone diseases.
Disclosures
All authors report no conflicts of interest.
ACKNOWLEDGMENTS
We thank Drs. Anne Delany and Joseph Lorenzo for reviewing the manuscript. We also thank Dr. Ernesto Canalis and Ms. Renata Rydzik for their assistance with μCT analysis. We express our sincere gratitude to Dr. Wen-Chao Song (University of Pennsylvania) for providing us with CD55 KO mice.
Authors' roles: BS and SKL designed the skeletal research studies. BS, HW, DJA, and SKL performed the research and analyzed the data. BS and SKL wrote the manuscript. BS and SKL take responsibility for data analysis. All authors approved manuscript submission.




