Increased Risk of Bone Fractures in Hemodialysis Patients Treated with Proton Pump Inhibitors in Real World: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS)
ABSTRACT
Long-term treatment with proton pump inhibitors (PPIs) is associated with an increased risk of fractures in the general population. PPIs are widely prescribed to dialysis patients but to date no study has specifically tested, by state-of-art statistical methods, the relationship between use of PPIs and fractures in this patient population. This study aimed to assess whether use of PPIs is associated with bone fractures (ie, hip fractures and fractures other than hip fractures) in a large international cohort of hemodialysis patients. We considered an observational prospective cohort of 27,097 hemodialysis patients from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Data analysis was performed by the Fine and Gray method, considering the competitive risk of mortality, as well as by a cause-specific hazards Cox model with death as a censoring event and matching patients according to the prescription time. Of 27,097 hemodialysis patients, 13,283 patients (49%) were on PPI treatment. Across the follow-up period (median, 19 months), 3.8 bone fractures × 100 person-years and 1.2 hip fractures × 100 person-years occurred. In multiple Cox models, considering the competitive risk of mortality, the incidence rate of bone (subdistribution hazard ratio [SHR] 1.22; 95% CI, 1.10 to 1.36; p < 0.001) and hip fractures (SHR 1.35; 95% CI, 1.13 to 1.62; p = 0.001) was significantly higher in PPI-treated than in PPI-untreated patients. These findings also held true in multiple, cause-specific, hazards Cox models matching patients according to the prescription time (bone fractures: HR 1.47; 95% CI, 1.23 to 1.76; p < 0.001; hip fractures: HR 1.85; 95% CI, 1.37 to 2.50; p < 0.001). The use of PPIs requires caution and a careful evaluation of risks/benefits ratio in hemodialysis patients. © 2019 American Society for Bone and Mineral Research.
Introduction
Proton pump inhibitors (PPIs) are commonly prescribed for gastrointestinal disorders in which the inhibition of gastric acid secretion is desirable, such as peptic ulcer disease, dyspepsia, and gastroesophageal reflux disease. The use of PPIs is widespread and has progressively increased since their introduction.1 In the ambulatory setting the prevalence of visits in which patients used PPIs increased from 4.0% in 2002 to 9.2% in 2009.2 Moreover, 63% of the patients using PPIs did not have gastrointestinal complaints or a specific indication for PPI use. Accordingly, PPIs were included among the most common potentially inappropriate medications, identified using the updated Beers criteria.3 For this reason, when prescribing this class of drugs, an accurate evaluation of the risks/benefits ratio of long-term use of PPI is formally recommended by the American Gastroenterological Association.4 In a nationwide Danish study, the prevalence of PPI use in adults increased from 2% in 2002 to 7.4% in 2014 and prolonged treatment was very common.5 Remarkably, PPI use increased with age; its prevalence among patients over 60 years old reaching 14.0% in men and 16.3% in women and exceeding 20% among patients aged ≥80 years.5 In stage 5D chronic kidney disease (CKD) patients, we found that PPI use is even higher, with 76% of patients receiving long-term treatment.6 Bone and mineral disorders, including secondary hyperparathyroidism and bone fractures, are more common among CKD patients than in people with normal renal function.7 In the general population, PPI use is associated with an increased risk of fractures.8, 9
The aim of this study was to assess the relationship between use of PPIs, and bone and hip fractures in hemodialysis patients in phases 2 to 4 of the observational Dialysis Outcomes and Practice Patterns Study (DOPPS), which included the systematic collection of fractures requiring hospitalization.
Patients and Methods
Patient population
The DOPPS is a prospective cohort study of hemodialysis practices based on the collection of observational longitudinal data for a random sample of patients from dialysis facilities in a representative and random sample of units in more than 20 countries.7 DOPPS 1 was from 1996 to 2001 in the United States, from 1998 to 2001 in Europe (France, Germany, Italy, Spain, and the United Kingdom), and from 1999 to 2001 in Japan. DOPPS 2 (2002 to 2004), DOPPS 3 (2005 to 2008), and DOPPS 4 (2009 to 2011), included the same DOPPS 1 countries, plus Australia, New Zealand, Canada, Belgium, and Sweden. The DOPPS phases 2 to 4 included 34,593 hemodialysis patients, of whom 27,857 (81%) were hospitalized for various reasons.
Study groups, outcome, and exposure definition
From a source population of 27,857 hospitalized patients, 75 were excluded because of missing baseline demographic characteristics (such as age, race, time of starting dialysis, and gender) and 685 patients were excluded because of missing information on PPI treatment. Thus, 27,097 hospitalized patients were available for the present analysis. We specifically focused on hospitalized patients to better capture medication data, thus minimizing the possibility of information bias.
The DOPPS investigators identified all first hospitalizations (defined by an overnight hospital stay) with an associated “bone fracture diagnosis code” according to international standards for hospital admissions. Bone fractures were coded as either “hip” or “other”; information on other types of fractures (eg, vertebral fractures) were not available. Thus, bone fractures include “hip-fractures” and fractures other than hip fractures. Hospitalizations and outpatient visits (including fracture-related visits) occurring during the study, along with diagnoses and procedures (ie, X-ray imaging) relevant to the hospitalization or outpatient visit, were reported chronologically for each patient. Capturing both hospitalization events and outpatient visits provided a reasonable expectation that the vast majority of fracture-related events were recorded in this study. Hip fractures were distinguishable from among all reported fracture-related events, whereas fracture types other than hip fracture were less well defined and consequently analyzed collectively. For fracture event rates, follow-up continued until death, fracture hospitalization, transplantation, renal replacement therapy modality switch, recovery of renal function, departure from the facility, or end of follow-up, whichever came first. PPI use was assessed on individual basis; ie, whether or not the PPI was administered in the patient concerned at the time of the visit. Prevalent PPI users were those who were already treated with the drug at the date of enrollment. Naive users or “new users” were those who started the treatment after the enrolment into the study.
Statistical analysis
Patients’ characteristics were summarized as mean ± standard deviation (SD), median and interquartile range (IQR), or as percent frequency, and comparisons between patient groups were made by independent t test, Mann-Whitney test, or chi-square test, as appropriate. The frequency of bone and hip fractures requiring hospitalization across countries was expressed as incidence rate (fractures/100 person-years) and 95% confidence interval. In the “time to the first event” survival analyses, the index date for naive PPI users was the date of starting treatment whereas for prevalent PPI users and nonusers the index date was the date of enrollment.
The crude and adjusted relationships between PPI treatment and incidence rate of bone and hip fractures, taking into account the competitive risk of mortality, were investigated by using the cumulative incidence function and the Fine and Gray approach,10 respectively. The effect of PPI on study outcomes was also investigated by a cause-specific hazards Cox model dealing with patient death as a censoring event and stratifying by country and study phase. To provide further insight into the pathophysiological implications of the study results, we calculated the etiological fraction or attributable risk (AR); ie, the proportion of fractures that would be prevented in our study cohort if the PPI treatment was eliminated in treated patients.
In multiple Cox models, we included PPI treatment as well as a series of potential confounders (ie, all variables listed in Table 1): age, gender, race, BMI, diabetes, smoking (past and current), background coefficient of variation (CV) comorbidities, dialysis vintage (ie, the time spanning from the date of dialysis initiation to the date of enrollment into the study), concomitant treatments (any form of vitamin D, phosphate binders, and calcium-based phosphate binders), and biochemical data (albumin, PTH, calcium, phosphate, alkaline phosphatase, and fractional urea clearance [Kt/V]). To account for prevalent users bias, a sensitivity analysis on naive patients versus no users was also performed. Concomitant therapies (any form of vitamin D, phosphate binders, and calcium-based phosphate binders) were defined differentially for the two study outcomes, depending on whether or not the start date of each specific treatment precedes the date of the outcome occurrence.
| PPI treatment | |||
| Characteristic | Whole cohort (n = 27,097) | Non-users (n = 13,814) | Prevalent users + naive patients (n = 13,283) |
| Age (years) | 63.9 ± 14.4 | 63.1 ± 14.6 | 64.8 ± 14 |
| BMI (kg/m2) | 25.2 ± 5.9 | 24.9 ± 5.8 | 25.6 ± 5.9 |
| Dialysis vintage (months) | 24 (5–64) | 23 (4–66) | 24 (5–62) |
| Male gender (%) | 58.4 | 59.7 | 57.1 |
| White (%) | 68.6 | 60.0 | 77.5 |
| African descent (%) | 7.2 | 8.1 | 6.3 |
| Asian/Indian (%) | 20.1 | 27.5 | 12.4 |
| Native American (%) | 0.8 | 0.72 | 0.76 |
| Other (%) | 3.3 | 3.6 | 3.0 |
| Smoking (%) | 48.4 | 46.8 | 50.1 |
| Background CV comorbidities | |||
| Diabetics (%) | 40.6 | 39.0 | 42.4 |
| Coronary artery disease (%) | 46.8 | 43.4 | 50.4 |
| Chronic heart failure (%) | 32.9 | 30.6 | 35.3 |
| Cardiovascular disease (%) | 36.7 | 34.5 | 38.9 |
| Cerebrovascular disease (%) | 18.0 | 16.4 | 19.8 |
| Peripheral artery disease (%) | 29.9 | 26.0 | 34.0 |
| Biochemical data | |||
| Albumin (g/dL) | 3.7 ± 0.4 | 3.7 ± 0.4 | 3.6 ± 0.5 |
| PTH (pg/mL) | 199(104–340) | 187(96–329) | 209(114–349) |
| Calcium (mg/dL) | 9.1 ± 0.7 | 9.1 ± 0.7 | 9.0 ± 0.6 |
| Phosphorus (mg/dL) | 5.2 ± 1.3 | 5.4 ± 1.3 | 5.1 ± 1.3 |
| Alkaline phosphatase (units/L) | 109(77–185) | 114(77–202) | 106(77–170) |
| Kt/Va | 1.48 ± 0.28 | 1.45 ± 0.28 | 1.50 ± 0.28 |
| Concomitant therapiesb | |||
| Any vitamin D (%)c | 65.2 | 62.9 | 67.5 |
| Phosphate binders (%) | 91.3 | 92.1 | 90.5 |
| Calcium-based phosphate binders (%) | 74.1 | 77.7 | 70.5 |
- Values are mean ± SD, median and interquartile range, or as percent frequency, as appropriate.
- a Fractional urea clearance (a dimensionless index of dialysis adequacy).
- b Concomitant therapies when investigating the hip fractures are as follows: whole cohort, any vitamin D: 65.4%; phosphate binders: 91.4%; calcium-based phosphate binders: 74.3%. Non-users, any vitamin D: 63.2%; phosphate binders: 92.2%; calcium-based phosphate binders: 77.8%. Prevalent users + naive users, any vitamin D: 67.7%; phosphate binders: 90.5%; calcium-based phosphate binders: 70.6%.
- c Includes either intravenous (alphacalcidol, paricalcitol, doxercalciferol, calcitriol, and other) or oral vitamin D.
Missing values for confounding variables were imputed by multiple imputation in which 30 completed data sets were generated and analyzed with standard combination rules for multiple imputation. Each variable was used as a confounder in the imputation model. In Cox models fitted according to the Fine and Gray approach, data were expressed as subdistribution hazard ratios (SHRs), 95% CIs, and p values. In cause-specific hazards Cox models dealing with patient death as a censoring event, stratifying by country and study phase and matching patients of the two arms according to the prescription time-distribution,11 data were expressed as hazard ratios (HRs), 95% CIs, and p values.
All analyses were performed by two standard statistical packages: SPSS for Windows Version 22 (IBM Corp., Armonk, NY, USA) and STATA/IC 13.0 (Stata Corporation, Inc., College Station, TX, USA).
Patient and public involvement
Full details about the involvement of patients and public in the DOPPS data collection are given elsewhere (see https://www.dopps.org/).
Results
The main demographic, clinical, and biochemical data of the whole study population (n = 27,097) as well as of PPI treated (n = 13,283, 49%) and untreated (n = 13,814, 51%) patients are given in Table 1. In individual countries, the prevalence of PPI-treated patients ranges widely from 28.6% in Japan to 73.5% in Spain (Supporting Fig. 1). Overall, patients from western countries represent 77% of the PPI-treated population and 60% of the PPI-untreated patients, whereas the corresponding data for Asians are 12% and 28%, respectively. The incidence rates of bone fractures were higher on average in PPI treated than in PPI untreated patients in all countries but Italy and Germany (Supporting Fig. 2). However, in Italy hip fractures were higher in PPI-treated than in untreated patients, whereas in Germany the opposite was observed.
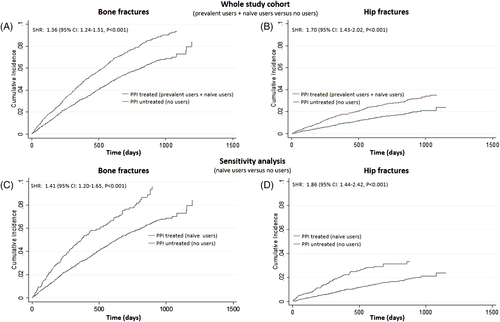
Across the follow-up period (median, 19 months; IQR, 10 to 28 months), 1592 patients experienced bone fractures (3.8 bone fractures/100 person-years; 95% CI, 3.6 to 3.9), and 528 patients had hip fractures (1.2 hip fractures/100 person-years; 95% CI, 1.1 to 1.3). Overall, 6249 patients died. The mortality rate was higher in PPI users than in non-users (25.8% versus 20.4%, p < 0.001), raising a problem of competitive risks. In crude Cox models (Fine and Gray approach) taking into account the competitive risk of mortality, the incidence rate of bone (SHR 1.36; 95% CI, 1.24 to 1.51; p < 0.001) and hip fractures (SHR 1.70; 95% CI, 1.43 to 2.02; p < 0.001) was significantly higher in PPI-treated than in PPI-untreated patients (Fig. 1 A, B). A sensitivity analysis in naive PPI users (Supporting Table 1) confirmed a higher incidence rate of bone and hip fractures in PPI-treated than in PPI-untreated patients (Fig. 1 C, D). The calculation of the AR showed an AR of 30% for bone fractures and 44% for hip fractures in the whole study population and an AR of 37% and 52%, respectively, in the sensitivity analysis on naive users versus untreated, suggesting that about one-half of hip fractures and more than one-third of bone fractures could be avoided if PPI use was eliminated in treated patients. Data adjustment for potential confounders, ie, for all variables listed in Table 1, did not materially affect the PPI study outcomes links either in the whole study cohort (“prevalent users + naive users” versus “no users”; see Table 2) or in a sensitivity analysis assessing the fractures risk of “naive users versus no users” (Table 3).
| Multiple Cox regression models taking into account the competing risk of death (Fine and Gray approach) | ||
| Variables (units of increase) | Bone fractures SHR (95% CI), p | Hip fractures SHR (95% CI), p |
| PPI treatment (0 = non-users; 1 = prevalent users + naive users) | 1.22 (1.10–1.36), p < 0.001 | 1.35 (1.13–1.62), p = 0.001 |
| Age (1 year) | 1.02 (1.01–1.02), p < 0.001 | 1.04 (1.03–1.05), p < 0.001 |
| Gender (0 = female; 1 = male) | 0.64 (0.57–0.71), p < 0.001 | 0.68 (0.58–0.83), p < 0.001 |
| African descent (0 = white; 1 = yes) | 0.61 (0.47–0.79), p < 0.001 | 0.72 (0.46–1.11), p = 0.13 |
| Asian/Indian (0 = white;1 = yes) | 0.89 (0.77–1.04), p = 0.15 | 0.41 (0.29–0.56), p < 0.001 |
| Native American (0 = white; 1 = yes) | 1.07 (0.62–1.85), p = 0.80 | 1.66 (0.74–3.72), p = 0.22 |
| Other (0 = white; 1 = yes) | 0.68 (0.47–0.97), p = 0.03 | 0.65 (0.32–1.30), p = 0.22 |
| BMI (kg/m2) | 0.99 (0.98–1.01), p = 0.14 | 0.98 (0.96–1.00), p = 0.05 |
| Diabetes (0 = no; 1 = yes) | 1.29 (1.16–1.44), p < 0.001 | 1.23 (1.01–1.50), p = 0.04 |
| Smoking (0 = no; 1 = yes) | 1.09 (0.98–1.23), p = 0.12 | 1.10 (0.90–1.34), p = 0.34 |
| CV comorbidities (0 = no; 1 = yes) | 1.03 (0.91–1.17), p = 0.66 | 1.21 (0.95–1.56), p = 0.13 |
| Dialysis vintage (years) | 1.02 (1.01–1.03), p < 0.001 | 1.04 (1.02–1.05), p < 0.001 |
| Any vitamin D (0 = no; 1 = yes) | 0.87 (0.78–0.97), p = 0.01 | 0.94 (0.78–1.14), p = 0.54 |
| Phosphate binders (0 = no; 1 = yes) | 0.91 (0.77–1.08), p = 0.27 | 0.88 (0.67–1.17), p = 0.38 |
| Albumin (1 g/dL) | 0.73 (0.65–0.83), p < 0.001 | 0.62 (0.50–0.77), p < 0.001 |
| PTH (100 pg/mL) | 1.01 (1.00–1.03), p = 0.05 | 1.02 (0.99–1.04), p = 0.15 |
| Calcium (1 mg/dL) | 1.01 (0.93–1.09), p = 0.80 | 1.05 (0.92–1.20), p = 0.49 |
| Phosphate (1 mg/dL) | 0.92 (0.88–0.97), p = 0.001 | 0.93 (0.85–1.01), p = 0.08 |
| Alkaline phosphatase (1 unit/L) | 1.01 (1.00–1.02), p = 0.006 | 1.00 (0.99–1.01), p = 0.23 |
| Kt/V (1 unit) | 1.33 (1.09–1.61), p = 0.004 | 1.27 (0.91–1.77), p = 0.16 |
- Data are SHR, 95% CI, and p values. Bold values are significant. Cox analyses including calcium-based phosphate binders instead of phosphate binders provided similar results (data not shown).
| Multiple Cox regression models taking into account the competing risk of death (Fine and Gray approach) | ||
| Variables (units of increase) | Bone fractures SHR (95% CI), p | Hip fractures SHR (95% CI), p |
| PPI treatment (0 = no users; 1 = naive users) | 1.29 (1.10–1.51), p = 0.002 | 1.62 (1.24–2.11), p < 0.001 |
| Age (1 year) | 1.02 (1.02–1.03), p < 0.001 | 1.06 (1.04–1.07), p < 0.001 |
| Gender (0 = female; 1 = male) | 0.56 (0.49–0.65), p < 0.001 | 0.56 (0.43–0.73), p < 0.001 |
| African descent (0 = white; 1 = yes) | 0.49 (0.35–0.70), p < 0.001 | 0.74 (0.43–1.28), p = 0.28 |
| Asian/Indian (0 = white; 1 = yes) | 0.79 (0.66–0.95), p < 0.001 | 0.34 (0.23–0.51), p < 0.001 |
| Native American (0 = white; 1 = yes) | 1.41 (0.74–2.68), p = 0.30 | 2.78 (1.14–6.79), p = 0.03 |
| Other (0 = white; 1 = yes) | 0.72 (0.46–1.12), p = 0.15 | 0.42 (0.13–1.32), p = 0.14 |
| BMI (1 kg/m2) | 0.99 (0.97–0.99), p = 0.04 | 0.96 (0.94–0.99), p = 0.01 |
| Diabetes (0 = no; 1 = yes) | 1.26 (1.09–1.46), p = 0.002 | 1.31 (1.01–1.70), p = 0.04 |
| Smoking (0 = no; 1 = yes) | 1.13 (0.96–1.32), p = 0.15 | 1.05 (0.80–1.38), p = 0.74 |
| CV comorbidities (0 = no; 1 = yes) | 1.05 (0.89–1.23), p = 0.58 | 1.24 (0.89–1.73), p = 0.20 |
| Dialysis vintage (years) | 1.02 (1.01–1.04), p < 0.001 | 1.03 (1.01–1.06), p = 0.002 |
| Any Vitamin D (0 = no; 1 = yes) | 0.92 (0.80–1.07), p = 0.28 | 1.19 (0.91–1.54), p = 0.20 |
| Phosphate binders (0 = no; 1 = yes) | 0.83 (0.66–1.05), p = 0.12 | 0.79 (0.53–1.19), p = 0.26 |
| Albumin (1 g/dL) | 0.79 (0.67–0.93), p = 0.005 | 0.72 (0.53–0.99), p = 0.04 |
| PTH (100 pg/mL) | 1.01 (0.99–1.03), p = 0.32 | 0.99 (0.94–1.03), p = 0.57 |
| Calcium (1 mg/dL) | 0.95 (0.86–1.05), p = 0.34 | 1.01 (0.84–1.23), p = 0.88 |
| Phosphate (1 mg/dL) | 0.92 (0.86–0.97), p = 0.005 | 0.94 (0.85–1.06), p = 0.30 |
| Alkaline phosphatase (1 unit/L) | 1.00 (1.00–1.01), p = 0.03 | 1.00 (0.99–1.01), p = 0.41 |
| Kt/V (1 unit) | 1.24 (0.95–1.61), p = 0.11 | 0.97 (0.60–1.57), p = 0.90 |
- Data are SHR, 95% CI, and p values. Bold values are significant. Cox analyses including calcium-based phosphate binders instead of phosphate binders provided similar results (data not shown).
In multiple, country and phase–stratified, cause-specific hazards Cox models (adjusting for the same set of variables listed in Tables 2 and 3) in patients of the two arms matched according to the prescription time (n = 14,136; of whom 3276 were treated with PPI and 10,860 were untreated), PPI treatment confirmed as a strong and independent risk factor of study outcomes (bone fractures: HRcountry and phase stratified 1.47; 95% CI, 1.23 to 1.76; p < 0.001; hip fractures: HRcountry and phase stratified 1.85; 95% CI, 1.37 to 2.50; p < 0.001). Of note, diabetes consistently emerged as a strong risk factor of bone and hip fractures in the study population (see Tables 2 and 3) independently of PPI use and other potential confounders.
Discussion
In a large, international cohort of hospitalized hemodialysis patients, the use of PPI associates with an increased risk of bone and hip fractures independently of the competitive risk of mortality and potential confounders including demographic, clinical, and biochemical data as well as concomitant therapies. Thus, the study provides evidence supporting the notion that PPI use is a risk factor of bone and hip fractures in the dialysis population.
PPIs are among the most widely prescribed medications worldwide. The average prevalence of PPI use in the DOPPS population is 49%, a figure markedly higher than that in the general population,6 where several studies have highlighted the association between PPI treatment and bone fractures.8, 9, 12 In a meta-analysis including 11 observational studies,12 the reported relative risk for hip fractures associated with PPI use was 1.30 (95% CI, 1.19 to 1.43) and such an association was of similar magnitude to that we found in the whole dialysis population comparing prevalent + naive users versus no users (SHR 1.35; 95% CI, 1.13 to 1.62; Table 2) but lower than that emerged in the sensitivity analysis comparing naive users versus no users (SHR 1.62; 95% CI, 1.24 to 2.11) indicating that the prevalent users bias should be taken into account when investigating the effect of PPI on adverse clinical outcomes in hemodialysis patients. In the same meta-analysis,12 the relative risk associated with PPI users was also increased for spine (RR 1.56; 95% CI, 1.31 to 1.85) and any-site fractures (RR 1.16; 95% CI, 1.04 to 1.30). Based on growing and compelling evidences reported in literature, the US Food and Drug Administration (FDA) issued a drug safety communication warning about the possibility of increased risk of fractures of the hip, wrist, and spine with the long-term use of both prescription and over-the-counter PPIs in the general population.13 This recommendation is of obvious public health importance because fractures per se are not only disabling clinical events but also a risk factor for mortality. Indeed, a population-based study highlighted a relationship between bone fractures and an increased risk of death and reported a mortality rate of 20% in the first year after a hip fracture.14 Similar data on mortality associated with hip fractures were also reported in hemodialysis patients: postfracture mortality rates exceeded 500/1000 patient years and fractured patients had higher unadjusted rates of death (3.7-fold) than the overall nonfractured dialysis population.7 On the other hand, mortality is exceedingly higher in hemodialysis patients than in the general population and for this reason it could act as a competitive risk while investigating the relationship between PPI use and bone fractures. Remarkably, in our study the relationship between PPI use and bone fractures remained significant when the competitive risk of death for both bone and hip fractures was also considered, either on univariate or on multivariable Cox analyses.
Mechanisms linking PPI use and bone fractures are still poorly understood, but there are several potentially plausible explanations. Importantly, fractures may be facilitated not only by reduction in bone density, but also by derangement of bone quality, and both bone quantity and quality could be affected by PPI use. Inhibition of gastric acid secretion can adversely affect the absorption of several nutrients, vitamins, and drugs.15 A reduced intestinal absorption of calcium and magnesium could lead to osteoporosis and fractures. However, in our study blood biochemistry results did not affect the association between PPI and fractures. Our results are consistent with accurate metabolic studies showing that PPI-associated hypochlorhydria does not decrease fractional calcium absorption following 30 days of continuous PPI use.16 Although we did not measure magnesium level in the study cohort, the homeostasis of this cation seems to be crucial for bone health. Magnesium deficiency, a well-known adverse effect of PPI use,17 contributes to bone impairment, both directly by acting on crystal formation and on bone cells, and indirectly by interfering with the activity of parathyroid hormone and 1,25(OH)2-vitamin D synthesis, as well as by promoting low-grade inflammation.18 Magnesium is also deposited in large quantities in bone, being essential for bone health. In addition, because magnesium is acting as inhibitor of extraskeletal calcification, PPI-induced hypomagnesemia may worsen vascular calcifications in patients with CKD.19 Interestingly, in the DOPPS cohort treated with PPIs peripheral artery disease was more common (34% of patients) compared to untreated patients (26%). PPIs interfere with the active transport of magnesium, and clinically significant phenomena are observed in the carriers of heterozygotic mutations of the ion channels transient receptor potential melastatin 6 (TRPM6) and TRPM7, which have a relevant role in the maintenance of magnesium homeostasis.17, 19 Recently, Sakaguchi and colleagues20 investigated 113,683 patients undergoing hemodialysis over a 2-year follow-up, finding an incidence of 2% for new hip fractures. The crude incidence rate was significantly higher among patients in the lower quartiles of serum magnesium levels (2.63%, 2.08%, 1.76%, and 1.49% in Q1 to Q4, respectively). After adjustment for demographic and clinical factors, patients in Q1 had a 1.23-fold higher risk for hip fracture than those in Q4 (95% CI, 1.06 to 1.44; p = 0.01).20 Of course, given the fact that we did not dose magnesium levels in the DOPPS patients, these considerations, although biologically plausible, are purely speculative.
Vitamin B12 deficiency has been associated with PPI use.21 Low vitamin B12 levels increase homocysteine levels, impairing cross-linking of bone collagen,22 and might increase the risk of bone fractures.23 In addition, peripheral neuropathy is also a consequence of vitamin B12 deficiency, increasing the risk of falls and, consequently, of bone fractures. The association between PPI use and increased risk of falls has been clearly demonstrated.24 Moreover, a direct PPI action on bone is also a possibility.25 Osteoclast function is dependent on proton pumps, which may be directly inhibited by PPIs, reducing bone resorption and turnover. This action was initially considered a potential treatment for osteoporosis,26 but altering bone turnover can deteriorate bone quality and increase the risk of fractures.
PPI treatment has been associated with other relevant side effects, supporting the concept of significant adverse biologic effects on the body, besides the intended effects in the gastrointestinal system.27 Microbiome alterations with bacterial overgrowth may affect absorption of nutrients, including proteins and vitamin K, with potential long-term adverse effects on bone health and increased fracture risk.28 The potential interaction between PPIs and vitamin K is of interest, because vitamin K intake is associated with a protective effect on bone fractures.29
The large difference in the incidence rates of bone fractures among different countries, as well as the finding that in Italian and German patients the side effect of PPI was not visible, remain unexplained and they deserve further studies, to identify which factors might prevent bone fractures.
Study strengths and limitations
The strengths of our study are the large patient-population and the fact that the PPI-fractures relationship remains significant in Cox models including potential confounders and taking into account the competitive risk of death by the Fine and Gray approach10 as well as in multiple, cause-specific hazards Cox models dealing with patient death as a censoring event, stratifying by country and study phase, and matching patients of the two arms according to the prescription time-distribution. Furthermore, the potential distortion attributable to prevalent users’ bias on the study results was investigated by performing a detailed analysis in naive patients versus untreated. Remarkably, such a sensitivity analysis provides results even more convincing than those obtained in the whole study population comparing prevalent + naive PPI users versus nonusers. Last, the observational nature of our cohort represents another strength, rather than a weakness, of our study, because observational studies are recognized to be essential for investigating the safety profile of medications.30 Although in Cox models we adjusted for a series of well-known potential confounders (including demographic and clinical variables and bone biomarkers), the possibility that we did not adjust for “unmeasured confounders” (including other drugs) cannot be excluded. However, the magnitude of the excess risk of bone and hip fractures (ranging from +22% to +62%) in PPI-treated patients as compared to those untreated indicates that such a possibility is rather unlikely. Furthermore, it is important to note that the HR of PPI for bone fractures we found in our study (see Table 2) was very similar (1.22 versus 1.19) to that reported in a secondary analysis of the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial investigating the effect of cinacalcet and other risk factors on the risk of bone fractures in dialysis patients.31 In this post hoc analysis,31 in a multiple Cox model adjusting for a series of potential confounders, the effect of PPI on bone fractures did not achieve formal statistical significance (p = 0.09), most likely because of the low number of patients treated with PPI in the experimental EVOLVE trial (only 1141 patients versus 13,283 patients in our real-life study). Our results are also in keeping with those reported in a recent case-control study by Vangala and colleagues32 in hemodialysis patients included in the USA Renal Data System. This study shows that the odds ratio of hip fractures is higher in PPI-treated than in untreated patients, independently of a series of potential confounders. In the study by Vangala and colleagues,32 the adjusted excess risk of hip fractures of PPI use versus non-use ranged from +16% to +21% (dependent on the frequency of PPI administration), figures lower than those found by us in primary (+35%) and sensitivity (+62% and + 85%) analyses. This underestimation of the PPI effect on hip fractures may depend on the fact that Vangala and colleagues32 did not take into account the potential distortion due to prevalent users’ bias, did not include in multivariate models circulating levels of bone biomarkers, and did not collect time to event data, all methodological issues that we specifically considered in our study, which also has the strength of including an international cohort of hemodialysis patients.
Conclusions
Considering the major health and economic burden of bone fractures, it is of utmost importance to adopt strategies for prevention of bone fractures in a fragile population such as hemodialysis patients. The growing and convincing evidence of a harmful effect of PPI on bone health,33, 34 the internal coherence of the results emerged in our study, as well as the magnitude of the negative effect of PPI use on the risk of bone and hip fractures in hemodialysis patients suggest caution and a careful evaluation of risks/benefits ratio when prescribing this class of drugs in this patient population.
Disclosures
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
We thank Jennifer Hartwig for editorial assistance.
Authors’ roles: Study design: MF, GT, and MG. Study conduct: MF, GD, AP, GT, and MG. Data collection: MF, GD, AP, GT, MG, FT, BR, BB, and KM. Data analysis: MF, GD, AP, GT, and MG. Data interpretation: MF, GD, AP, GT, MG, MP, FF, SG, GI, AA, FT, BR, BB, and KM. Drafting and reviewing the manuscript: MF, GD, AP, GT, MG, MP, FF, SG, GI, AA, FT, BR, BB, and KM. Approval of the final manuscript: all authors. MF is the guarantor who takes responsibility for the integrity of the data analysis. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.




