Long-term cadmium exposure and the association with bone mineral density and fractures in a population-based study among women
Abstract
All people are exposed to cadmium (Cd) via food; smokers are additionally exposed. High Cd exposure is associated with severe bone damage, but the public health impact in relation to osteoporosis and fractures at low environmental exposure remains to be clarified. Within the population-based Swedish Mammography Cohort, we assessed urinary Cd [U-Cd, µg/g of creatinine (cr)] as a marker of lifetime exposure and bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA) among 2688 women. Register-based information on fractures was retrieved from 1997 to 2009. Associations were evaluated by multivariable regression analyses. In linear regression, U-Cd was inversely associated with BMD at the total body (p < .001), femoral neck (p = .025), total hip (p = .004), lumbar spine (p = .088), and volumetric femoral neck (p = .013). In comparison with women with U-Cd < 0.50 µg/g of cr, those with U-Cd ≥ 0.75 µg/g of cr had odds ratios (ORs) of 2.45 [95% confidence interval (CI) 1.51–3.97] and 1.97 (95% CI 1.24–3.14) for osteoporosis at the femoral neck and lumbar spine, respectively. Among never-smokers, the corresponding ORs were 3.47 (95% CI 1.46–8.23) and 3.26 (95% CI 1.44–7.38). For any first fracture (n = 395), the OR was 1.16 (95% CI 0.89–1.50) comparing U-Cd ≥ 0.50 µg/g of cr with lower levels. Among never-smokers, the ORs (95% CIs) were 2.03 (1.33–3.09) for any first fracture, 2.06 (1.28–3.32) for first osteoporotic fracture, 2.18 (1.20–3.94) for first distal forearm fracture, and 1.89 (1.25–2.85) for multiple incident fractures. U-Cd at low environmental exposure from food in a general population of women showed modest but significant association with both BMD and fractures, especially in never-smokers, indicating a larger concern than previously known. © 2011 American Society for Bone and Mineral Research.
Introduction
The influence of environmental pollutants on human bone health has not received much consideration. Cadmium (Cd) is a widespread food contaminant, with cereals, vegetables, root vegetables, and potatoes being the major contributors (80%) to dietary Cd intake.1 Smokers are additionally exposed as a result of the high cadmium content of tobacco smoke.2 Cd is highly toxic and carcinogenic.2 The negative effect of Cd on bone became evident following the outbreak of Itai-Itai disease in Japan more than 50 years ago. This severe disease was characterized by renal and bone damage, with bone deformities and multiple fractures occurring as a consequence.2 Recently, Cd has been associated with low bone mass of the forearm3-6 and the hip7 at considerably lower exposure levels in some but far from all studies.8-11 Therefore, a possible role of Cd in the public health context of osteoporosis needs to be evaluated. One previous large study has addressed this issue and reported a modestly increasing risk of osteoporosis at the total hip with increasing urinary Cd (U-Cd) in a general population of women.7 No studies have assessed the association between Cd and bone mineral density (BMD) at total body or at the lumbar spine. Also, studies on fracture risk are sparse and limited to populations living in the vicinity of cadmium-emitting industries.4, 12 Furthermore, it is not known whether the association is independent of tobacco smoking.
The aim of this study was to determine the association between long-term Cd body burden, as assessed by U-Cd, and BMD in a large, well-characterized population-based cohort of women with no known occupational or significant environmental exposure to Cd. We specifically assessed the risk of osteoporosis at the femoral neck, total hip, and lumbar spine—sites particular susceptible to osteoporotic fractures with high public health importance. In addition, we assessed the risk of fractures both retrospectively and prospectively.
Methods
Study population
The Swedish Mammography Cohort (SMC) was established in 1987–1990, when all 90,303 women residing in two counties (Uppsala and Västmanland) in central Sweden and born between 1914 and 1948 received a mailed invitation to be screened by mammography. Enclosed with this invitation was a six-page questionnaire covering diet, body size, education, and parity, and 66,651 (74%) of the women completed the questionnaire. In the autumn of 1997, a second questionnaire, expanded to include approximately 350 questions concerning diet and other lifestyle factors, was sent to all 56,030 participants who were still living in the study area (response rate 70%).13
Starting in 2003, we invited all women in the cohort living in the town of Uppsala to undergo BMD measurements, to provide blood and urine samples, to have height and weight measurements taken, and to complete a detailed questionnaire on diet and lifestyle factors (participation rate of 65%). For this study, all women younger than 70 years of age (at the time of clinical examination) were selected from this group. This age cutoff was used to avoid exposure misclassification caused by the distortion of kidney Cd levels that may occur in old age.14 By the end of 2008, 2820 women younger than 70 years of age had undergone this examination. After excluding women with diabetes (n = 132) based on self-reports and on computerized linkage of the cohort to the National Patient Registry,15 2688 women were included in the study. Diabetics were excluded because they might distort our associations owing to their intricate general increased risk of fractures that is not explained by low BMD.16, 17
The study was approved by the Regional Ethical Review Board in Stockholm, Sweden, and written informed consent was obtained from each participant.
Biomarker assessment
Long-term Cd exposure was assessed using U-Cd because this reflects the cadmium accumulation in the kidney cortex, that is, the body burden. The concentration in the kidney cortex increases with age, and U-Cd thus is a more appropriate marker of lifelong exposure than the Cd concentration in blood, which mainly reflects current exposure.2, 18 To minimize the risk of Cd contamination of the urine, the women received detailed sampling instructions, and the first voided morning urine was collected in cups that had tested free from Cd contamination.
The urine samples were diluted with 10 parts 1% HNO3 (Merck, Darmstedt, Germany), and the Cd concentrations (111Cd) then were measured using inductively coupled plasma mass spectrometry (ICPMS; Agilent 7500ce, Agilent Technologies, Waldbronn, Germany) with the collision reaction cell in helium mode.19 For quality-control purposes, the commercial reference materials Seronorm Trace Elements for Urine (SERO A/S, Billingstad, Norway) and the Certified Standard Reference Material (National Institute of Standards and Technology [NIST], Gaithersburg, MD, USA) were analyzed. Mean Cd concentrations (±SD) in the Seronorm samples were 4.7 ± 0.19 µg/L (n = 130; recommended value 5.1 ± 0.22 µg/L) and 0.37 ± 0.030 µg/L (n = 21; recommended value 0.35 ± 0.08 µg/L), respectively. For low and high NIST samples, the mean Cd concentrations obtained were 0.070 ± 0.0092 µg/L (n = 18; recommended value 0.059 ± 0.0034 µg/L) and 5.2 ± 0.061 µg/L (n = 18; recommended value 4.7 ± 0.084 µg/L). The coefficients of variation (CVs) were 4.1% and 8.0%, respectively, for Seronorm samples and 13% and 1.2% for low and high NIST samples, respectively. The limit of detection (LOD), calculated as 3 × SD of the mean blank values, was 0.002 µg/L.
Calcium and magnesium also were measured in urine. The analytical measurements were performed the same way as for Cd, and the quality controls were satisfactory.
U-Cd, urinary calcium, and urinary magnesium were adjusted to creatinine (cr) excretion. To compensate for an effect of muscle mass on cr excretion, all multivariable-adjusted models were adjusted for lean body mass (dual-energy X-ray absorptiometry [DXA] measurements).
Assessment of covariates
From the questionnaires, we obtained information on the use of postmenopausal hormones and oral corticosteroids, on smoking habits, and on the types and amounts of alcoholic beverages consumed. The total daily physical activity [multiples of the metabolic equivalent (MET), kcal/kg × hour] was estimated by the reported duration of predefined activities with varying degrees of assigned intensity. The estimates of total physical activity had reasonable validity; deattenuated concordance correlations comparing the questionnaire with accelerometers were 0.38 and 0.64 compared with activity records.20 The use of certain predefined dietary supplements was reported to be regular (3 to 7 tablets/week), occasional (≤2 tablets/week), or no use.
Information on history of inflammatory joint diseases (tenth revision of the International Classification of Disease [ICD-10]: M05 to M14, also based on self-reported information), kidney diseases (ICD-10: N00 to N19), liver diseases (ICD-10: K70 to K77), and malabsorption (ICD-10: K90) was obtained by computerized linkage of the cohort to the National Hospital Discharge Registry.
Assessment of BMD and fractures
BMD and bone mineral content (BMC) at the total body (n = 2673), femoral neck, total hip (equivalent to proximal femur; n = 2648), and lumbar spine (vertebrae L2–L4; n = 2686), as well as total fat and total lean mass, were assessed by DXA (DPX Prodigy, Lunar Corp., Madison, WI, USA). When applicable, measurements from both extremities were used in the calculation (in 2% of the women, only one-sided measurements at the femoral neck and total hip were available). The precision error of the DXA BMD measurements, based on triple measurements in 15 of the participants, varied depending on sites between 0.8% and 1.5%. Total fat mass had a precision error of 1.5% and total lean mass 1.0%. Daily scans of a lumbar spine phantom were performed. The long-term precision CV for L2–L4 was less than 1% during the study period. Volumetric BMD (g/cm3) was estimated by assuming the femoral neck to be a cylinder. The average diameter at the femoral neck was obtained from Lunar software that uses a fixed length (k = 1.5 cm). Thus volumetric BMD (BMC per unit volume) ≅ [(BMD2/BMC) × (4k/π)].21 Osteoporosis was defined as a T-score of < –2.5, that is, 2.5 SD22 below the mean values of the reference population provided by the manufacturer.
Information on all fractures (ICD-10 codes S12, S22, S32, S42, S52, S62, S72, S82, and S92) was obtained from mid-September 1997 throughout March 2009 by computerized linkage of the cohort via the individual personal registration number provided to all Swedish citizens to the regional hospital diagnosis registries and to the National Patient Registry covering both outpatient and inpatient treated fractures. Fractures were categorized as any first fracture, first classic osteoporotic fracture (ie, hip, spine, distal forearm, proximal humerus, and pelvic fractures), and the most common first fracture (distal forearm). For multiple fractures (ie, multiple incident fracture occasions), a validated method with a prediction model was used to identify incident injuries admissions from resubmissions.23
Statistical methods and analyses
Associations between U-Cd and BMD were assessed using Spearman's rank correlation (rs, univariate assessments) and multiple linear regression analysis (BMD as a continuous dependent variable). We assessed the risk of osteoporosis (T-score < –2.5) and fractures using binary logistic regression and the risk of multiple fractures using ordinal logistic regression. Residual analyses indicated no major deviation from a linear pattern in the linear regression. We used analysis of covariance (ANCOVA) to assess the adjusted mean of BMD by three categories of U-Cd, and statistical differences were assessed using Fisher's least significant difference (LSD) test. The U-Cd categories: <0.50 (77%), 0.50 to 0.75 (17%), and ≥0.75 (6%) µg/g of cr were chosen to facilitate comparisons with previous studies3, 7 and because few subjects had U-Cd > 1 µg/g of cr (n = 46). In logistic regression, U-Cd was included in the models either as a continuous variable rescaled to a 0.42 µg/g of cr (equivalent to 2 SD increase), allowing the coefficients to be interpreted directly in the same way as binary inputs,24 or as categories. Odds ratios (ORs) based on tertiles of U-Cd in the study population (<0.28, 0.28 to 0.43, and ≥0.43 µg/g of cr) are given for comparison. Given the limited number of cases, we used only two exposure categories, <0.50 and ≥0.50 µg/g of cr, besides U-Cd in continuous form in the analysis of fracture risk. Binary logistic regression models were tested for a Hosmer and Lemeshow test (>0.05) and ordinal logistic regression for a Pearson goodness-of-fit and proportional odds assumption (>0.05).
To gain additional insights into potential nonlinearity of the association, we modeled the nonlinear trend in the risk of osteoporosis by a restricted cubic-spline logistic regression analysis with three “knots” by U-Cd percentiles [10 (=0.20 µg Cd/g of cr), 50 (=0.35 µg Cd/g of cr), and 90 (=0.65 µg Cd/g of cr)],25 including 99% of the women. The result is (with U-Cd 0.50 µg/g of cr as a reference)3, 7 presented as a smoothed plot with 95% confidence interval (95% CI) for the overall risk of osteoporosis.
Multivariable models were adjusted for age, education, height, total fat mass, lean body mass, parity, ever use of postmenopausal hormones, ever use of corticosteroids, total physical activity, smoking status, alcohol consumption, inflammatory joint diseases, kidney diseases, liver diseases, and malabsorption based on data obtained 2004–2008. We additionally adjusted for urinary calcium (mg/g of cr), urinary magnesium (mg/g of cr), and the use of dietary supplements, including vitamin D, calcium, magnesium, multivitamins, and minerals or combined use of these supplements. In analysis of fracture risk, all covariates with the exception of the U-Cd measurements were based on data from the 1997 questionnaire (start of fracture follow-up).
All tests were two-sided, and the statistical analyses were carried out using SPSS (PASW), Version 18.0 (SPSS, Inc., Chicago, IL, USA) and Stata 10.1 (Stata Corporation, Inc., Collage Station, TX, USA).
Results
The median concentration of U-Cd was 0.34 (5th to 95th percentile 0.15 to 0.79) µg/g of cr in all women and 0.29 (0.14 to 0.64) µg/g of cr in women who never smoked. In total, 8.2% of the women were classified as having osteoporosis at the femoral neck, 2.1% at the total hip, 9.9% at the lumbar spine, and 15% at the hip or spine. During 11.5 years, 395 first cases of any fracture, 248 cases of first osteoporotic fracture, and 137 cases of first fracture of the distal forearm occurred; 116 women had two or more fractures. The median concentration of U-Cd was 26% higher in women with osteoporosis at the femoral neck and 12% higher in those with osteoporosis at the lumbar spine than in those without osteoporosis. Data on major covariates in relation to categories of U-Cd are shown in Table 1. In general, BMD decreased and prevalence of osteoporosis increased with increasing Cd exposure. Accordingly, U-Cd was inversely associated with BMD at all sites and positively associated with urinary calcium and magnesium in the univariate analysis (Table 2).
| U-Cd, <0.50 µg/g of cr 0.30 (0.14–0.47)a n = 2067 | U-Cd, 0.50–0.75 µg/g of cr 0.59 (0.51–0.72) n = 449 | U-Cd, ≥0.75 µg/g of cr 0.87 (0.76–1.5) n = 172 | |
|---|---|---|---|
| Population characteristicsa | |||
| Age (years) | 63 (60–69) | 64 (60–69) | 63 (60–69) |
| Education >9 years (%) | 55 | 54 | 56 |
| Total fat mass (kg) | 26 (14–42) | 26 (14–42) | 25 (12–39) |
| Lean body mass (kg) | 39 (33–47) | 40 (33–47) | 38 (32–45) |
| Parity (no. of children) | 2 (0–4) | 2 (0–4) | 2 (0–4) |
| Ever use of postmenopausal hormones (%) | 75 | 70 | 77 |
| Ever use of oral corticosteroids (%) | 5.9 | 6.0 | 5.8 |
| Total physical activity (MET-hours/day) | 41 (35–48) | 41 (34–49) | 41 (33–49) |
| Smoking: never/ever (%) | 53/47 | 26/74 | 19/81 |
| Alcohol consumption (g ethanol/day) | 5.9 (0–21) | 5.0 (0–22) | 5.7 (0–20) |
| Biomarkers | |||
| Urinary calcium (mg/g of cr) | 146 (47–333) | 161 (53–354) | 157 (50–418) |
| Urinary magnesium (mg/g of cr) | 96 (48–166) | 98 (46–173) | 96 (42–193) |
| Bone mineral density | |||
| Total body (g/cm2) | 1.1 (0.97–1.3) | 1.1 (0.95–1.3) | 1.1 (0.92–1.2) |
| Femoral neck (g/cm2) | 0.89 (0.73–1.1) | 0.88 (0.69–1.1) | 0.85 (0.67–1.1) |
| Total hip (g/cm2) | 0.94 (0.75–1.2) | 0.92 (0.72–1.1) | 0.88 (0.68–1.1) |
| Lumbar spine (g/cm2) | 1.1 (0.86–1.5) | 1.1 (0.85–1.4) | 1.1 (0.77–1.5) |
| Volumetric femoral neck (g/cm3) | 0.35 (0.28–0.44) | 0.34 (0.27–0.44) | 0.33 (0.24–0.45) |
| Osteoporosis (T-score < –2.5) | |||
| Femoral neck (%) | 6.5 | 13 | 17 |
| Total hip (%) | 1.6 | 2.7 | 5.9 |
| Lumbar spine (%) | 9.1 | 11 | 17 |
| Hip or spine (%) | 13 | 19 | 24 |
- a Median (5th to 95th percentiles) or percent.
| Spearman rank correlation coefficient (p Value) | |
|---|---|
| Bone mineral density | |
| Total body | −0.088 (<.001) |
| Femoral neck | −0.057 (.003) |
| Total hip | −0.068 (<.001) |
| Lumbar spine | −0.052 (.007) |
| Volumetric femoral neck | −0.059 (.002) |
| Skull | −0.098 (<.001) |
| Arm | −0.068 (<.001) |
| Leg | −0.092 (<.001) |
| Urinary calcium (mg/g of cr) | 0.104 (<.001) |
| Urinary magnesium (mg/g cr) | 0.071 (<.001) |
To elucidate whether the observed associations between U-Cd and BMD were influenced by other factors, we performed multiple linear regression analyses. In the fully adjusted model, U-Cd as a continuous variable was inversely associated with BMD at the total body (p < .001), femoral neck (p = .025), total hip (p = .004), and volumetric femoral neck (p = .013) and close to statistically significantly associated with BMD at the lumbar spine (p = .088; Table 3). Adding urinary calcium, urinary magnesium, or the use of dietary supplements (ie, vitamin D, calcium, magnesium, or multivitamins and minerals or combined use of these supplements) into the statistical model did not affect the results, and therefore, these variables were not included in subsequent models.
| Bone mineral density | Regression coefficient (95% CI) | p Value | Adjusted R2 |
|---|---|---|---|
| Total body | |||
| U-Cda | −0.044 (−0.060; −0.028) | < .001 | 0.038 |
| U-Cdb | −0.027 (−0.042; −0.012) | < .001 | 0.23 |
| Femoral neck | |||
| U-Cda | −0.038 (−0.058; −0.017) | < .001 | 0.038 |
| U-Cdb | −0.023 (−0.044; −0.0029) | .025 | 0.17 |
| Total hip | |||
| U-Cda | −0.050 (−0.072; −0.028) | < .001 | 0.027 |
| U-Cdb | −0.031 (−0.052; −0.010) | .004 | 0.18 |
| Lumbar spine | |||
| U-Cda | −0.043 (−0.076; −0.010) | .011 | 0.005 |
| U-Cdb | −0.028 (−0.061; +0.0042) | .088 | 0.14 |
| Volumetric femoral neck | |||
| U-Cda | −0.015 (−0.024; −0.0057) | .001 | 0.048 |
| U-Cdb | −0.012 (−0.021; −0.0025) | .013 | 0.11 |
- a Age-adjusted estimate for U-Cd.
- b Multivariable-adjusted for age (years), education (≤9 and >9 years; yes/no), height (cm), total fat mass (kg), lean body mass (kg), parity (0–6), use of postmenopausal hormones (yes/no), ever use of corticosteroids (yes/no), total physical activity (MET-hours/day), smoking status (never/ever), alcohol intake (g ethanol/day), inflammatory joint diseases (yes/no), kidney diseases (yes/no), liver diseases (yes/no), malabsorption (yes/no).
The adjusted mean of BMD was lower in the highest U-Cd category (≥0.75 µg/g of cr) compared with the lowest (<0.50 µg/g of cr) at all sites (Fig. 1A). At the femoral neck, the magnitude of difference in BMD was similar to that observed for a 4.9-year increase in age or a 9.4-kg lower body weight. For total hip this difference was similar to that observed for a 7.3-year increase in age or a 9.3-kg lower body weight and for lumbar spine an 11-year increase in age or a 5.6-kg lower body weight, respectively. We also assessed the adjusted mean of BMD among never-smoking women only. The lowest BMD values were observed for women in the highest U-Cd category (Fig. 1B).
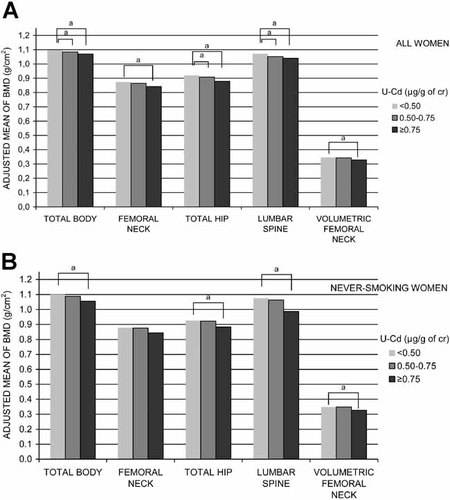
Adjusted mean of bone mineral density based on categorized U-Cd in all women (A) and in never-smoking women (B). Models were adjusted to the median age, height, total fat mass, lean body mass, alcohol intake, parity, total physical activity (MET-hours/day), >9 years education, never use of postmenopausal hormones, never use of corticosteroids, never-smoking status, and absence of the following diseases: inflammatory joint diseases, kidney diseases, liver diseases, and malabsorption. aDifferences (p < .05) compared with the lowest exposure group according to ANCOVA and Fisher's least significant difference (LSD) test.
To elucidate the risk of osteoporosis at the femoral neck, total hip, lumbar spine, and hip or spine in relation to Cd exposure, we performed binary logistic regression analyses with continuous as well as categorized U-Cd (Table 4). The risk was estimated per 0.42 µg/g of cr (equivalent to 2 SD) increment of U-Cd, and we observed a 40% to 60% statistically significantly increased risk at all sites. The highest exposure group (≥0.75 µg/g of cr) compared with the lowest (<0.50 µg/g of cr) was associated with two- to threefold increased risk of osteoporosis at all three sites. For femoral neck and hip or spine, the second highest U-Cd exposure group (0.50 to 0.75 µg/g of cr) also was associated with higher risk of osteoporosis. To enable comparison with previous studies, we also calculated the risk of osteoporosis per 1 µg/g of cr in U-Cd, and a two- to threefold statistically significantly increased risk was observed for the three sites (data not shown). Restricted cubic spline models showed no presence of a threshold in the risk of osteoporosis at the femoral neck (Fig. 2A) or lumbar spine (Fig. 2B).
| Odds ratioa (95% CI) | ||
|---|---|---|
| All women | Never-smokers | |
| Femoral neck | n = 216 | n = 101 |
| U-Cd per 0.42 µg/g of crb | 1.58 (1.22–2.05) | 1.95 (1.21–3.16) |
| U-Cd, categories | ||
| <0.50 | 1.00 (ref) | 1.00 (ref) |
| 0.50–0.75 | 2.17 (1.51–3.11) | 2.09 (1.12–3.93) |
| ≥ 0.75 | 2.45 (1.51–3.97) | 3.47 (1.46–8.23) |
| p Trendc | < .001 | .001 |
| Total hip | n = 55 | n = 22 |
| U-Cd per 0.42 µg/g of crb,d | 1.61 (1.09–2.38) | Numbers too small to perform analyses |
| U-Cdd, categories | ||
| <0.50 | 1.00 (ref) | |
| 0.50–0.75 | 1.49 (0.75–2.97) | |
| ≥0.75 | 3.01 (1.41–6.43) | |
| p Trendc | .005 | |
| Lumbar spine | n = 267 | n = 141 |
| U-Cd per 0.42 µg/g of crb | 1.41 (1.10–1.81) | 1.35 (0.86–2.12) |
| U-Cd, categories | ||
| <0.50 | 1.00 (ref) | 1.00 (ref) |
| 0.50–0.75 | 1.30 (0.91–1.86) | 1.17 (0.64–2.15) |
| ≥0.75 | 1.97 (1.24–3.14) | 3.26 (1.44–7.38) |
| p Trendc | .003 | .013 |
| Hip or spine | n = 400 | n = 201 |
| U-Cd per 0.42 µg/g of crb | 1.43 (1.15–1.78) | 1.55 (1.05–2.29) |
| U-Cd, categories | ||
| <0.50 | 1.00 (ref) | 1.00 (ref) |
| 0.50–0.75 | 1.61 (1.20–2.16) | 1.27 (0.75–2.14) |
| ≥0.75 | 1.95 (1.30–2.93) | 4.24 (1.99–9.04) |
| p Trendc | < .001 | .001 |
- a Multivariable-adjusted model (for covariates, see Table 3).
- b, b,d To convert OR per 2 SD (0.42 µg/g of cr) increment in U-Cd to per 1 µg/g of cr; e(ln OR)/0.42.
- c Linear trends across categories were tested using the median U-Cd within categories as a continuous variable.
- d Model only adjusted for significant covariates (age, height, total fat mass, lean body mass, and ever use of corticosteroids) because the Hosmer and Lemeshow test indicated instability when all covariates were included.
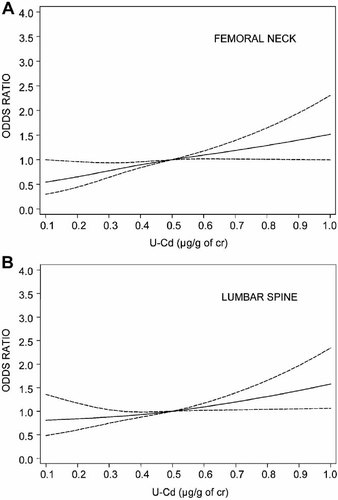
Multivariable-adjusted odds ratio and its 95% confidence interval at the femoral neck (A) or lumbar spine (B) evaluated using restricted cubic spline with three knots at 0.20, 0.35, and 0.65 µg/g of cr in 99% of the women.
We performed sensitivity analyses to test the robustness of our results. First, we excluded subjects with extreme creatinine concentrations (outside 0.3 and 3.0 g/L, n = 65); this had marginal effects on our estimates. In addition, we included the creatinine concentration as a covariate in the multivariable-adjusted models; this had no impact on the results. Second, we assessed all models using Cd adjusted to the mean urinary density instead of creatinine to minimize an effect of muscle mass on the adjustment of U-Cd; even this approach had only a marginal effect on the results. In addition, to test whether the cutoffs chosen for the U-Cd categories influenced the observed associations, we performed analyses with tertiles of U-Cd exposure. The highest tertile (≥0.43 µg/g of cr) compared with the lowest tertile (<0.28 µg/g of cr) had associated ORs of osteoporosis at the femoral neck of 2.02 (95% CI 1.39–2.94), at the lumbar spine of 1.53 (95% CI 1.08–2.13), and at the hip or spine of 1.65 (95% CI 1.24–2.20).
We also performed subgroup analyses among never-smokers in order to minimize any possible non-Cd mediated negative effects of tobacco smoking on bone. In the multivariable-adjusted model for never-smokers, the OR of osteoporosis at the femoral neck was 1.95 (95% CI 1.21–3.16) and at the hip or spine was 1.55 (95% CI 1.05–2.29; Table 4) for every 0.42 µg/g of cr increment in U-Cd. The highest exposure category of U-Cd (≥0.75 µg Cd/g of cr) was associated with a three- to fourfold statistically significant increased risk of osteoporosis at the femoral neck, lumbar spine, and hip or spine. Among ever-smokers, the multivariable-adjusted OR of osteoporosis at the femoral neck was 1.46 (95% CI 1.07–1.99) and at the hip or spine was 1.40 (95% CI 1.07–1.82) for every 0.42 µg/g of cr increment in U-Cd (data not shown).
We also evaluated urinary calcium excretion as a dependent variable in relation to U-Cd (Cd exposure is associated with proximal tubular damage).26 Multivariable-adjusted urinary calcium excretion was significantly positively associated with U-Cd (p < .001).
In the analysis of any first fracture, we observed a multivariable adjusted OR of 1.15 (95% CI 0.92–1.43) among all women and a borderline statistically significant higher OR of 1.48 (95% CI 1.00–2.17) per 0.42 µg/g of cr increment in U-Cd among never-smokers. For categorized U-Cd, the risk of fracture among all women also was nonsignificant, with an OR of 1.16 (95% CI 0.89–1.50), whereas a higher risk was observed among never-smokers, with an OR of 2.03 (95% CI 1.33–3.09), comparing U-Cd ≥ 0.50 µg/g of cr with lower levels (Table 5). Similar results were observed in the analysis of any first osteoporotic fracture and first fracture of the distal forearm, as well as in the analysis of multiple incident fractures (Table 5).
| Odds ratioa (95% CI) | ||
|---|---|---|
| All women | Never-smokers | |
| Any first fracture | n = 395 | n = 190 |
| U-Cd per 0.42 µg/g of crb | 1.15 (0.92–1.43) | 1.48 (1.00–2.17) |
| U-Cd, categories | ||
| <0.50 | 1.00 (ref) | 1.00 (ref) |
| ≥0.50 | 1.16 (0.89–1.50) | 2.03 (1.33–3.09) |
| Additional adjustment for total-body BMD | ||
| U-Cd per 0.42 µg/g of crb,c | 1.05 (0.83–1.31) | 1.31 (0.88–1.94) |
| U-Cd, categoriesc | ||
| <0.50 | 1.00 (ref) | 1.00 (ref) |
| ≥0.50 | 1.03 (0.79–1.34) | 1.76 (1.14–2.72) |
| First osteoporotic fractured | n = 248 | n = 129 |
| U-Cd per 0.42 µg/g of crb | 1.05 (0.79–1.38) | 1.64 (1.06–2.55) |
| U-Cd, categories | ||
| <0.50 | 1.00 (ref) | 1.00 (ref) |
| ≥0.50 | 1.15 (0.83–1.58) | 2.06 (1.28–3.32) |
| Additional adjustment for total-body BMD | ||
| U-Cd per 0.42 µg/g of crb,c | 0.92 (0.69–1.23) | 1.38 (0.88–2.17) |
| U-Cd, categoriesc | ||
| <0.50 | 1.00 (ref) | 1.00 (ref) |
| ≥0.50 | 0.98 (0.71–1.37) | 1.64 (0.99–2.71) |
| First distal forearm fracture | n = 137 | n = 74 |
| U-Cd per 0.42 µg/g of crb | 1.21 (0.86–1.72) | 2.14 (1.28–3.60) |
| U-Cd, categories | ||
| <0.50 | 1.00 (ref) | 1.00 (ref) |
| ≥0.50 | 1.23 (0.81–1.86) | 2.18 (1.20–3.94) |
| Additional adjustment for total-body BMD | ||
| U-Cd per 0.42 µg/g of crb,c | 1.04 (0.72–1.50) | 1.75 (1.03–2.97) |
| U-Cd, categoriesc | ||
| <0.50 | 1.00 (ref) | 1.00 (ref) |
| ≥0.50 | 1.02 (0.67–1.57) | 1.68 (0.91–3.13) |
| Multiple fracturese | n = 279f/n = 116g | n = 132f/n = 58g |
| U-Cd per 0.42 µg/g of crb | 1.16 (0.94–1.44) | 1.40 (0.96–2.05) |
| U-Cd, categories | ||
| <0.50 | 1.00 (ref) | 1.00 (ref) |
| ≥0.50 | 1.17 (0.90–1.51) | 1.89 (1.25–2.85) |
- a Multivariable-adjusted for age (years), education (≤9 and >9 years; yes/no), body mass index (< or ≥20 kg/m2), parity (0–6), use of postmenopausal hormones (yes/no), ever use of corticosteroids (yes/no), total physical activity (MET-hours/day), smoking status (never/ever), alcohol intake (g ethanol/day), inflammatory joint diseases (yes/no), kidney diseases (yes/no), liver diseases (yes/no), malabsorption (yes/no).
- b, b,c To convert OR per 2 SD (0.42 µg/g of cr) increment in U-Cd to per 1 µg/g of cr, e(ln OR)/0.42.
- c Additionally adjusted for total-body BMD (g/cm2).
- d Includes fractures of the hip, spine, distal forearm, proximal humerus, and pelvis.
- e Only adjusted for the major covariates: age (median split), use of postmenopausal hormones (yes/no), body mass index (< or ≥20 kg/m2), and smoking status (never/ever).
- f Individuals with one fracture.
- g Individuals with ≥2 fractures.
We finally explored whether the Cd-associated risk of fractures was mediated via lowered bone mass. Inclusion of total-body BMD in the fracture models, however, only partly remove the higher risks (Table 5).
Discussion
In this population-based study among 56- to 69-year-old women with low environmental Cd exposure, we observed associations between U-Cd, a marker of long-term Cd exposure, and lower BMD of the total body as well as at sites particularly susceptible to osteoporotic fractures. Specifically, we observed a 40% to 60% increased risk of osteoporosis per 0.42 µg/g of cr (equivalent to 2 SD) increment of U-Cd at the femoral neck, total hip, and lumbar spine with no observed threshold effect. The observed associations were independent of tobacco smoking, which is both an important source of Cd and a risk factor for osteoporosis. The results on risk of fractures support the conclusion of the BMD results. Thus our observations suggest that Cd exposure from diet alone (mainly from cereals, vegetables, and potatoes) may contribute to the risk of osteoporosis and fractures.
Our study has several strengths, such as a large age-homogeneous study group from a well-characterized population-based female cohort, with no known occupational or significant environmental exposure to Cd and with extensive data on all relevant factors important for bone health. We assessed BMD by DXA (reference standard for BMD measurements)27 at sites not previously studied in relation to Cd, as well as the risk of various types of fractures, including multiple incident fractures. U-Cd was assessed with high analytical precision giving low uncertainty even in our low-dose range. In addition, we were able to control for calcium excretion, reducing the possibility that our results were due to confounding by a parallel increased excretion of calcium. The sensitivity analyses performed indicated that our results are robust.
The main limitation of our study is the cross-sectional design, where U-Cd and BMD were measured at the same time, hampering inference with respect to causality. On the other hand, U-Cd is a marker of long-term Cd accumulation in the body. In contrast, the assessment of fracture risk was based on fractures that had occurred both before and after the collection of biologic samples, and the fracture analysis was further limited by few cases. Nevertheless, we find it unlikely that the U-Cd exposure level is affected by fracture status. Spondylosis at the lumbar spine (common in the elderly) may result in falsely elevated bone density and, as a consequence, lead to dilution of the observed associations. Another potential limitation may be related to the use of the predefined U-Cd categories,3, 7 which resulted in only 17% and 6% of the women falling within the second highest and highest exposure groups, respectively. However, the results for BMD and risk of osteoporosis were found to be consistent when exposure tertiles were used in the analysis. The dose-dependent and linear increased risk detected in the analysis also supports the robustness and consistency of the findings.
In general, our results on the associations between U-Cd and BMD are in agreement with previous studies restricted to forearm BMD in populations with a higher exposure.3-6 It should be noted, however, that our results indicate an increased risk of osteoporosis at considerably lower Cd exposures. In addition, the results for BMD at the lumbar spine may indicate a greater public health concern than recognized previously. The National Health and Nutrition Examination Survey (NHANES) study, based on women 50 to 90 years of age, observed an OR of 1.15 (95% CI 1.00–1.33) per 1 µg/g of cr increment in U-Cd for the hip.7 The corresponding result observed in this study was 3.11 (95% CI 1.23–7.88). Based on categorized exposure, we found a statistically significant dose-dependent increased risk of osteoporosis across U-Cd groups, whereas the NHANES study7 found a statistically significant but not dose-dependent increased risk of osteoporosis at the total hip: OR = 1.43 (95% CI 1.02–2.00) for U-Cd 0.50 to 1.0 µg/g of cr and OR = 1.40 (95% CI 0.97–2.03) for U-Cd > 1.0 µg/g of cr compared with U-Cd ≤ 0.50 µg/g of cr. Moreover, we also observed, in contrast to the NHANES data, a statistically significant dose-dependent increased risk among never-smokers. The reason for the observed differences between our study and the NHANES study is not known; it can only be speculated that by restricting our study to women younger than 70 years of age, we might have reduced the possibility that exposure misclassification owing to old age attenuated our associations.
The associations between U-Cd and fracture risk observed in this study are generally in agreement with the two previous studies, although they were conducted in areas with higher environmental Cd exposure.4, 12 Alfvén and colleagues12 found a relative risk of 1.18 (95% CI 1.01–1.37) for forearm fractures per 1 µg/g of cr increment in U-Cd in men and women. In the study by Staessen and colleagues,4 the relative risks associated with doubled urinary cadmium were 1.73 (95% CI 1.16–2.57, p = .007) for fractures in women and 1.60 (95% CI 0.94–2.72, p = .08) for height loss in men. No separate results were presented for never-smokers in the two studies.
The mechanisms for Cd-induced bone effects are not fully understood. Experimental data demonstrate a direct effect of Cd on bone with decreased bone formation and increased bone resorption at Cd concentrations relevant to human exposures (see review by Bhattacharyya28). Cd-exposed osteoblast-like cells decreased their bone-forming activity and secreted prostaglandin E2 (PGE2), which, in turn, can increase the formation and activity of osteoclasts. In a study of bone marrow cells, Cd both accelerated the differentiation and enhanced the activity of new osteoclasts. Cd-induced demineralization of the bone begins almost directly after exposure, for example, after 24 hours in mice, supporting the idea that the effect is independent of kidney damage. Female animals seem more vulnerable to Cd-induced bone effects. Human data support Cd-induced increased bone resorption rather than an effect on bone formation.6, 29, 30
Our results on fracture risk suggest, however, that Cd's effect is only partly mediated via lowered BMD. It can be speculated that Cd exposure also results in specific effects on cortical and trabecular bone or in alterations of biomechanical properties that are not detected by DXA; this is also supported by experimental studies.28, 31, 32 Other possible mechanisms underlying the effects of Cd on bone in humans include secondary effects owing to Cd-induced kidney damage. Decreased circulating levels of 1,25-dihydroxyvitamin D3, however, seem unlikely to be the proposed link between Cd-induced effects on kidney and bone.33 Furthermore, the positive association between U-Cd and urinary calcium observed previously26, 29, 30 and in this study has been considered a result of Cd-induced tubular damage causing hypercalciuria but may well be a result of Cd-induced increased bone resorption.29, 30 U-Cd also has been shown to be associated with both increased bone resorption and decreased parathyroid hormone concentrations,6, 26 suggesting that the effect on bone was not mediated primarily by Cd-induced tubular damage.
In conclusion, this study indicates that the long-term low-level exposure to Cd from food, mainly cereals, vegetables, and potatoes, is associated with a higher risk of osteoporosis and fractures. Efforts should be taken to reduce dietary Cd exposure.
Disclosures
This article reflects only the authors' views. The European Community is not liable for any use that may be made of the information contained therein. All the authors state that they have no conflicts of interest.
Acknowledgements
We thank Margaretha Grandér and Brita Palm at the Karolinska Institutet and Carina Fredriksson and Siv Tengblad at Uppsala University for skillful technical assistance.
Financial support was obtained from the Swedish Research Council/Medicine and Longitudinal Studies and from the European Union through its Sixth Framework Program for RTD (Contract No. FOOD-CT-2006-016253).




