Increased Heterogeneity of Bone Matrix Mineralization in Pediatric Patients Prone to Fractures: A Biopsy Study
ABSTRACT
Idiopathic osteoporosis (IOP) in children is characterized by fragility fractures and/or low bone mineral density in otherwise healthy individuals. The aim of the present work was to measure bone mineralization density distribution (BMDD) based on quantitative backscattered electron imaging (qBEI) in children with suspected IOP. Entire cross-sectional areas of transiliac bone biopsy samples from children (n = 24, 17 boys; aged 6.7–16.6 years) with a history of fractures (n = 14 with at least one vertebral fracture) were analyzed for cancellous (Cn) and cortical (Ct) BMDD. Outcomes were compared with normal reference BMDD data and correlated with the patients' clinical characteristics and bone histomorphometry findings. The subjects had similar average degree but significantly higher heterogeneity of mineralization in both Cn and Ct bone (Cn.CaWidth +23%, Ct.CaWidth +15%, p < 0.001 and p = 0.002, respectively), together with higher percentages of low mineralized cancellous (Cn.CaLow +35%, p < 0.001) and highly mineralized cortical bone areas (Ct.CaHigh +82%, p = 0.032). Ct.CaWidth and Ct.CaLow were positively correlated with mineralizing surface per bone surface (MS/BS; a primary histomorphometric determinant of bone formation) and with serum bone turnover markers (all p < 0.05). The correlations of the mineralization heterogeneity with histomorphometric and serum bone turnover indices suggest that an enhanced variation in bone turnover/formation contributes to the increased heterogeneity of mineralization. However, it remains unclear whether the latter is cause for, or the response to the increased bone fragility in these children with suspected IOP. © 2014 American Society for Bone and Mineral Research.
Introduction
The diagnosis of idiopathic osteoporosis (IOP) in children is based on the occurrence of fragility fractures and/or low bone mineral density (BMD) in otherwise healthy individuals with no evidence for secondary causes of osteoporosis. Mäyränpää and colleagues1 have previously shown using bone histomorphometry that apparently healthy, fracture-prone children with suspected IOP have a great variation in bone volume and turnover. Bone mass, biochemical values, and bone turnover markers provided little help in evaluating susceptibility to fractures in this patient cohort.1
In addition to bone mass, the quality of the bone material, including collagen and mineral properties as well as the degree and heterogeneity of mineralization, plays an important role in defining the mechanical quality of the entire organ. In particular, the mineral content is an important determinant of bone stiffness.2 Bone material is highly heterogeneous; it is composed of bone packets (“bone structural units”) that have different mineral content depending on their tissue ages. This characteristic of mineralization can be quantified by the bone mineralization density distribution (BMDD), which is a measure of the degree and the heterogeneity of calcium concentrations. In healthy cancellous bone of the adult skeleton, the BMDD was shown to have only minor variation with biological factors, such as age, gender, ethnic origin, or skeletal site.3 In children, the BMDD of transiliac bone showed somewhat higher variation but no age-dependency from 1.5 to 23 years.4 Based on this, a reference BMDD for children could be established.
In our previous study using Fourier transform infrared spectroscopic imaging (FTIRI), we investigated a potential difference in material properties between children with suspected IOP having sustained vertebral fractures to those with nonvertebral fractures. A higher collagen maturity and a lower carbonate-to-phosphate ratio were found in the vertebral fracture group.5 However, it remained unknown how the bone material properties in these patients compared to those in healthy children. In the present study, we wanted to further evaluate the bone material properties in children with fractures. The aim was to measure the BMDD in 24 pediatric patients with suspected IOP and to compare the outcomes with the normative BMDD data for children.4 The BMDD findings were correlated with fracture history, biochemical data, BMD by dual-energy X-ray absorptiometry (DXA) and with bone histomorphometry.
Patients and Methods
Patients
Transiliac bone biopsy samples were obtained from children (n = 24) with suspected IOP. Primary osteoporosis was suspected based on history of increased fragility, ie, several nonvertebral fractures and/or low-energy vertebral fracture(s), and the exclusion of secondary causes of osteoporosis.1 Patients had sustained altogether 67 nonvertebral fractures, including forearm (29), upper arm (9), hand (9), crus (8), clavicle (7), foot (3), upper leg (1), and pelvic (1) fractures. In some but not all patients, bone-age adjusted lumbar spine bone mineral density (BMD) Z-scores were low (≤–2.0). Vertebral compression fractures were observed in 14 (58%) patients in plain spinal radiographs.1, 5 Data relating to patients' history of fractures, and diagnosis or treatment of other illnesses were collected from hospital records and by parental interview. The patients were clinically assessed for overall health, growth, and skeletal phenotype including possible deformities, disproportion, joint laxity, and features of OI; no genetic testing was performed to exclude mild OI. The pubertal stage was clinically assessed by a pediatric endocrinologist (OM) according to Tanner.6 Body mass index (BMI) Z-scores were calculated using age-and-sex-specific reference data provided by the WHO (http://www.who.int/en/).
Bone densitometry and biochemistry
Areal BMD for the lumbar spine (L1–L4), proximal femur, and whole body was measured by DXA (Hologic Discovery A; Bedford, USA, pediatric software version 12.4) within 6 months of the biopsy. Values were transformed into BMD Z-scores by using age-and-sex-specific reference data for the equipment.1 Biochemical data were collected for all children including plasma parathyroid hormone (P-PTH), plasma alkaline phosphatase (P-ALP), serum 25-hydroxyvitamin D (S-25-OHD), the serum formation marker N-terminal propeptide of type I procollagen (P1NP), and the bone resorption marker C-terminal telopeptide of type I collagen (1CTP). Serum concentrations of follicle stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (in girls over 8 years) and testosterone (in boys over 10 years) were measured by standard assays. Urine was analyzed for calcium (U-Ca), creatinine (U-Crea), and U-Ca/U-Crea ratio. All were determined as described.1
Bone biopsies
Transiliac bone biopsy and tetracycline labeling procedure has been described.1 The iliac crest biopsies were usually taken within 12 months of the most recent fracture (n = 19 patients); in a few cases 12 to 18 months had passed since the fracture (n = 5). The samples were fixed in 70% ethanol for at least 48 hours before embedding in polymethylmethacrylate (PMMA). The use of the samples was approved by the local healthcare authorities and ethics committee (Research Ethics Board, Helsinki University Hospital, Dnro 442/E7/2004).
Bone histomorphometry
Analysis of bone histomorphometry has been described.1 In addition to the published cancellous histomorphometric parameters, we analyzed the cortical width (Ct.Wi) based on backscattered electron images acquired for BMDD analysis (as described below). In these images, the two cortices were individually isolated from the trabecular compartment of the biopsy by following a line where the compact bone characteristics changed to cancellous bone. Ct.Wi values were compared to published age-matched normative data.7
Quantitative backscattered electron imaging
The quantitative backscattered electron imaging (qBEI) technique is well-established and the details of the method have been published.8 Sample blocks were trimmed using a low-speed diamond saw (Isomet-R; Buehler Ltd., Lake Buff, IL, USA). Sectioned bone surfaces were sequentially ground with sandpaper with increasing grit number followed by polishing with diamond grains (size down to 1.0 µm) on hard polishing cloths by a precision polishing device (PM5 Logitech, Glasgow, Scotland). Finally, the sample surface was carbon coated by vacuum evaporation (SCD 004 Balzers, Lichtenstein). A digital electron microscope (DSM 962; Zeiss, Oberkochen, Germany) equipped with a four-quadrant semiconductor backscattered electron BE detector was used for imaging of the entire cross-sectional biopsy areas. The BE-signal (grayscale) was calibrated using the “atomic number contrast” between carbon (C, Z = 6) and aluminum (Al, Z = 13) as reference materials. Carbon was set to gray level index 25 and aluminum to 225. This allows a scaling also into weight % (wt%) Ca, whereby osteoid (Z ≅ 6) has 0 wt% Ca and pure hydroxyapatite (Z = 14.06) has 39.86 wt% Ca according to its composition. Thus, one gray level step corresponds to 0.17 wt% Ca as a consequence of this calibration protocol.
- CaMean, the weighted average Ca concentration of the mineralized tissue area, obtained from the integrated area under the BMDD curve (Cn.CaMean = cancellous CaMean, Ct.CaMean = cortical CaMean).
- CaPeak, the peak position of the BMDD histogram showing the most frequently occurring (mode) wt% Ca (Cn.CaPeak = cancellous CaPeak, Ct.CaPeak = cortical CaPeak).
- CaWidth, the width at half-maximum of the BMDD histogram curve indicating the heterogeneity of mineralization (Cn.CaWidth = cancellous CaWidth, Ct.CaWidth = cortical CaWidth).
- CaLow, the fraction of low mineralized tissue areas having a mineral content below 17.68 wt% Ca, corresponding to young, not fully mineralized bone areas (Cn.CaLow = cancellous CaLow, Ct.CaLow = cortical CaLow).
- CaHigh, the fraction of highly mineralized bone tissue having a calcium content above 25.30 wt% Ca (corresponding mainly to interstitial bone) (Cn.CaHigh = cancellous CaHigh, Ct.CaHigh = cortical CaHigh).
Statistical analysis
BMDD values in healthy children were used for comparisons with our study cohort4 using Mann-Whitney U test. Comparison of Cn versus Ct BMDD variables is based on paired Wilcoxon signed rank tests. Further, Spearman's correlation coefficients were calculated for Cn versus Ct BMDD variables and for Cn and Ct BMDD variables versus clinical characteristics and other bone parameters. The nonparametric Kruskal Wallis test was used to test differences between multiple subgroups and Mann-Whitney U test was used to test differences between two study groups. Bonferroni correction was used when testing differences between the groups based on Tanner stage. Statistical analyses were performed with the SPSS Statistics software (version 19.0.0; SPSS Inc., Chicago, IL, USA). The limit of statistical significance in all statistical tests was set to p < 0.05.
Results
Patient characteristics
Clinical characteristics are shown in Table 1. The cohort included 24 fracture-prone children with suspected IOP (17 males; 71%). Their age varied from 6.7 to 16.6 years (median age 12.0 years). Pubertal maturation, as assessed by Tanner stage and hormonal variables, was appropriate for age in all subjects. Normal stature (height Z-score between –2.0 and +2.0) was found in 23 children (96%); only 1 boy had a height Z-score of +2.1. BMI Z-score was normal in 18 children (75%). Two children were underweight (BMI Z-score < –2.0) and 4 children were obese (BMI Z-score > +2.0).
| Parameter | Patients mean ± SD (n = 24) |
|---|---|
| Age (years) | 12.0 ± 2.6 |
| Range (years) | 6.7–16.6 |
| Male/female, n | 17/7 |
| Fracture history | |
| At least 1 vertebral fracture | 14 |
| At least 1 nonvertebral fracturea | 22 |
| Height Z-score | 0.2 ± 1.2 |
| BMI Z-score | 0.4 ± 1.4 |
| Lumbar spine BMD Z-score | –1.2 ± 1.1 |
| 25-Hydroxyvitamin D (nmol/L)b | 47 ± 19 (decreased in n = 14) |
| Parathyroid hormone (ng/L)c | 44 ± 22 (increased in n = 2) |
| Alkaline phosphatase (U/L)d | 274 ± 80 (increased in n = 2) |
| P1NP (µg/L)e | 594 ± 221 (increased in n = 2) |
| 1CTP (µg/L)e | 16.5 ± 5.5 (increased in n = 6) |
- BMI = body mass index; BMD = bone mineral density; P1NP = N-terminal propeptide of type I procollagen; 1CTP = C-terminal telopeptide of type I collagen.
- a A total of 67 fractures (median 2.5; range, 0–7 per patient).
- b Normal >50 nmol/L.
- c Normal range, 8–73 ng/L.
- d Reference range is age-and-sex-dependent.
- e Reference range is age-dependent.
BMDD outcomes in cancellous and cortical transiliac bone
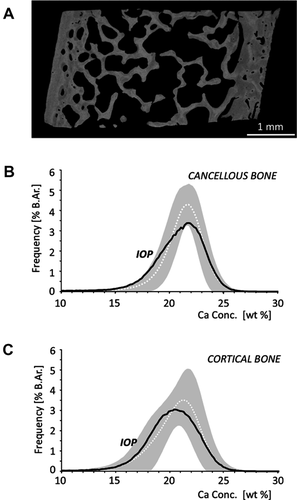
A typical qBEI image and the corresponding Cn and Ct BMDD curves are shown in Fig. 1. Comparison of BMDD variables in the entire cohort of fracture-prone children with previously published normative values for healthy children revealed no significant differences in Cn or Ct CaMean (average calcium concentration) and CaPeak (mode calcium concentration) (Table 2). However, a significantly higher heterogeneity of mineralization in Cn and Ct bone (Cn.CaWidth +23%, Ct.CaWidth +15%, p < 0.001 and p = 0.002, respectively) was observed in the fracture-prone children (Table 2). The fracture-prone children also had increased percentages of low mineralized Cn bone area (Cn.CaLow +35%, p < 0.001) and of highly mineralized Ct bone areas (Ct.CaHigh +82%, p = 0.032) compared to normal. Ct.CaLow and Cn.CaHigh were not different from normal.
| BMDD variable | Cancellous bone | Cortical bone | ||||
|---|---|---|---|---|---|---|
| Suspected IOP (n = 24) | Reference valuea (n = 54) | pb | Suspected IOP (n = 24) | Reference valuea (n = 53) | pb | |
| CaMean (wt% Ca) | 20.80 (0.67) | 20.95 (0.57) | NS | 20.41c (0.86) | 20.45 [19.68; 21.04] | NS |
| CaPeak (wt% Ca) | 21.76 (0.68) | 21.66 (0.52) | NS | 21.22c (1.07) | 21.14 [20.62; 21.75] | NS |
| CaWidth (Δwt% Ca) | 4.27 (0.66) | 3.47 [3.12; 3.64] | <0.001 | 4.48 (0.59) | 3.81 [3.38; 4.38] | 0.002 |
| CaLow (%) | 9.38 (3.36) | 6.14 [4.90; 7.99] | <0.001 | 12.02c (5.52) | 9.06 [6.22; 15.00] | NS |
| CaHigh (%) | 1.29 [0.48; 2.16] | 0.89 [0.43; 1.47] | NS | 0.84 [0.39; 1.78] | 0.46 [0.28; 1.22] | 0.032 |
- Data show mean (SD) or median [25th; 75th percentiles].
- BMDD = bone mineralization density distribution; IOP = idiopathic osteoporosis; NS = not significant; CaMean = weighted average Ca concentration of the mineralized tissue area, obtained from the integrated area under the BMDD curve; CaPeak = peak position of the BMDD histogram showing the most frequently occurring (mode) wt% Ca; CaWidth = width at half-maximum of the BMDD histogram curve indicating the heterogeneity of mineralization; CaLow = fraction of low mineralized tissue areas having a mineral content below 17.68 wt% Ca, corresponding to young, not fully mineralized bone areas; CaHigh = fraction of highly mineralized bone tissue having a calcium content above 25.30 wt% Ca (corresponding mainly to interstitial bone).
- a Reference values from Fratzl-Zelman and colleagues (4).
- b Comparison to pediatric reference data are based on Mann-Whitney test.
- c p < 0.05 versus cancellous bone (paired comparison using Wilcoxon signed rank test).
The comparison between Cn and Ct BMDD in the fracture-prone children revealed that CaMean and CaPeak of cortical bone were lower than those of cancellous bone (p = 0.010 and p = 0.011, respectively), whereas CaLow was higher in the cortex (p = 0.01). CaWidth and CaHigh were not different for the two bone compartments. All Cn BMDD variables were well correlated with the corresponding Ct BMDD variables (Spearman rank order coefficients R from 0.52 to 0.73, p values from 0.009 to <0.001).
Correlations of BMDD outcomes with clinical characteristics
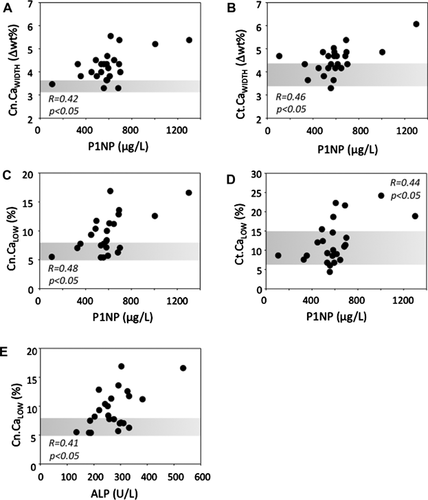
None of the BMDD variables correlated with age, height, weight, BMI, BMI Z-score, BMD Z-score at femoral neck or lumbar spine, S-25-OHD, P-PTH, or 1CTP. There was no statistical difference in BMDD between male and female patients or between children with vertebral and with nonvertebral fractures only (data not shown). When grouping the samples based on Tanner stage, children in early puberty (Tanner stage 2–3, n = 9) had lower Ct.CaMean (p = 0.027) and higher Ct.CaLow (p = 0.013) than children in late puberty (Tanner stage 4–5, n = 4). These subgroups did not differ in BMDD from the children with Tanner stage 1 (n = 11). Cn.CaWidth and Ct.CaWidth (R = 0.42 and 0.46, p < 0.05) as well as Cn.CaLow and Ct.CaLow (R = 0.48 and 0.44, p < 0.05) were all positively correlated with P1NP (Fig. 2). P-ALP correlated positively with Cn.CaLow (R = 0.41, p = 0.045).
Correlations between BMDD and histomorphometric indices of bone turnover
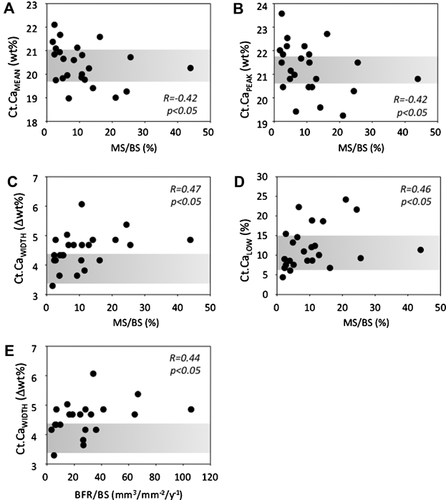
Children with normal cancellous bone volume (BV/TV > –1.0 SD) versus those with lower cancellous bone volume (BV/TV < –1.0 SD), based on bone histomorphometry, showed no significant differences in BMDD parameters (data not shown). Ct BMDD variables but not Cn BMDD variables were correlated with mineralizing surface (MS/BS). Weak, negative correlations were found for Ct.CaMean and Ct.CaPeak with MS/BS (both R = –0.42, p < 0.05). Ct.CaWidth (R = 0.47, p < 0.05) and Ct.CaLow (R = 0.45, p < 0.05) correlated positively with MS/BS (Fig. 3). Bone formation rate (BFR/BS) (n = 21 because 3 samples were lacking fluorescence double labels) correlated positively with Ct.CaWidth (R = 0.44, p = 0.046).
Correlations of cortical width and BMD by DXA
Average cortical width (Ct.Wi) was 948 ± 328 μm. This was in accordance with normal cortical development as observed by comparing with age-matched normative data. Most Ct.Wi Z-scores were within their normal age range, with few exceptions (n = 4) having Ct.Wi Z-scores < –1.0 (the lowest value was –1.8, which was the only Z-score < –1.5 in our cohort) and 1 child who had an increased Ct.Wi Z-score of +3.1. Ct.Wi Z-score correlated positively with whole-body BMD Z-score (R = 0.58, p = 0.003).
Discussion
In this study, we evaluated BMDD in fracture-prone children with suspected IOP in comparison with healthy controls and correlated the findings with clinical and histomorphometric outcomes. The BMDD findings for the patients revealed a higher intraindividual variety (heterogeneity) of mineralization in cancellous and cortical bone compartments than in healthy children.4
The patients comprised a heterogeneous group of children who all had sustained low-energy fracture(s) and more than half of the patients had prevalent vertebral compressions in radiographs. Growth was normal. Earlier histomorphometric analysis showed great variability in bone volume and bone turnover; however, both were on average normal.1 Cortical width was normal in most and somewhat decreased (Z-score < –1) in 4 of the patients and not different between patients with and without vertebral fractures. Interestingly, we found a positive correlation of the cortical width Z-score with whole-body BMD Z-score, indicating a relationship between structural properties at the biopsy site with the BMD of the entire skeleton. Three biopsy samples were lacking double labels and mineral apposition rate was decreased in many. Together with the biochemical markers of bone turnover (normal in the vast majority of patients), these results suggest that our study cohort has on average normal to low-normal bone turnover. Generally, fracture healing is known to increase bone turnover systemically for up to 12 months after a fracture in adult patients.9 Because all our patients were biopsied within 18 months (most within 1 year) after fracture, we might have expected some influence on the serum markers. However, only 2 children had higher serum bone formation markers and 6 had higher bone resorption markers than normal.1 This might suggest that the bone turnover in the children before they had sustained the fracture was on average even lower.
In principle, the BMDD (obtained from the entire cross-sectional area of the transiliac bone biopsy samples) is determined by the bone turnover rate and by the time course of mineral accumulation within the bone matrix (mineralization kinetics). An increase in bone turnover rate will cause elevated amounts of new bone packets and a reduction of the time period of secondary mineralization in numerous bone packets, thus leading to decreased average tissue age and a shift to the left and broadening of the BMDD.8, 10, 11
In accordance with the normal to low-normal bone turnover in the present cohort, we found no significant changes in the average (CaMean) or mode (CaPeak) calcium concentrations in cancellous or cortical bone. However, the most striking and also surprising outcome was the significant increase in CaWidth of the BMDD, indicating a higher heterogeneity in matrix mineralization. Compatible with the latter are the higher fractions of low mineralized cancellous (CaLow) and highly mineralized cortical bone (CaHigh). The increase in CaWidth without a concomitant decrease in CaMean or CaPeak is pointing toward transient changes in either mineralization kinetics and/or bone turnover rates.
Such transient changes in the mineralization kinetics and concomitant changes in bone turnover rates could, for instance, be caused by larger variations in vitamin D levels. Indeed, those of our patients with decreased vitamin D levels had relatively higher (but within normal range) bone turnover.1 However, low vitamin D levels are a common finding in the young, healthy Finnish population,12 and the findings in children with repeated fractures were not different.1 In this study, no correlation was seen between BMDD parameters and vitamin D levels. Therefore, variations in vitamin D levels as a cause for increased bone mineralization heterogeneity per se cannot be ruled out but is rather unlikely.
A more likely scenario would be a general systemic transient increase in bone turnover due to fractures, which might also increase the mineralization heterogeneity in patients with lower turnover. This has been demonstrated after 1 year teriparatide treatment in postmenopausal women who had previously been treated with bisphosphonates13 and confirmed by computed modeling of the BMDD.11 In line with the assumption that changes in bone turnover rates increase the heterogeneity of mineralization in our cohort, we found weak positive correlations of Ct.CaWidth with MS/BS and of both Cn.CaWidth and Ct.CaWidth with P1NP.
Similar to the findings in healthy controls, there was no age-dependency in the BMDD variables in our patients.4 Grouping by Tanner stage revealed some dependency of cortical CaMean and CaLow on pubertal stage and might reflect growth spurt during puberty. However, there is no information on the dependency of the BMDD variables on Tanner stage in healthy children. Further, cortical bone matrix mineralization was lower than that of cancellous bone, which was also observed for healthy children.4 This fact mirrors the lateral growth/modeling drift of the iliac crest and is another indication for normal growth in these fracture-prone children. Moreover, Cn and Ct BMDD variables were significantly correlated as previously observed also in the healthy children4 and is likely to indicate a normal coupling of bone modeling/remodeling in these two compartments.
Apart from the fact that fracture healing processes may have influenced the BMDD, it might be hypothesized that increased mineralization heterogeneity was present even before the fracture. The aforementioned histomorphometric and biochemical data indicate that many of these children may have low bone turnover, which together with impaired material properties might make them more prone than healthy children to bone microdamage. This seems to be supported by FTIRI data. Lower carbonate-to-phosphate ratios have been found in the areas of microdamage in bone.14 In our previous study5 the subgroup with vertebral factures also revealed lower carbonate-to-phosphate ratios and increased mature collagen crosslinks compared to the subgroup with nonvertebral fractures (both indicators for tissue ageing). Local microdamage repair is a mechanism that might also result in increased heterogeneity of mineralization.
It is difficult to judge whether, and how, the increased heterogeneity of mineralization affects bone mechanical properties. Too homogenously mineralized bone is mechanically not favorable, because it might favor crack propagation, as has been discussed, for instance, in the context of bisphosphonate therapy.15 A heterogeneity in material properties is described to be advantageous for preventing the propagation of cracks but it may also favor crack initiation.16, 17
Our study is limited by the relatively low number of samples, in particular for the subgroup analyses. The patient cohort, although clinically similar, may have heterogeneous background for bone fragility. Only the rather low interindividual variation of the BMDD variables in healthy children and the high accuracy/sensitivity of the technique in the measurement of calcium concentrations made the detection of the BMDD differences from normal possible.
In conclusion, this cohort of fracture-prone children has a normal degree of bone mineralization but a significantly higher heterogeneity of mineralization. This can be caused by transiently increased bone turnover during fracture healing or due to repair of microdamage resulting from inherent skeletal fragility. The latter would point toward an unsuccessful compensatory adaptation to increased fragility in these children.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
We acknowledge financial support from Kuopio University Hospital, the Academy of Finland, National Doctoral Programme of Musculoskeletal Disorders and Biomaterials, the Finnish Cultural Foundation, the Päivikki and Sakari Sohlberg Foundation, the Foundation for Pediatric Research, the Sigrid Juselius Foundation, the Folkhälsan Research Foundation, the Helsinki University Hospital research funds, and the strategic funding of the University of Eastern Finland. This study was also supported by the AUVA (Austrian Social Insurance for Occupational Risk), the WGKK (Social Health Insurance Vienna). We also thank Daniela Gabriel, Petra Keplinger, Sonja Lueger, and Phaedra Messmer for qBEI analysis at the bone material laboratory of the Ludwig Boltzmann Institute of Osteology in Vienna.
Authors' roles: Study design: IT, BM, PR, HK, KK, and OM. Study conduct: BM and PR. Data collection: BM, PR, IT, MM, and OM. Data analysis: IT, BM, PR, and MK. Data interpretation: IT, BM, PR, KK, MT, HI, and OM. Drafting manuscript: IT, BM, PR, and OM. All of the authors had access to raw data and statistical analyses. All authors have revised the manuscript content and approved the final version of manuscript.




