The Association Between Protein Intake by Source and Osteoporotic Fracture in Older Men: A Prospective Cohort Study
ABSTRACT
Dietary protein is a potentially modifiable risk factor for fracture. Our objectives were to assess the association of protein intake with incident fracture among older men and whether these associations varied by protein source or by skeletal site. We studied a longitudinal cohort of 5875 men (mean age 73.6 ± 5.9 years) in the Osteoporotic Fractures in Men (MrOS) study. At baseline, protein intake was assessed as percent of total energy intake (TEI) with mean intake from all sources = 16.1%TEI. Incident clinical fractures were confirmed by physician review of medical records. There were 612 major osteoporotic fractures, 806 low-trauma fractures, 270 hip fractures, 193 spine fractures, and 919 non-hip non-spine fractures during 15 years of follow-up. We used Cox proportional hazards models with age, race, height, clinical site, TEI, physical activity, marital status, osteoporosis, gastrointestinal surgery, smoking, oral corticosteroids use, alcohol consumption, and calcium and vitamin D supplements as covariates to compute hazard ratios (HRs) with 95% confidence intervals (CIs), all expressed per unit (SD = 2.9%TEI) increase. Higher protein intake was associated with a decreased risk of major osteoporotic fracture (HR = 0.92; 95% CI, 0.84 to 1.00) with a similar association found for low-trauma fracture. The association between protein and fracture varied by protein source; eg, increased dairy protein and non-dairy animal protein were associated with a decreased risk of hip fracture (HR = 0.80 [95% CI, 0.65 to 0.98] and HR = 0.84 [95% CI, 0.72 to 0.97], respectively), whereas plant-source protein was not (HR = 0.99 [95% CI, 0.78 to 1.24]). The association between protein and fracture varied by fracture site; total protein was associated with a decreased risk of hip fracture (HR = 0.84 [95% CI, 0.73 to 0.95]), but not clinical spine fracture (HR = 1.06 [95% CI, 0.92 to 1.22]). In conclusion, those with high protein intake (particularly high animal protein intake) as a percentage of TEI have a lower risk of major osteoporotic fracture. © 2016 American Society for Bone and Mineral Research.
Introduction
Osteoporotic fractures, particularly hip fractures, are a significant health care burden in older adults. Between 1986 and 2005, the annual incidence of hip fractures among those over 65 years of age was 9.6 per 1000 for women and 4.1 per 1000 for men with subsequent mortality of nearly one in four women and one in three men.1 Hip fractures are also associated with decreased health-related quality of life2 and substantial health care costs.3, 4 The Institute of Medicine has formulated an acceptable macronutrient distributions range for adults, which specifies that protein intake among adults should range between 10% to 35% of total energy intake (TEI).5 This range is not based on an assessment of protein requirements to maintain optimal musculoskeletal health among older adults. Causal links between protein intake and bone health are contentious due to many conflicting reports. Some of this discordance might be related to protein source (animal versus plant, dairy versus non-dairy). In particular, several studies (all in women) have observed heterogeneity by source of protein.6-8 A recent study assessing protein and fracture risk in both men and women found no statistically significant heterogeneity between protein and fracture risk by source of protein.9 The same study found that the association between protein and bone mineral density (BMD), a key risk factor for fracture, did vary by source of protein. A plausible explanation for this finding might well have been the insufficient number of fracture outcomes to observe heterogeneity by source, particularly in men. Another potential explanation for the discordant fracture results could be heterogeneity of effect by skeletal site of fracture. A recent study of Beasley and colleagues10 in postmenopausal women found that high protein intake was associated with a lower risk of hip fracture and forearm fracture, but was not associated with a lower risk of clinical spine fracture.
Thus, our primary objective was to assess the association of protein intake overall and by dietary source (dairy, non-dairy animal, and plant) with fracture risk (major osteoporotic, low-trauma, and skeletal site-specific) among older men. Our secondary objective was to assess potential mediation by other factors including BMD. Our primary hypothesis was that higher protein intake would be associated with a lower risk of fracture, with possible attenuation of effect with increasing intake. We also hypothesized that the effect of protein intake would depend on source of intake, likely due to the mediation by total hip BMD.
Subjects and Methods
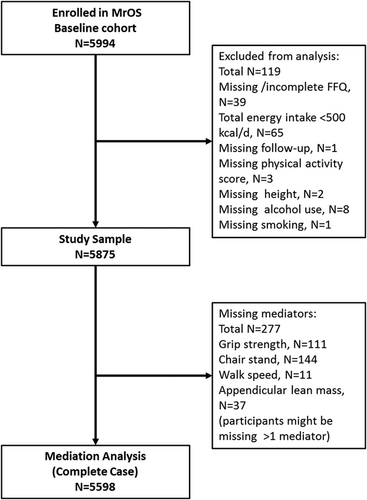
From 2000 to 2002, the Osteoporotic Fractures in Men (MrOS) study enrolled 5994 ambulatory men aged 65 years and older living in one of six US metropolitan areas. The baseline clinical exam included height (Harpenden stadiometer), weight (balance beam or electronic scale), and dual-energy X-ray absorptiometry (DXA) scans. Body mass index (BMI) was calculated from measured height and weight using the formula BMI = weight (kg)/height (m)2. The study sample for this analysis included 5875 men who completed a food frequency questionnaire (FFQ) at baseline with fewer than 10% missing responses, who had plausible reported energy intake (>500 kcal/day), and who had no missing data on covariates used in the main analysis (see Fig. 1). Further details concerning study cohort recruitment and methods have been published elsewhere.11, 12 All participants gave written informed consent; the study was conducted in accord with the Helsinki Declaration, and ethics approval was granted through institutional review boards at the respective study centers.
Protein intake
Participants completed a modified version of the original Block FFQ at study baseline.13 The FFQ asked 69 individual food item questions, including an additional 13 questions about food preparation and low-fat foods which were used to refine nutrient calculations. There were nine categories of frequency responses for foods and beverages and four categories of portion size responses. A graphic representation of standard portion sizes was included with the questionnaire. Total energy intake, total protein intake, and protein intake by source were derived from the responses to the questionnaire by Block Dietary Data Systems (Berkeley CA, USA), with dietary reference data from the US Department of Agriculture Database for Standard Reference for Version 12 and the 1994–1996 Continuing Survey of Food Intakes by Individuals database. We considered the following subcategories of intake: protein from dairy products, non-dairy animal protein (eg, meat, fish, poultry, eggs), and protein from plant sources (eg, legumes, grains, nuts).
Fracture outcomes
Participants were contacted every 4 months and queried whether they had a fracture. The radiographic reports pertaining to the fracture event were obtained and reviewed at the MrOS Coordinating Center to adjudicate incident fracture outcomes. For incident spine fractures, spine images taken at the time of the clinical encounter were obtained and reviewed by the study radiologist, who used the Genant semiquantitative method to confirm that the community imaging study showed an increase of 1 SQ grade in one or more vertebrae compared with the study baseline lateral spine radiographs.14 The main fracture outcome was major osteoporotic fracture (hip, clinical spine, forearm/wrist, or humerus). A secondary fracture outcome was low-trauma fracture, excluding those of the head, hands, or feet. Low-trauma fractures were defined to be a fractures due to: (1) a fall from standing height or less; (2) moderate trauma other than a fall (eg, collisions with objects during normal activities); or (3) minimal trauma other than a fall (eg, turning over in bed). Other secondary fractures outcomes were hip fracture, clinical spine fracture, and non-hip, non-spine fractures (regardless of trauma). Note that the main outcome and the secondary outcomes were not mutually exclusive.
Total hip BMD (mediator and secondary outcome)
Participants had BMD at the hip measured using QDR 4500 fan-beam densitometers (Hologic, Bedford, MA, USA). The study used standardized procedures for position and scanning and certification of machine operators to ensure reproducibility. A set of whole-body, spine, hip, and linearity phantoms were circulated between centers; however, variation between centers was within acceptable limits and no corrections were required.
Other covariates
Information on demographics, lifestyle, and medical and family history was obtained by questionnaire and interview by trained clinical staff. Race/ethnicity was self-identified. Participants were classified into current, past, or never smokers. Self-reported alcohol intake was divided into four categories: none/occasional (<1 drink/week), light (1 to 6 drinks/week), moderate (7 to 13 drinks/week), and heavy (≥14 drinks/week). Physical activity was measured by computing the Physical Activity Scale for the Elderly (PASE). Gait speed was determined from the using the best time to complete a 6-m walk and expressed as m/s. Time to complete five chair stands was measured and expressed as number of stands/s. Grip strength (kg) was measured in four trials (two in each hand) using a handheld Jamar dynamometer (Sammons Preston Rolyan, Bolingbrook, IL, USA) and maximum grip strength was taken to be the maximum of all measures. Those who were unable to perform the physical performance tests (gait speed, chair stand, and grip strength) were assigned the value 0 in the respective speed or strength measure. Participants were asked to bring all current (any use within the past 30 days) prescription medications with them to the clinic. All prescription medications were recorded in an electronic medication inventory database and matched to its ingredients(s) based on the Iowa Drug Information Service drug vocabulary (College Pharmacy, University of Iowa, Iowa City, IA, USA). Information on calcium and vitamin D supplement use was determined from supplement questions on the modified Block FFQ. Appendicular lean mass was measured by Hologic densitometers (the same machines used to determine BMD).
Statistical methods
We used ANOVA for comparisons of continuous variables by protein intake quartile and chi square tests for comparisons of categorical variables by protein intake category. We used a time-to-event (Cox) model to determine the association between protein intake and risk of fracture. Participants were followed until a fracture, death, loss to follow-up, or end of study follow-up, whichever occurred first. We considered age-adjusted and full confounder-adjusted models. Total protein was the primary exposure variable, whereas protein intake by source (dairy, non-dairy, animal, plant) were secondary exposure variables. Our analysis considered protein intake (and protein intake by source) as a percent of total energy intake (TEI), whereas TEI was included as an adjustment variable in all multivariate models to account for the correlations between energy intake and the exposure and outcome. We assessed all continuous variables for possible nonlinearity using higher order terms and fractional polynomials. Heterogeneity by source (dairy versus non-dairy, animal versus plant) was tested by looking at the coefficient of the difference variables (eg, percent dairy protein minus percent non-dairy protein) with threshold p < 0.05 with adjustment for all covariates previously listed. If this testing indicated heterogeneity, source-specific estimates were estimated using a single model including all three protein source variables. We did not exclude participants with missing supplement or medication data, but rather used derived variables with a separate category for those with missing data. We found that missing data for these variables was not in fact associated with either the exposure (protein intake) or fracture outcomes. If the source of trauma was unknown, we classified the fracture as low-trauma, because we presumed that participants would have recall of all high-trauma fracture events. We performed a sensitivity analysis repeating the low-trauma fracture analysis and excluding the 71 fractures for which there was no recall of associated level of trauma, and results were unchanged.
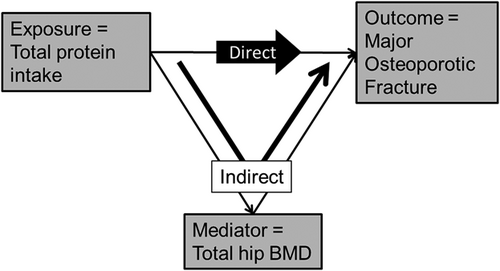
Mediation was assessed using two methods on the subcohort with measures for all mediators (complete case analysis). Mediation was first informally assessed by including the appropriate covariates in the Cox proportional hazards models and looking at the change (attenuation) in point estimates. We also performed a second formal mediation analysis to estimate direct effects (independent of mediators) and indirect effects (via one or more mediators). Figure 2 shows the directed acyclic graph linking exposure, mediator, and outcome; in this case the mediator is total hip BMD. The total effect is decomposed into two separate components: the direct component, which occurs through causal mechanisms other than total hip BMD; and the indirect component, which occurs through the posited mediator(s). The indirect component is a sequential effect, and thus is not null when both exposure-mediator and mediator-outcome associations are not null. We used inverse odds ratio weights to measure the direct and indirect effects and used a bootstrap to estimate standard errors.15 The exposure coefficient in such a weighted regression estimates the natural direct effect of exposure on the outcome, and indirect effects are identified by subtracting direct effects from total effects. Weighting renders exposure and mediators independent, thereby deactivating indirect pathways of the mediators. Hypothesized mediators of the association of protein intake with fracture risk included BMI, total hip BMD, appendicular lean mass, history of falls, and physical performance measures (grip strength, usual walking speed, chair stand speed). Total hip BMD was found to be the most important mediating covariate with statistically significant indirect effect; therefore, we further analyzed the sequential relationship by considering total hip BMD as an outcome in a linear regression model. Effect size was defined to be the beta coefficient from a regression model where both exposure and outcome were parameterized to have mean = 0 and SD = 1. For comparison purposes, models considering protein by source were parameterized to have the same units as for total protein; ie, 1 unit = 2.9%TEI. Several post hoc sensitivity analyses were performed: (1) exclusion of men taking medications likely to impact bone mineral metabolism (androgens, androgen-agonists, bisphosphonates); (2) competing risk analysis for mortality using Fine-Grey models); (3) explicit inclusion of non-dairy sources of calcium and vitamin D (food and supplements); and (4) truncation of analysis at shorter time intervals (5 and 10 years). Analysis was performed using Stata Version 14.0 (College Station, TX, USA).
Results
Among the cohort of 5875 men, the mean ± SD age was 73.6 ± 5.9 years, the mean ± SD energy intake was 1630 ± 635 kcal/day, and the mean ± SD total protein intake was 16.1 ± 2.9 %TEI. The distributions (mean ± SD) of protein intake by source as a percent of total energy intake were as follows: dairy protein 3.5 ± 2.0 %TEI, non-dairy animal protein 6.2 ± 2.9 %TEI, and plant protein 6.3 ± 1.7 %TEI. The same parameters expressed as a percent of total protein intake were as follows: dairy protein 22.0% ± 11.2%, non-dairy animal protein 37.7% ± 13.2%, and plant protein 40.4% ± 11.9%. Baseline characteristics of the study sample stratified by total protein intake quartile are shown in Table 1. Participants in the higher quartiles of protein intake compared with those in the lower quartiles of protein intake were more likely to have post-secondary education and were more likely to report moderate/heavy alcohol use. Those in the lower quartiles of intake had slightly lower BMD, appendicular lean mass, grip strength, usual walking speed, and chair-stand speed than those in the upper quartiles of intake. BMI and smoking did not vary by protein intake quartiles.
| Protein intake quartile (%)a | |||||
|---|---|---|---|---|---|
| Variable | Q1 (6.0–14.1) (n = 1469) | Q2 (14.2–15.8) (n = 1469) | Q3 (15.9–17.7) (n = 1469) | Q4 (17.8–29.3) (n = 1468) | pb |
| Incident low-trauma fracture, n (%) | 221 (15.0) | 193 (13.1) | 194 (13.2) | 198 (13.5) | 0.39 |
| Incident major osteoporotic fracture, n (%) | 161 (11.0) | 154 (10.5) | 165 (11.2) | 133 (9.1) | 0.22 |
| Hip fracture, n (%) | 83 (5.7) | 70 (4.8) | 68 (4.6) | 49 (3.3) | 0.03 |
| Clinical spine fracture, n (%) | 44 (3.0) | 37 (2.5) | 54 (3.7) | 58 (4.0) | 0.12 |
| Non-hip non-spine fracture, n (%) | 229 (15.6) | 231 (15.7) | 231 (15.7) | 228 (15.5) | 0.99 |
| Age (years), mean ± SD | 73.6 ± 5.9 | 74.0 ± 5.8 | 73.6 ± 5.9 | 73.4 ± 5.9 | 0.03 |
| Non-Hispanic white, n (%) | 1282 (87.3) | 1329 (90.5) | 1336 (91.0) | 1324 (90.2) | 0.004 |
| Post-secondary degree, n (%)c | 665 (45.3) | 764 (52.0) | 842 (57.3) | 860 (58.6) | <0.001 |
| Married, n (%) | 1192 (81.1) | 1240 (84.4) | 1231 (83.8) | 1173 (79.9) | 0.003 |
| Current smoker, n (%)c | 61 (4.2) | 50 (3.4) | 39 (2.7) | 49 (3.3) | 0.17 |
| Alcohol use of ≥7 drinks/week, n (%)c | 301 (20.5) | 364 (24.9) | 402 (27.4) | 455 (31.0) | <0.001 |
| History of gastrointestinal surgery, n (%) | 112 (7.6) | 96 (6.5) | 130 (8.8) | 94 (6.4) | 0.04 |
| Osteoporosis diagnosis, n (%) | 49 (3.3) | 56 (3.8) | 41 (2.8) | 62 (4.2) | 0.18 |
| Calcium/Vitamin D supplement use, n (%)c | 478 (32.5) | 529 (36.0) | 555 (37.8) | 538 (36.7) | 0.02 |
| Corticosteroid medication use, n (%)c | 34 (2.3) | 30 (2.0) | 36 (2.5) | 24 (1.6) | 0.42 |
| Body mass index (kg/m2), mean ± SD | 27.3 ± 3.8 | 27.3 ± 3.6 | 27.4 ± 3.9 | 27.5 ± 4.1 | 0.27 |
| Total hip BMD (g/cm2), mean ± SD | 0.946 ± 0.141 | 0.958 ± 0.138 | 0.959 ± 0.135 | 0.968 ± 0.148 | <0.001 |
| Prior fall history, n (%) | 322 (21.9) | 323 (22.0) | 277 (18.9) | 320 (21.8) | 0.11 |
| PASE score, mean ± SD | 147.9 ± 69.1 | 145.0 ± 66.1 | 149.0 ± 67.9 | 144.1 ± 69.8 | 0.16 |
| Appendicular skeleton lean mass (kg), mean ± SD | 24.0 ± 3.4 | 24.4 ± 3.4 | 24.3 ± 3.4 | 24.4 ± 3.7 | 0.05 |
| Usual walking speed (m/s), mean ± SD | 1.23 ± 0.25 | 1.23 ± 0.26 | 1.26 ± 0.25 | 1.25 ± 0.26 | <0.001 |
| Maximum grip strength (kg), mean ± SD | 40.1 ± 9.8 | 40.8 ± 10.1 | 41.4 ± 10.0 | 41.5 ± 10.1 | <0.001 |
| Chair stand speed (stands/s), mean ± SD | 0.47 ± 0.14 | 0.48 ± 0.13 | 0.49 ± 0.14 | 0.49 ± 0.14 | 0.01 |
- PASE = Physical Activity Scale for the Elderly.
- a Expressed as a percentage of total energy intake (TEI).
- b ANOVA for continuous variables, chi-square for categorical variables.
- c For simplicity, categories used in analysis have been collapsed in this table. Education: <12 years (377), high school degree (2367), college degree (1698), graduate studies (1433). Smoking: current (199), past (3472), or never smokers (2204). Alcohol intake: <1 drink/week (2803), 1–6 drinks/week (1550), 7–13 drinks/week (839), ≥14 drinks/week (683). Calcium/vitamin D supplement use: non-user (3559), user (2100), missing (216). Corticosteroid use: non-user (5517), user (124), missing (234).
Protein and fracture outcomes
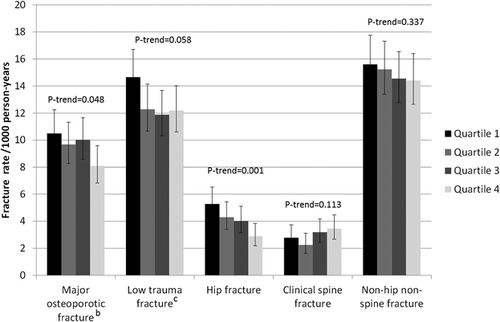
During a mean follow-up of 10.5 to 11.2 years (depending on outcome) there were 613 incident major osteoporotic fractures, 806 low-trauma fractures, 270 hip fractures, 193 clinical spine fractures, and 919 non-hip non-spine fractures. Unadjusted incident fracture rates by protein intake quartile are shown in Fig. 3. There was a statistically significant trend by quartile of protein intake for major osteoporotic fracture (p = 0.048) and hip fracture (p = 0.001), where those with higher protein intake had a lower fracture incidence. In particular those in the highest quartile of protein intake had a hip fracture incidence of 2.91 (95% CI, 2.20 to 3.84)/1000 person-years, whereas those in the bottom quartile of protein intake had a hip fracture incidence of 5.27 (95% CI, 4.25 to 6.53)/1000 person-years. Table 2 shows the association between total protein intake (and by source) and incident fracture (major osteoporotic fracture, low-trauma fracture, and site-specific fracture). Higher total protein intake was associated with a decreased risk of major osteoporotic fracture (HR = 0.92 [95% CI, 0.84 to 1.00] per unit = 2.9%TEI increase in protein intake) and low-trauma fracture (HR = 0.92 [95% CI, 0.85 to 0.99]). Higher total protein intake was also associated with a decreased risk of hip fracture (HR = 0.84 [95% CI, 0.73 to 0.95]) and with a non-statistically significant (p = 0.07) decreased risk of non-hip non-spine fracture (HR = 0.94 [95% CI, 0.88 to 1.00]), but was not associated with a risk of clinical spine fracture (HR = 1.06 [95% CI, 0.92 to 1.22]).
| Hazard ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Fracture type | n | Total protein | Dairy protein | Non-dairy animal protein | Plant protein |
| Major osteoporotic fracturea | 613 | ||||
| Base modelb | 0.93 (0.86–1.01) | 0.94 (0.83–1.07) | 0.92 (0.84–1.01) | 0.96 (0.83–1.12) | |
| Full modelc | 0.92 (0.84–1.00) | 0.89 (0.78–1.01) | 0.92 (0.84–1.01) | 0.97 (0.83–1.13) | |
| Low-trauma fractured | 806 | ||||
| Base model | 0.94 (0.87–1.01) | 0.93 (0.84–1.04) | 0.94 (0.87–1.02) | 0.95 (0.83–1.08) | |
| Full model | 0.92 (0.85–0.99) | 0.89 (0.79–0.99) | 0.93 (0.86–1.01) | 0.95 (0.83–1.09) | |
| Hip fracture | 270 | ||||
| Base model | 0.81 (0.71–0.92) | 0.79 (0.66–0.96) | 0.80 (0.69–0.93) | 0.88 (0.70–1.10) | |
| Full model | 0.84 (0.73–0.95) | 0.80 (0.65–0.98) | 0.84 (0.72–0.97) | 0.99 (0.78–1.24) | |
| Clinical spine fracture | 193 | ||||
| Base model | 1.10 (0.96–1.27) | 1.14 (0.92–1.40) | 1.09 (0.94–1.28) | 1.08 (0.82–1.41) | |
| Full model | 1.06 (0.92–1.22) | 1.05 (0.85–1.31) | 1.06 (0.91–1.24) | 1.02 (0.77–1.35) | |
| Non-hip non-spine fracture | 919 | ||||
| Base model | 0.97 (0.90–1.03) | 0.94 (0.85–1.04) | 0.97 (0.91–1.05) | 0.99 (0.87–1.11) | |
| Full model | 0.94 (0.88–1.00) | 0.87 (0.78–0.97) | 0.96 (0.89–1.03) | 0.95 (0.83–1.07) | |
- Dietary protein intake is calculated as a percentage of total energy intake (%TEI) and scaled to have 1 unit change = 2.9%TEI = 1 SD (total protein intake). Source-specific protein intakes were included in a single model; ie, each one was adjusted for the other two source-specific intakes.
- a Hip, clinical spine, forearm, humerus
- b Base model adjusted for age
- c Full model adjusted for age, height, race/ethnicity, education, marital status, clinical center, total energy intake, smoking, alcohol use, self-reported physical activity (PASE), history of GI surgery, history of osteoporosis, calcium/vitamin D supplement use, corticosteroid use as covariates
- d Without trauma or due to trauma less than or equal to fall from standing height.
The association between protein and fracture varied by protein source; eg, increased dairy protein and non-dairy animal protein were both associated with a decreased risk of hip fracture (HR = 0.80 [95% CI, 0.65 to 0.98] and HR = 0.84 [95% CI, 0.72 to 0.97], respectively), whereas increased plant-source protein was not (HR = 0.99 [95% CI, 0.78 to 1.24]).
Potential mediators of association of protein intake with fracture outcomes
We further assessed the extent to which the association between protein intake and fracture was mediated by BMI, total hip BMD, appendicular lean mass, falls, and physical performance measures (grip strength, usual walking speed, and chair stand speed) in a subcohort of 5598 (95%) men in which these intermediate outcome variables were assessed. In the mediation subcohort, there were 568 incident major osteoporotic fractures, 753 low-trauma fractures, 246 hip fractures, 183 spine fractures, and 865 non-hip non-spine fractures. Table 3 shows there was an association between protein intake and major osteoporotic fracture in the mediation model that decomposed into an estimated (albeit nonsignificant) direct effect (HR = 0.93 [95% CI, 0.85 to 1.01] per unit = 2.9%TEI increase in protein intake) and estimated indirect (via one or more mediators) effect (HR = 0.97 [95% CI, 0.95 to 1.00]). A similar analysis considering total hip as the sole intermediate variable showed an estimated (albeit nonsignificant) direct effect (HR = 0.93 [95% CI, 0.85 to 1.02]) and estimated indirect (via total hip BMD) effect (HR = 0.97 [95% CI, 0.96 to 0.99]). The only statistically significant direct effects observed were for hip fracture. There was a statistically significant indirect effect of total hip BMD for all fracture outcomes, but this effect was strongest for hip fracture. The direct effects estimated with inverse odd ratio weighting were nearly identical to the estimates from Cox proportional hazards models, which included either total hip or all mediators as covariates (data not shown).
| Hazard ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Fracture type | n | Total effect | Direct effect (not through intermediate variablea) | Indirect effect (via 1 or more intermediate variablea) | Direct effect (not through total hip BMD) | Indirect effect (via total hip BMD alone) |
| Major osteoporotic fractureb | 568 | 0.90 (0.83–0.99) | 0.93 (0.85–1.01) | 0.97 (0.95–1.00) | 0.93 (0.85–1.02) | 0.97 (0.96–0.99) |
| Low-trauma fracturec | 753 | 0.91 (0.84–0.98) | 0.93 (0.86–1.01) | 0.98 (0.95–1.00) | 0.93 (0.86–1.01) | 0.98 (0.96–0.99) |
| Hip fracture | 246 | 0.82 (0.71–0.95) | 0.86 (0.75–0.98) | 0.95 (0.92–0.99) | 0.86 (0.74–0.98) | 0.96 (0.93–0.98) |
| Clinical spine fracture | 183 | 1.04 (0.89–1.20) | 1.08 (0.93–1.25) | 0.96 (0.93–0.99) | 1.07 (0.92–1.23) | 0.97 (0.95–0.99) |
| Non-hip non-spine fracture | 865 | 0.94 (0.87–1.01) | 0.95 (0.89–1.03) | 0.98 (0.96–1.00) | 0.95 (0.89–1.02) | 0.98 (0.97–0.99) |
- Model adjusted for age, height, race/ethnicity, education, marital status, clinical center, total energy intake, smoking, alcohol use, self-reported physical activity (PASE), history of GI surgery, history of osteoporosis, calcium/vitamin D supplement use, and corticosteroid use as covariates. Protein intake is calculated as a percentage of total energy intake (%TEI) and scaled to have 1 unit change = 2.9%TEI = 1 SD (total protein intake).
- a Intermediate variables (mediators) included BMI, total hip BMD, falls, appendicular lean mass, usual walk speed, grip strength, and chair stand speed.
- b Hip, clinical spine, forearm, and humerus.
- c Without trauma or due to trauma less than or equal to fall from standing height.
Protein and total hip BMD
Higher total protein intake was associated with higher total hip BMD with effect size (beta = 0.06; 95% CI, 0.04 to 0.09). Similar to the findings for the association of protein intake with fracture, the association between protein intake and total hip BMD varied according to source of protein. With regard to protein source, higher intake of protein from dairy sources was associated with higher total hip BMD (beta = 0.10; 95% CI, 0.07 to 0.14), as was higher protein intake from non-dairy animal sources (beta = 0.06; 95% CI, 0.03 to 0.08), but higher intake from plant sources was not (beta = −0.01; 95% CI, –0.06 to 0.04), with all betas calculated per unit = 2.9%TEI increase in protein intake.
We performed several post hoc sensitivity analyses. Exclusion of men taking androgens, androgen-agonists, or bisphosphonates yielded similar results (difference <0.01 for all sites except the spine). For the spine there was a slight change in point estimate (1.04 versus 1.06) that did not affect the overall conclusions. The results of the Fine-Grey competing risk analysis were largely concurrent with the main analysis with only a slight attenuation of the point estimates; eg, HR = 0.93 (95% CI, 0.85 to 1.01) for major osteoporotic fracture and HR = 0.85 (95% CI, 0.75 to 0.96) for hip fracture. We considered auxiliary variables (vitamin D from non-dairy foods, calcium from non-dairy foods, vitamin D from supplements, and calcium from supplements) but none of these variables were associated with hip fracture. Finally, we considered truncation of follow-up. The association between protein intake and hip fracture was HR = 0.84 (95% CI, 0.65 to 1.08) per SD increase when truncated at 5 years and HR = 0.81 (95% CI, 0.69 to 0.95) per SD increase when truncated at 10 years.
Discussion
We found that older men with higher protein intake as a percentage of TEI had a decreased risk of major osteoporotic, low-trauma, and hip fracture, but not clinical vertebral fracture. We did not find evidence of a threshold effect for any of the fracture outcomes. Higher protein intake was also associated with higher total hip BMD. These associations varied according to source of protein; higher protein intake from animal sources (both dairy and non-dairy) was associated with higher hip BMD and lower major osteoporotic and hip fracture risk, but higher intake from plant sources was not related to these outcomes. We also found that the association between protein and fracture could be decomposed into a direct association (independent of major risk factors) and an indirect association (mediated by one or more major risk factors). The indirect component was small in magnitude, but statistically significant and largely attributable to the serial correlations between protein intake, total hip BMD, and fracture risk.
The results are consistent with the larger cohort studies that examined the association of protein intake with fracture risk, but do not confirm findings of some other smaller studies. In particular, Mussolino and colleagues16 showed that higher protein intake and higher calcium intake were each independently associated with a lower risk of hip fracture based on a population-based cohort of non-Hispanic white men, concurrent with the present analysis linking source of protein (dairy versus non-dairy) and risk of hip fracture. The fracture site heterogeneity in the present study was also consistent with that noted in the Women's Health Initiative (WHI), a large prospective study of US women which determined total biomarker-calibrated protein among women and found that protein intake was associated with a decreased risk of hip and forearm fracture, but was not associated with the risk of clinical spine fracture.10 Fracture risk factors may vary by skeletal site due to different contributions of cortical versus trabecular bone to bone strength parameters and failure mechanisms. As an example, a study of postmenopausal women found several prominent risk factors for hip fracture; in particular weight, BMI, and neuromuscular disability were not related to incident spine fracture.17 Thus, it is quite possible in the present case that higher protein intake may preserve lean mass and muscle strength, consequently preserving cortical integrity of the proximal femur, and that these risk factors are not a key component of vertebral fracture risk.
The present study expands upon a previous paper from the MrOS cohort that reported that low protein intake was an independent risk factor for hip fracture in that it considers source of intake, longer follow-up and consideration of skeletal site and level of trauma.18 These results are somewhat concordant with results for major osteoporotic fracture and low-trauma fracture in the Canadian Multicentre Osteoporosis Study (CaMos) cohort9 and hip fracture outcomes in the Framingham Osteoporosis Study cohort.19 The CaMos study did not find protein source heterogeneity for fracture outcomes, but did not specifically evaluate hip fracture, the fracture site where protein source heterogeneity appeared to be the strongest. The Framingham study evaluated hip fracture but was limited by the low total number of fractures in men (n = 80 in women, n = 20 in men).
Some earlier studies assessing protein and fracture were less clear in identification of an association between protein and fracture. One study assessing protein intake and the risk of hip fracture in Norwegian men and women was largely inconclusive, but noted an increased risk among those with high intakes of non-dairy animal protein intake.6 Another study assessing protein intake among participants in the Nurses’ Health Study (United States) also noted an increased risk of forearm fractures in women, but found no association for hip fracture.20 A third study assessing the association among US men and women had mixed results; high protein intake was associated with decreased fracture risk among men and women ages 50 to 69 years but not among men and women ages 70 to 89 years.21 The present study did not confirm any of these previous results, but might not be entirely inconsistent in view of the markedly different target populations and noted heterogeneity by source and outcome in the present study.
A novelty of the present study is that it identifies total hip BMD as a key mediator in the association between protein intake and fracture. A meta-analysis assessing the relation between protein intake and BMD or bone mineral content at the main clinically relevant sites found that increased protein intake was associated with increased BMD with 1% to 2% of variation in BMD explained by protein intake.22 One underlying biologic mechanism linking protein intake to BMD is increased calcium absorption. Based on experimental studies, Kerstetter and colleagues23 proposed that there is a biological interaction between protein and calcium intake whereby protein increases calcium absorption in the gut. A controlled feeding study showed that increasing protein intake from 10% to 20% of TEI increased fractional calcium absorption among those with low calcium intake.24 Greater fractional calcium absorption was associated with a lower risk of hip fracture in an older cohort of women.25 Calcium protein interactions have been observed in many studies. Of note, Dawson-Hughes and Harris26 found that higher protein intake was associated with attenuated bone loss, but only among those randomized to calcium and vitamin D supplementation. Other analyses have shown similar protein-calcium interactions, such as the study of Zhong and colleagues27 that showed protein intake was associated with lower risk of fracture, but only for postmenopausal women with total calcium intake of more than 1200 g/day. In the study of Dawson-Hughes and Harris,26 the addition of calcium and vitamin D (a randomized exposure) appears to modify the putative effect of protein intake. Assuming this to be true we would expect that dairy versus non-dairy sources of protein to have a stronger association with bone health outcomes such as BMD and fracture as observed in the present study.
Given the pattern of heterogeneity noted in the present study for both BMD and fracture outcomes, it is likely that the heterogeneity is due to several source-dependent nutrient differences. One such difference noted previously is the calcium and vitamin D content of dairy products. The second is the different underlying amino acid profiles of the three protein sources considered in this study. An experimental study in male rats by Gaffney-Stromberg and colleagues28 found systematic differences in markers of bone mineral metabolism specific to eight different experimental scenarios (normal versus high protein, milk protein versus soy protein, ad libitum versus energy restriction). Energy restriction had a consistent effect on gain in body mass with strong secondary effects on markers of bone mineral metabolism. Rats assigned to a high protein group had overall lower parathyroid hormone (PTH) and bone turnover and increased trabecular volumetric BMD versus those in a normal protein group. Finally, PTH was higher and bone turnover was lower in rats assigned to a milk protein group versus those assigned to a soy protein group. A longitudinal study in men and women demonstrated that both low protein intake and low animal-source protein intake were both associated with increased bone loss at the lumbar spine and femoral neck.29 More recent results from the CaMos study also confirmed a strong source-specific association between protein intake and BMD change whereby high intake of plant protein was associated with greater BMD loss, but high intake of protein from animal sources was not associated with greater BMD loss.9 Kerstetter and colleagues30 showed that supplemental whey protein was not associated with change in lumbar spine BMD, but was associated with preservation of fat-free mass in a randomized placebo-controlled trial. Protein intake has also been shown to be related to appendicular lean mass in other studies.31, 32 However, appendicular lean mass was not found to be an independent mediating variable in the present analysis, and therefore the results of this study are not attributable to this hypothesized mediator. Likewise, protein intake has been associated with physical performance measures in a cohort of older women,33 but again physical performance itself was not shown to be an independent mediating variable in the present analysis.
The strengths of this study are the inclusion of major potential confounders and a large sample of community-dwelling older men with a long follow-up and more than adequate number of fracture outcomes to detect small effects. We assessed protein intake by source, accounting for different nutrient profiles (ie, micronutrients and distribution of specific amino acids) of the three major sources (dairy, non-dairy animal, and plant). We further strengthened the analysis by considering intermediate variables that were biologically relevant. Finally, we performed several sensitivity analyses which all showed that the main results were robust to consideration of medications, non-dairy sources of calcium and vitamin D, competing mortality, and truncation of follow-up.
Study limitations include the use of a FFQ at a single point in time versus a life-course analysis to assess long-term dietary intake and its relationship to bone health. FFQs have limitations in the assessment of absolute and relative intake with likely bias toward the null. The study design was an observational cohort study, and therefore limitations include the possibility of selection bias and residual confounding. The generalizability of the present study is limited to healthy community-dwelling older men. The cohort was also mostly non-Hispanic white, and therefore we were unable to assess potential racial/ethnic differences, and this will also limit generalizability. Finally, we were unable in this analysis to assess whether the differential effects for dairy versus non-dairy sources of protein is attributable to the calcium (and vitamin D) or due to different amino acid profiles. Milk, yogurt, and hard cheese all have similar calcium/protein ratios (30 to 36 mg per gram protein) but have different profiles of other nutrients and hence intakes of these items cannot be used to find differential effects of calcium versus protein. Products with substantially different ratios include soft cheese, Greek yogurt, and calcium, and/or protein fortified dairy products. None of these items was specifically queried on the baseline questionnaire, which was a questionnaire designed to assess only the most commonly consumed food items.
In summary, we found that protein intake (particularly protein from animal sources) as a percentage of TEI is an independent and potentially modifiable risk factor for major osteoporotic fracture (with the exception of spine fracture). In particular, each 1 SD increase in protein intake was associated with an 8% decrease in major osteoporotic fracture risk and a 16% decrease in hip fracture risk. To put the association in context for potential intervention, 1 SD or 2.9%TEI corresponds to an absolute increase of 12 g protein for those with average energy intake in this cohort of older men, or equivalently, one to two protein-rich food servings per day. These types of dietary interventions could easily be incorporated into an older person's diet.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System.
Authors’ roles: Study design and conduct: LL, JMS, PMC, JAC, ESO, and KEE. Data collection: JMS, PMC, JAC, ESO, and KEE. Data analysis: LL and TNV. Data interpretation: LL, JMS, PMC, JAC, BCT, TNV, DCB, ESO, JTS, and KEE. Drafting and revising manuscript: LL, JMS, and KEE. Critical review and approval of manuscript: LL, JMS, PMC, JAC, BCT, TNV, DCB, ESO, JTS, and KEE. LL takes responsibility for the integrity of the data analysis.




