Role of TGF-β in a Mouse Model of High Turnover Renal Osteodystrophy
ABSTRACT
Altered bone turnover is a key pathologic feature of chronic kidney disease-mineral and bone disorder (CKD-MBD). Expression of TGF-β1, a known regulator of bone turnover, is increased in bone biopsies from individuals with CKD. Similarly, TGF-β1 mRNA and downstream signaling is increased in bones from jck mice, a model of high-turnover renal osteodystrophy. A neutralizing anti-TGF-β antibody (1D11) was used to explore TGF-β's role in renal osteodystrophy. 1D11 administration to jck significantly attenuated elevated serum osteocalcin and type I collagen C-telopeptides. Histomorphometric analysis indicated that 1D11 administration increased bone volume and suppressed the elevated bone turnover in a dose-dependent manner. These effects were associated with reductions in osteoblast and osteoclast surface areas. Micro-computed tomography (µCT) confirmed the observed increase in trabecular bone volume and demonstrated improvements in trabecular architecture and increased cortical thickness. 1D11 administration was associated with significant reductions in expression of osteoblast marker genes (Runx2, alkaline phosphatase, osteocalcin) and the osteoclast marker gene, Trap5. Importantly, in this model, 1D11 did not improve kidney function or reduce serum parathyroid hormone (PTH) levels, indicating that 1D11 effects on bone are independent of changes in renal or parathyroid function. 1D11 also significantly attenuated high-turnover bone disease in the adenine-induced uremic rat model. Antibody administration was associated with a reduction in pSMAD2/SMAD2 in bone but not bone marrow as assessed by quantitative immunoblot analysis. Immunostaining revealed pSMAD staining in osteoblasts and osteocytes but not osteoclasts, suggesting 1D11 effects on osteoclasts may be indirect. Immunoblot and whole genome mRNA expression analysis confirmed our previous observation that repression of Wnt/β-catenin expression in bone is correlated with increased osteoclast activity in jck mice and bone biopsies from CKD patients. Furthermore, our data suggest that elevated TGF-β may contribute to the pathogenesis of high-turnover disease partially through inhibition of β-catenin signaling. © 2014 American Society for Bone and Mineral Research.
Introduction
Skeletal complications associated with renal failure have been well documented in individuals with chronic kidney disease (CKD) from stage 3 through dialysis.1-5 Furthermore, evidence is accumulating to suggest that at least some individuals with stage 2 CKD also have poor bone health.5-8 These findings are relevant given that poor bone health and cardiovascular disease appear interrelated, and cardiovascular events are the major cause of mortality in CKD patients.9-11 Importantly, agents that enhance bone quality also reduce cardiovascular disease.12-15 In late stages of the disease, elevated parathyroid hormone (PTH) and serum phosphorus (Pi) can be mechanistically linked to bone and cardiovascular disease through well-characterized mechanisms.16 As anticipated, treatments that lower PTH and/or serum phosphorus provide positive benefits on bone and cardiovascular health.3, 17-22 Nonetheless, as elevations in PTH and Pi are rather late events in CKD progression, these pathologies may not be sufficient to explain the growing evidence for declining bone and cardiovascular health occurring before their elevation. An understanding of the pathologic mechanisms associated with these early skeletal and vascular changes may lead to the generation of transformative therapies.
Given the challenges of performing longitudinal studies based on clinical bone biopsies, we and others have begun to assess early bone changes in genetic rodent models that develop progressive CKD.4, 6, 23 We recently described molecular and phenotypic bone changes throughout the development of renal osteodystrophy in the CKD model, jck mice. Jck mice develop polycystic kidney disease as a result of a mutation in Nek8, a protein responsible for trafficking of two cilia-associated proteins, polycystin 1 and 2.24, 25 Despite a clear role for cilia in normal bone remodeling,26, 27 we have previously demonstrated that the underlying defect in jck mice is insufficient to directly influence bone health in the absence of reduced renal function.6 Our data also showed that early repression of osteocyte Wnt/β-catenin signaling was associated with increased osteoclast activity, which was independent of detectable PTH changes. Furthermore, we also demonstrated that osteocyte Wnt/β-catenin signaling is altered in bone biopsies from individuals with CKD, underscoring the relevance of this newly characterized model of renal osteodystrophy. Finally, we showed that biphasic changes in Wnt/β-catenin antagonist expression also occur both in jck mouse and clinical bone biopsies.6 This evidence is supported by clinical epidemiologic studies demonstrating increased serum levels of sclerostin, an antagonist of the Wnt/β-catenin pathway in CKD and dialysis patients.28, 29
The factors responsible for early changes in sclerostin and β-catenin signaling have not yet been identified, but one viable candidate is TGF-β1, the most abundant bone cytokine.30 Under physiological conditions, TGF-β1 is a major modulator of bone turnover that plays diverse roles throughout the remodeling cycle. It regulates bone remodeling by recruiting mesenchymal stem cells to bone remodeling sites, enhancing differentiation of bone marrow mesenchymal stem cells, enhancing osteoblast precursor proliferation, and inhibiting osteoblast differentiation.31-36 Pharmacologic inhibition of TGF-β receptor signaling in osteoblasts increases bone mass by reducing the rate of remodeling, providing additional evidence for TGF-β's role in bone health.37 Furthermore, TGF-β antagonism via a neutralizing antibody leads to significant enhancement of bone quality in normal mice.38
TGF-β1 protein is elevated in bone biopsies from individuals with end-stage renal disease where it is thought to contribute to increased fibrosis associated with cortical bone porosity.39, 40 Given the important role of this factor in bone biology, it is conceivable that high bone levels of TGF-β in CKD may contribute to renal osteodystrophy (ROD). The availability of a neutralizing PAN–anti-TGF-β antibody provided the means to directly explore the potential role of this cytokine in the development of renal osteodystrophy in both jck mice and adenine-induced rat models of CKD.6, 25, 41 Our data demonstrate that TGF-β signaling is significantly increased in osteoblasts and early osteocytes and 1D11 attenuates high-turnover disease, at least in part through specific effects on osteoblast lineage cells by promoting enhanced β-catenin signaling. These data support a role of TGF-β in the pathogenesis of high-turnover renal osteodystrophy.
Materials and Methods
In vivo evaluation of 1D11 effects on bone
Wild-type (WT) (C57BL/6J) and jck mice were originally obtained from Jackson Laboratory (Bar Harbor, ME, USA). Eight- to 9-week-old male NTac:SD rats were purchased from Taconic and Yecuris Corporation (Germantown, NY, USA). All animals for the studies were maintained in a virus- and parasite-free barrier facility with a 12-hour light/dark cycle. All animal procedures were conducted in accordance with approved Institutional Animal Care and Use Committee (IACUC) protocols. Unless otherwise specified, both mice and rats were maintained on standard rodent chow diet (PicoLab Rodent Diet 20, LabDiet, St. Louis, MO, USA) containing 0.63% phosphate, 0.81% calcium, with 2.2 IU/g vitamin D3.
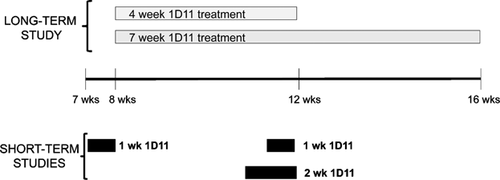
At 5 weeks of age, jck mice were switched to a casein-based diet containing 0.4% calcium and 1.0% phosphate (Diet # D08112306; Research Diets Inc., New Brunswick, NJ, USA) to induce hyperphosphatemia. To explore the role of TGF-β on the progression of renal osteodystrophy, the neutralizing anti-TGF-β antibody, 1D11 (Genzyme) or vehicle was given to jck mice at 8 to 9 weeks of age via intraperitoneal injection 3 times per week at 0, 2, or 5 mg/kg. Because a potential mechanism of 1D11 was unknown at the initiation of these studies, we designed two studies that spanned the different mechanistic phases. Because bone turnover in mouse is about 3 weeks, we designed studies that spanned one or two bone remodeling periods and chose 4- and 7-week treatment periods to evaluate 1D11 effects on bone histomorphometric parameters. Blood and tissues were collected after 4 or 7 weeks of treatment (n = 15 for the 4-week treatment study and n = 12 for the 7-week treatment study). In contrast to histomorphometric changes, primary effects on gene expression and signaling occur more rapidly. To evaluate 1D11's influence on mechanisms associated with bone turnover, we utilized bone samples from 12-week-old mice given 4 injections at 5 mg/kg for 1 week. Bones were collected 7 days after the first injection for gene expression and immunoblot analysis, as described in Results and in figure legends.
To induce uremia in rats, 10-week-old male NTac:SD rats were fed standard rodent diet containing 0.6% adenine for 1 week, followed by a diet containing 0.3% adenine for 5 weeks, and then standard rodent diet without adenine for the remaining 4 weeks of the study. The evaluation of 1D11's effects in the rat adenine model included three groups (n = 8 per group): 1) normal control: rats on the standard diet; 2) adenine group: adenine + vehicle (PBS); 3) 1D11 group: adenine + 1D11. 1D11 or vehicle was administered to the adenine-treated rats from the beginning of the study via intraperitoneal injection 3 times per week at 10 mg/kg for 10 weeks. Serum and tissue samples were collected after 10 weeks of treatment.
Blood and urine biochemistry
Whole blood was collected under isofluorane anesthesia via retro-orbital bleed, incubated for 20 minutes at room temperature, and then centrifuged at 4°C. Serum was aliquoted and frozen at –80°C for subsequent analysis. Serum phosphate (Pi), calcium (Ca), blood urea nitrogen (BUN), and creatinine (enzymatic method) were measured on an Integra 400 bioanalyzer (Roche Diagnostics, Indianapolis, IN, USA). Intact PTH (Immutopics, San Clemente, CA, USA), 1, 25-dihydroxyvitamin D (1,25(OH)2D3; IDS, Fountain Hills, AZ, USA), serum osteocalcin (OCN; Biomedical Technologies Inc., Stoughton, MA, USA), serum c-telopeptide of type I collagen (CTX; IDS), N-terminal propeptide of type I procollagen (P1NP; IDS); intact FGF23 (Kainos, Tokyo, Japan) ELISAs, and Mouse/Rat SOST Immunoassay Quantikine ELISA (R&D Systems, Minneapolis, MN, USA) were performed according to manufacturer's instructions.
Tissue harvest and histology
Tissues were harvested, fixed in 10% neutral-buffered formalin (NBF) for 24 to 48 hours, and then placed in 70% ethanol. Processed tissues were paraffin embedded and sectioned. Sections were dewaxed in xylene, rehydrated by ethanol gradient, and stained in hematoxylin and eosin. For bone immunostaining, femurs were decalcified, processed, and embedded in paraffin and sectioned. For dynamic bone histomorphometric analysis, mice were given intraperitoneal injections of Alizarin complexone (ACROS, Fair Lawn, NJ, USA) 7 days before death (40 mg/kg) and calcein (15 mg/kg) 2 days before death. Femurs were fixed in 40% alcohol, processed, and embedded in methyl methacrylate. Each femur was sectioned in the ventral/dorsal plane. Two levels per block separated by 100 microns were selected for staining. Goldner's Trichrome and von Kossa sections were analyzed for static measurements. Unstained serial sections were used for dynamic measurements using the Osteo II Bioquant system (Bioquant, Nashville, TN, USA). Nomenclature is in agreement with recommendations by Parfitt and colleagues.42
Cortical bone resorption score were evaluated based on number and size of the resorption canals. The samples were evaluated blind by a certified veterinary pathologist. The scoring system was defined as follows: 0, no significant lesions; 1, a few scattered small resorption canals; 2, more frequent, small to medium-sized resorption canals; 3, more prominent cortical resorption canals, beginning to coalesce; 4, numerous large coalescing cortical resorption canals, sometimes extending full thickness through the cortex.
Immunohistochemistry and TRAP staining of mouse bones
Five-micrometer paraffin dewaxed and rehydrated sections were used. TRAP staining was performed using standard protocol (Sigma-Aldrich, St. Louis, MO, USA). The number of TRAP-positive osteoclasts in femurs was counted using the Osteo II Bioquant system. The region of interest (ROI) was defined by tracing an area from the growth plate to 500 microns below with exclusion of cortical bone. The number of TRAP-positive osteoclasts was normalized to bone surface.
Immunostaining was also performed using a standard protocol. Briefly, the sections were incubated with Trypsin Retrieval Solution (BioGenex, Fremont, CA, USA) at 37°C for 20 minutes followed by PBS washes. The sections were then treated with 0.3% H2O2 in methanol for 30 minutes and blocked with 2.5% normal horse serum. Slides were incubated overnight at 4°C in blocking buffer containing the primary antibody, goat anti-pSMAD2/3 (1:100) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). After washing in PBST, the slides were incubated in ImmPRESS Reagent anti-goat IgG (Vector Laboratories, Burlingame, CA, USA), washed in PBST, developed with diaminobenzidine substrate (Vector Laboratories), and counterstained with Mayer's hematoxylin. The slides were then washed in tap water for 5 minutes, dehydrated, cleared, and mounted with Cytoseal XYL (Thermo Scientific, Rockford, IL, USA). Images were visualized on a Zeiss (Thornwood, NY, USA) Axiovert 200.
TUNEL staining
Osteocyte apoptosis was detected by TdT-mediated dUTP nick-end labeling (TUNEL) using a modified version of a commercial kit (Calbiochem, Gibbstown, NJ, USA) in longitudinal sections of the femur counterstained with 2% methyl green, as previously described.43, 44 Osteocyte apoptosis and empty lacunae were separately assessed in the cancellous and cortical bone of the distal metaphysis. The metaphysis was defined as the area starting 0.5 mm from the most proximal part of the growth plate and extending 0.5 mm toward the diaphysis.
Micro-computed tomography (µCT) analysis of bone
Tibias were imaged by µCT using isotopic voxels at a resolution of 3.5 microns (AG Micro-CT 35, Scanco Medical, Brüttisellen, Switzerland). Fifty slices of mid-diaphyseal cortical bone (175 microns total thickness) were analyzed with a threshold of 565 mg/cc. The following variables were calculated using Scanco software routines and compared between groups: bone volume fraction (BV/TV), apparent density (density of cortical bone including resorption spaces and voids) (data not shown), and material density (BMD) (threshold set for cortical bone density only). The nomenclature, symbols, and units used correspond to those described by Bouxsein and colleagues.45
Strength testing
Tibias were tested by 3-point bending, and the strength parameter (maximum load at failure) was assessed with a Test Resources (Shakopee, MN, USA) DDL200 axial loading machine outfitted with an Interface SMT1-22 force transducer. Cross-head displacement rate was 0.1 mm/s. Tests were conducted on the mid-diaphyses with the bones resting on two supports 5 mm apart and the tibial anterior margins facing upward toward the actuator.
Colony formation unit (CFU) assay and osteoclastogenesis in vitro
Bone marrow was collected from 12-week-old female WT or jck animals treated with PBS or 1D11 at 2 or 5 mg/kg, 3 times a week for 2 weeks, and cultured in αMEM containing 15% heat-inactivated fetal bovine serum (HI-FBS), and penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA). Media were replaced 3 days postadherence and refreshed 7 days thereafter. After 13 days, adherent cells were plated at 5000 cells/cm2 in triplicate in six-well plates and cultured in differentiation media, αMEM, 50 µg/mL ascorbic acid, 10% HI-FBS, penicillin-streptomycin. Media were replaced every 4 days. At day 8, cells were fixed and stained for alkaline phosphatase activity with 10 mg naphthol AS-MX, 20 mg Fast Blue BB salt, 1 mL N,N-dimethylformamide, in 19 mL of 0.1 M Tris (pH 9.2) (all from Sigma) to detect CFU-osteoblast (CFU-OB). Colonies greater than 1 mm in diameter were counted. CFU-fibroblasts (CFU-F) were detected by staining the same plates with 0.2% crystal violet in 2% ethanol for 1 hour. Plates were washed with water and then air-dried. All colonies greater than 1 mm in diameter were counted to yield the percentage of CFU-OB/CFU-F. To induce differentiation of HSCs to osteoclasts, bone marrow cells were cultured in αMEM containing 10% HI-FBS, 5 ng/mL macrophage colony-stimulating factor (M-CSF) (Sigma) and penicillin-streptomycin (Invitrogen). Forty-eight hours later, the nonadherent cells were collected, counted, and plated in 96-well plates at a density of 1.5 × 105 cells/well in αMEM containing 10% HI-FBS, 60 ng/mL M-CSF, 50 ng/mL receptor activator of NF-κB ligand (RANKL), and penicillin-streptomycin for 9 days. Cells were then stained for TRAP staining kit (Sigma). TRAP-positive cells with 3 or more nuclei were considered osteoclasts and counted.
Gene expression analysis
Bone shafts were collected, epiphysis removed, bone marrow displaced via centrifugation, and the shafts placed in Trizol (Sigma). RNA was extracted using the Trizol, chloroform, and isopropanol precipitation method. The extracted RNA was treated with DNase, purified on a Qiagen (Valencia, CA, USA) column, and eluted in RNAse-free water. A reverse transcriptase reaction was performed. The generated cDNA was used in single Taqman assays or customized Taqman low-density arrays (TLDA) (Applied Biosystems, Carlsbad, CA, USA) containing genes of interest.
For whole genome mRNA expression analysis, 7- and 11-week-old jck mice received injections of PBS or 1D11 (5 mg/kg) at days 1, 3, 5, and 7. Long bones were collected from these animals at day 8 (8 or 12 weeks old) and age-matched WT mice to serve as controls. Six animals were used in each group. Total RNA was isolated from bone shafts using Trizol (Invitrogen). RNA sequencing libraries were constructed using Illumina (San Diego, CA, USA) mRNA sequencing kits by BGI Americas (Cambridge, MA, USA). Libraries were subsequently sequenced on Illumina Hiseq2000 sequencer by BGI to a depth of 30 million total reads (paired end and 90 bps read length). Fastq files containing clean sequence reads (PCR primer and adapter sequences removed) were then loaded into ArrayStudio (OmicSoft, Cary, NC, USA) for QC and analysis. Low-quality reads were removed and then aligned against mouse genome B37 and gene model Ensembl R65. Total read counts were summarized using the gene model, and RPKM (reads per kilobase per million reads) values were generated for each gene. Differential gene expression fold differences and p values were then generated by ANOVA analysis. Significantly expressed gene list (fold cut-off 1.2 and p < 0.05) were then loaded into IPA (Ingenuity, Redwood City, CA, USA) pathway analysis software for downstream functional analysis.
Immunoblot analysis
Bone marrow and bone shafts from tibia and femur were frozen in liquid nitrogen, pulverized, and protein was extracted with 50 mM Tris buffer (pH 7.4), containing 0.1 M sodium chloride and 0.1% Triton X-100. Bone marrow or bone extracts were denatured at 70°C for 10 minutes and then separated by electrophoresis on 8% Bis-Tris gels (Invitrogen). Separated proteins were electrotransferred to nitrocellulose (NC) membranes followed by incubation in blocking buffer (5% nonfat dry milk in Tris-buffered saline, pH 7.4, and 0.3% Tween 20). Membranes were then incubated overnight at 4°C in blocking buffer containing one of the following primary antibodies: rabbit polyclonal antiserum pSMAD2 (Cell Signaling Technology, Inc., Danvers, MA, USA) (1:500), rabbit polyclonal anti-β-catenin (phospho S37) antibody (Abcam, Cambridge, UK) (1:500) or mouse monoclonal primary antiserum SMAD2 (1:1000). The membrane was washed 5 times with TBS containing 0.1% Tween 20 (TBST) for 10 minutes each wash. For detection of pSMAD2 or p-β-catenin, the secondary antibody incubation was performed for 60 minutes at room temperature using anti-rabbit IgG conjugated to horseradish peroxidase (Cell Signaling Technology, Inc.) at dilution of 1:5000. The secondary anti-mouse IgG conjugated to horseradish peroxidase (Cell Signaling Technology, Inc.) at dilution of 1:5000 was used as secondary antibody for SMAD2 detection. After final washing, the immunoreactive bands were detected using an enhanced chemiluminescence reagent (SuperSignal West Dura, Thermo Scientific), read on film, and quantitated using Image J software. Antiserum to β-actin (Abcam) was incubated room temperature for 1 hour at 1:50,000 dilution, washed, quantified, and used as a loading control.
Statistical analysis
All values are reported as the mean ± standard error of the mean (SEM) and were analyzed by one-way ANOVA or t test using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA).
Results
TGF-β1 expression and signaling are increased in bone of jck mice, a high-turnover model of CKD-MBD
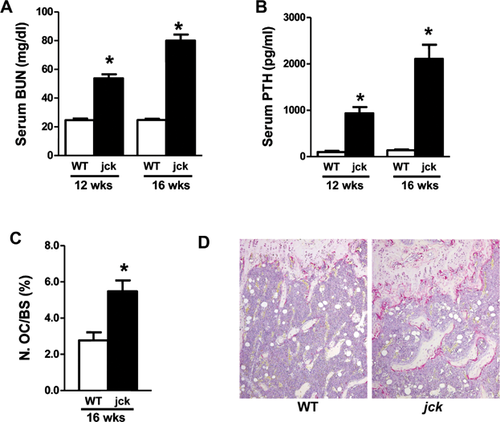
The jck mouse is a genetic model of polycystic kidney disease (PKD) that has a progressive decline in renal function.25 We have recently demonstrated that these mice also exhibit prominent features of CKD-MBD, including altered serum chemistries, high-turnover ROD, and cardiovascular disease.6 In addition to the previously reported increases in BUN and serum PTH, an increased number of surface TRAP-positive osteoclasts was observed in femur sections, which is consistent with high bone turnover (Fig. 1).
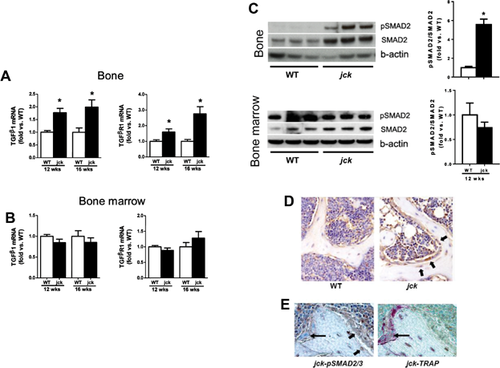
As a first step in exploring whether TGF-β contributes to the pathogenesis of high-turnover ROD, we first measured mRNA expression of the major isoform, TGF-β1, and its receptor, TGFβR1, in bone. TGF-β1 mRNA expression was significantly increased by about 1.7-fold in jck by 9 weeks of age (data not shown) and sustained throughout disease progression (Fig. 2A). TGFβR1 mRNA expression was also elevated in bones of jck compared with WT mice at both 12 and 16 weeks of age (Fig. 2A). However, neither the cytokine nor its receptor was significantly elevated in bone marrow (Fig. 2B). Immunoblot analysis confirmed that increased expression of TGF-β1 and TGFβR1 was associated with activation of SMAD signaling based on increased expression of pSMAD2 and total SMAD2 protein in bone but not bone marrow (Fig. 2C). The ratio of pSMAD2 and SMAD proteins (pSMAD/SMAD) measured by quantitative immunoblot analysis was also increased in bone but not in bone marrow (Fig. 2C). To further identify the specific cell types within bone that respond to TGF-β1, pSMAD2/3 immunostaining was performed on decalcified bone sections. pSMAD2/3 staining appeared more intense in osteoblasts and early osteocytes in jck bone. In addition, osteoblasts in jck were more cuboidal relative to those observed in WT-derived bones (Fig. 2D). Importantly, pSMAD2/3 expression did not colocalize with TRAP-positive osteoclasts (Fig. 2E).
The neutralizing anti-TGF-β antibody (1D11) attenuated the increased bone turnover in jck mice
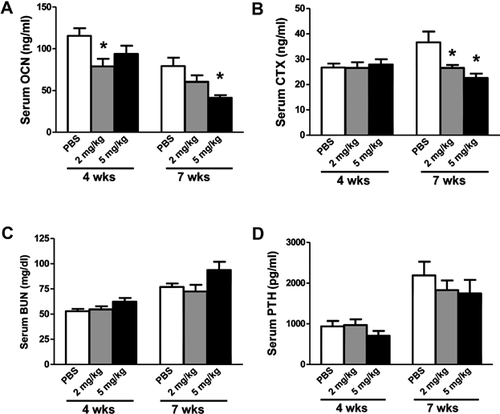
A neutralizing anti-TGF-β antibody, 1D11, was administered to jck mice starting at 8 weeks of age for 4 or 7 weeks (Fig. 3). We have previously shown that 8-week-old jck mice have evidence of early stages of high-turnover disease.6 To determine the effect of anti-TGF-β antibody on turnover, serum biomarkers were measured. 1D11 (5 mg/kg) resulted in a trend toward reduced OCN but not CTX at 4 weeks and significantly attenuated the elevation of both serum bone turnover markers after 7 weeks of treatment (Fig. 4A, B). A significant suppression of CTX and a trend toward suppression of serum OCN after 7 weeks of treatment were observed at 2 mg/kg. Consistent with its effects on serum OCN, 1D11 at 5 mg/kg also attenuated the elevation of serum P1NP levels in the 7-week treatment study (Table 1). 1D11 had no effect on either serum BUN or PTH levels, indicating that 1D11 did not significantly affect kidney function or PTH production/secretion in this study (Fig. 4C, D). Similarly, 1D11 did not influence the known alterations in serum Pi, Ca, 1,25(OH)2D3, or FGF23 levels (Table 1).
| 4-Week treatment | 7-Week treatment | |||||
|---|---|---|---|---|---|---|
| PBS | 1D11 (2 mg/kg) | 1D11 (5 mg/kg) | PBS | 1D11 (2 mg/kg) | 1D11 (5 mg/kg) | |
| BUN (mg/dL) | 53 ± 2 | 55 ± 3 | 62 ± 4 | 77 ± 3 | 72 ± 7 | 93 ± 8 |
| Creatinine (mg/dL) | 0.26 ± 0.02 | 0.27 ± 0.02 | 0.24 ± 0.01 | 0.27 ± 0.07 | 0.26 ± 0.05 | 0.29 ± 0.02 |
| Phosphate (mg/dL) | 11.1 ± 0.6 | 10.1 ± 0.6 | 8.8 ± 0.4* | 11.2 ± 0.7 | 9.8 ± 0.7 | 11.1 ± 0.9 |
| Calcium (mg/dL) | 8.7 ± 0.2 | 8.6 ± 0.2 | 8.9 ± 0.2 | 7.1 ± 0.2 | 7.6 ± 0.1 | 6.8 ± 0.2 |
| FGF23 (pg/mL) | 27,967 ± 7442 | 39,979 ± 11067 | 15,706 ± 2777 | 8818 ± 2413 | 9369 ± 2705 | 9813 ± 2104 |
| PTH (pg/mL) | 935 ± 132 | 968 ± 143 | 707 ± 119 | 2190 ± 335 | 1828 ± 240 | 1745 ± 334 |
| 1,25-(OH)2D (pg/mL) | 239 ± 13 | 242 ± 12 | 233 ± 14 | 365 ± 38 | 448 ± 37 | 351 ± 37 |
| Osteocalcin (ng/mL) | 115 ± 9 | 72 ± 6* | 94 ± 10 | 79 ± 10 | 60 ± 8* | 41 ± 3* |
| CTX (ng/mL) | 27 ± 2 | 27 ± 2 | 28 ± 2 | 37 ± 4 | 27 ± 1 | 23 ± 2* |
| P1NP (ng/mL) | ND | ND | ND | 30 ± 6 | 23 ± 4 | 11 ± 2* |
- Data are shown as mean ± SEM.
- ND = not determined.
- * p < 0.05 versus PBS-treated age-matched jck mice by one-way ANOVA and Tukey's multiple comparison test (n ≥ 12 in the 4-week treatment groups; n ≥ 8 in the 7-week treatment groups).
Dynamic and static bone histomorphometric analysis was performed on nondecalcified femur sections and reported using the TMV (turnover, mineralization, and bone volume) classification recommended by KDIGO (Fig. 5A–C) along with the full analysis (Table 2).1 Consistent with the observed changes in serum bone turnover biomarkers, 1D11 dose-dependently suppressed bone turnover measured by bone formation rates at 12 weeks of age after 7 weeks of treatment (Fig. 5A). Despite suppression of bone turnover, bone volume was significantly increased without impacting bone mineralization lag time in each treatment group (2 and 5 mg/kg) (Fig. 5B, C; Table 2). Evaluation of the decalcified bone sections with H&E and TRAP staining showed a trend toward improvement in cortical bone resorption in jck mice by 1D11treatment (5 mg/kg) (Fig. 5D, E).
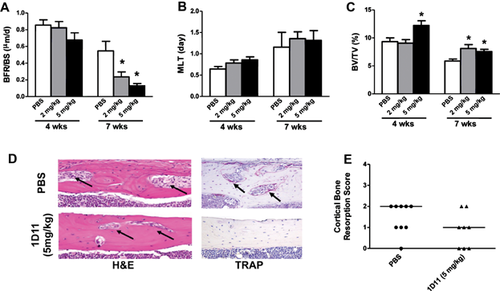
| 4-Week treatment | 7-Week treatment | |||||
|---|---|---|---|---|---|---|
| PBS | 1D11 (2 mg/kg) | 1D11 (5 mg/kg) | PBS | 1D11 (2 mg/kg) | 1D11 (5 mg/kg) | |
| TV/BV (%) | 9.3 ± 0.7 | 9.1 ± 0.6 | 12.2 ± 0.8* | 5.9 ± 0.4 | 8.1 ± 0.6* | 7.6 ± 0.4* |
| Tb.Th. (mm) | 0.065 ± 0.002 | 0.065 ± 0.002 | 0.072 ± 0.002* | 0.058 ± 0.003 | 0.068 ± 0.004 | 0.065 ± 0.004 |
| Tb.N.(1/mm2) | 5.2 ± 0.1 | 5.3 ± 0.1 | 5.5 ± 0.1 | 4.9 ± 0.1 | 5.1 ± 0.1 | 5.2 ± 0.1 |
| Tb.Sp (mm) | 0.13 ± 0.003 | 0.13 ± 0.003 | 0.11 ± 0.003 | 0.15 ± 0.004 | 0.13 ± 0.004 | 0.13 ± 0.004 |
| MS/BS (%) | 0.24 ± 0.19 | 0.23 ± 0.04 | 0.20 ± 0.02 | 0.25 ± 0.04 | 0.18 ± 0.02 | 0.14 ± 0.27 |
| MAR (mm/day) | 3.18 ± 0.17 | 2.70 ± 0.24 | 2.56 ± 0.25 | 1.65 ± 0.28 | 1.16 ± 0.17 | 0.90 ± 0.05 |
| BFR/BS (mm3/mm2/year) | 0.77 ± 0.08 | 0.76 ± 0.08 | 0.54 ± 0.08 | 0.55 ± 0.11 | 0.24 ± 0.06 | 0.13 ± 0.29 |
| MLT (day) | 1.16 ± 0.35 | 1.36 ± 0.16 | 1.32 ± 0.23 | 0.64 ± 0.06 | 0.79 ± 0.07 | 0.86 ± 0.07 |
| OV/BV (%) | 0.017 ± 0.004 | 0.012 ± 0.003 | 0.010 ± 0.003 | 0.66 ± 0.38 | 0.58 ± 0.38 | 0.001 ± 0.00 |
| N.Ob./BS (%) | 18.8 ± 2.2 | 14.4 ± 1.7 | 10.5 ± 1.8* | 19.6 ± 3.6 | 18.8 ± 2.8 | 10.2 ± 2.0* |
| N.Oc./BS(%) | 1.9 ± 0.15 | 1.3 ± 0.16* | 1.1 ± 0.17* | 5.5 ± 0.61 | 4.0 ± 0.79 | 2.4 ± 0.45* |
- Data are shown as mean ± SEM from histomorphometric analysis.
- BV/TV = bone volume/tissue volume; Tb.Th = trabecular thickness; Tb.N = trabecular number; Tb.Sp = trabecular separation; MS/BS = mineralizing surface/bone surface; MAR = mineral apposition rate; BFR/BS = bone formation rate/bone surface; MLT = mineralization lag time; OV/BV = osteoid volume/bone volume; N.Ob./BS = number of osteoblasts/bone surface; N.Oc./BS = number of osteoclasts/bone surface.
- * p < 0.05 versus PBS-treated age-matched jck mice by one-way ANOVA and Tukey's multiple comparison test (n ≥ 12 in the 4-week treatment groups; n ≥ 7 in the 7-week treatment groups).
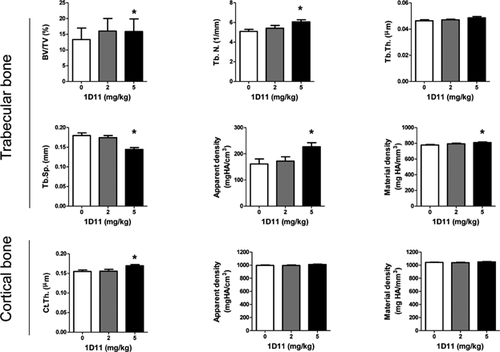
In agreement with findings from histomorphometric analysis, µCT analysis on femurs after 4 weeks of treatment (5 mg/kg 1D11) also demonstrated increased fractional bone volume (BV/TV) and trabecular number (Tb.N) with a corresponding decrease in trabecular separation (Tb.Sp) (Fig. 6). 1D11 administration was also associated with increased bone mineral density (both apparent and material density) and cortical thickness (Fig. 6). Finally, 1D11 administration was associated with an increased trend in bone strength as determined by 3-point bending test. The yield strengths were measured at 14.4 ± 1.1 N and 15.2 ± 0.7 N in jck treated with PBS and 1D11 (5 mg/kg), respectively.
1D11 suppresses osteoblast and osteoclast function in jck mice
We have previously demonstrated that in addition to increased bone formation rates, jck bones have increased osteoblast and osteoclast numbers. 1D11 suppressed both osteoblast and osteoclast numbers on trabecular bone surfaces at 5 mg/kg after 4 and 7 weeks of treatment (Fig. 7A). Gene expression analysis by RT-PCR (7-week treatment) confirmed that 1D11 administration was associated with significant reductions in mRNA for markers of osteoblast differentiation, including osteocalcin (Bglap), osterix (Sp7), Runx2, and alkaline phosphatase (Alpl), and the osteoclast marker Trap5 (Acp5) (Fig. 7B). Additionally, trends toward reduced expression of the osteoclast genes, cathepsin K (Ctsk) and Rank (Tnfrsf11a), were also observed.
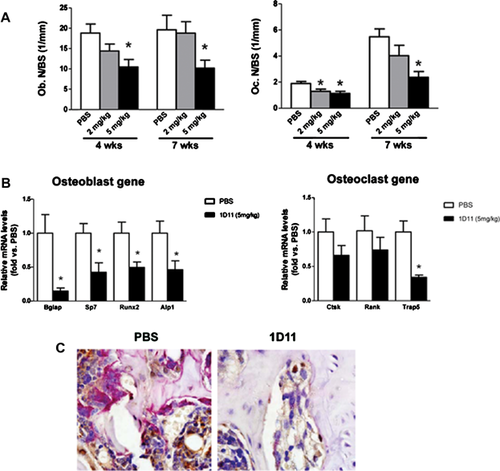
Normal coupling between osteoclast and osteoblast activities at the sites of remodeling was demonstrated by colocalization of TRAP staining with immunostaining for the osteoblast marker, osterix (Fig. 7C). Both TRAP and anti-osterix staining were significantly suppressed by 1D11, further demonstrating that inhibition of TGF-β reduced both osteoblastogenesis and osteoclastogenesis (Fig. 7C). These data indicated that 1D11 inhibited bone turnover without significantly disrupting the coupling between bone resorption and formation.
1D11 attenuates high turnover bone disease in rat adenine induced- CKD model
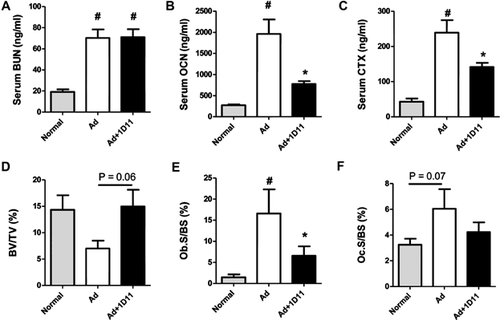
To confirm that the role of TGF-β in jck mice represents a general phenomenon in ROD and is not specific to the jck mouse model, we evaluated 1D11's influence on bone in a moderate rat adenine-induced CKD model in which animals were fed diets that had declining doses of adenine as described in Materials and Methods. Adenine treatment resulted in renal damage as demonstrated by increased serum BUN levels. Importantly, similar to our findings in the jck mouse model, 1D11 treatment had no significant effect on renal function (Fig. 8A). Consistent with observations in jck mice, serum bone turnover markers, OCN and CTX, were significantly elevated in the adenine-treated group and were both significantly attenuated by 1D11 treatment (Fig. 8B, C). Bone histomorphometric analysis confirmed an increase in both osteoblast and osteoclast surface areas consistent with increased bone turnover that were attenuated with 1D11 treatment (Fig. 8E, F). Similar to findings in jck mice, 1D11 treatment was also associated with a strong trend toward improved bone volume (p = 0.06) (Fig. 8D).
1D11 reduces SMAD2 signaling in bone
To explore the mechanism by which 1D11 influences osteoblast and osteoclast number and/or function, we first examined 1D11-induced early changes in the canonical TGF-β signaling pathway SMAD2/3. Eleven-week-old jck mice received 4 doses of 1D11 (5 mg/kg) before death over a course of 1 week (Fig. 3). Quantitative immunoblot analysis of protein extracted from the midshaft of both tibia and femur confirmed the initial observation that the pSMAD2/SMAD2 ratio was increased in jck mice and demonstrated that a 1-week treatment with 1D11 significantly attenuated the increase (Fig. 9A, B).
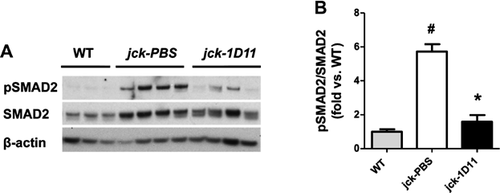
As described above, changes in pSMAD2/SMAD2 were not observed via immunoblot of whole bone marrow. To explore the possibility that 1D11 may influence the subset of osteoprogenitor cells, we assessed the influence of antibody treatment on the differentiation potential of bone marrow mesenchymal (MSC) and hematopoetic stem cells (HSC). 1D11 (5 mg/kg) was administered to jck starting at 10 weeks of age (Fig. 3). After 2 weeks of treatment (12 weeks of age), bone marrow was collected from age-matched WT, treated and untreated jck, and cultured under conditions that favored either MSC differentiation to osteoblasts or HSC differentiation to osteoclasts. CFU-OB/CFU-F ratio was significantly increased from jck mice compared with WT mice. 1D11 at 5 mg/kg significantly suppressed the CFU-OB/CFU-F ratio in jck mice (Fig. 10A), whereas the number of osteoclast progenitors was similar between WT and jck mice. 1D11 treatment had no influence on the osteoclastic differentiation potential (Fig. 10B).
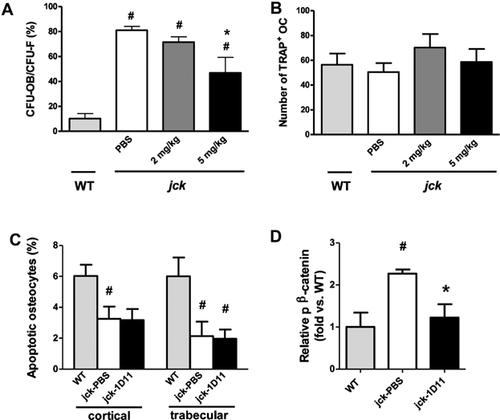
We also determined whether the higher number of osteoclasts observed in bones of jck mice were a consequence of increased osteoclast recruitment by apoptotic osteocytes, and if so whether 1D11 could attenuate these changes (Fig. 10C). However, our data did not support this hypothesis as the number of TUNEL-positive osteocytes was significantly decreased in jck compared with age-matched WT mice. Furthermore, no differences were observed between 1D11-treated versus -untreated jck mice.
1D11 treatment restores β-catenin pathway changes
We have previously shown that an underlying decrease in β-catenin signaling is associated with ROD in jck bones and human bone biopsies.6 Immunoblot analysis confirmed the increased expression of the inactive form of β-catenin (p-β-catenin) in protein isolated from jck femurs compared with WT bones at 12 weeks of age. Importantly, 1 week of 1D11 administration was associated with a decrease in p-β-catenin protein to a level similar to that observed in WT mice (Fig. 3 and Fig. 10D). Modulation of Wnt/β-catenin pathway components and downstream gene expression changes were further explored using whole genome RNA sequence analysis on bone samples from the 1-week study at 12 weeks of age (Fig. 3). As shown in Table 3, expression of genes whose products are within or downstream of the canonical Wnt/β-catenin pathway are consistent with an overall repression of this pathway in jck mice relative to WT mice. Genes that were significantly downregulated included ligands (Wnt16, Wnt10b, and Wnt5b) and receptors/coreceptors (Frzb and Lrp5) of this pathway. β-catenin (Ctnnb1), the most important downstream component of the Wnt pathway, was also significantly suppressed. Within those genes that were upregulated in expression, Sfrp1, Sfrp4, and Dkkl1 are all negative regulators of Wnt signaling. In addition, consistent with previously described gene expression data from RT-PCR, Tgf-β1 and Tgfβr1 were significantly elevated. Furthermore, after 1 week of 1D11 administration, many genes within the Wnt/β-catenin pathway were regulated toward normal levels (Table 4). Among the genes upregulated by 1D11, the most important gene in the Wnt pathway, β-catenin (Ctnnb1), was completely normalized. In addition, Wnt receptors (Fzd5 and Smo), downstream elements (Dvl1 and Dvl3), and the positive regulator (Appl2) were upregulated after 1 week of 1D11 treatment. β-casein kinase 2 was the only downregulated gene in the pathway, which was upregulated in jck and completely normalized by 1D11. Importantly, β-catenin target genes such as Pkd1 were normalized by 1D11.
| Gene | Description | jck versus WT (fold) |
|---|---|---|
| Frzb | Frizzled-related protein | –3.54 |
| Wnt16 | Wingless-type MMTV integration site family, member 16 | –2.35 |
| Dkk3 | Dickkopf 3 homolog (Xenopus laevis) | –2.16 |
| Sost | Sclerostin | –2.00 |
| Tgfb3 | Transforming growth factor, beta 3 | –1.90 |
| Wnt10b | Wingless-type MMTV integration site family, member 10B | –1.80 |
| Wnt5b | Wingless-type MMTV integration site family, member 5B | –1.77 |
| Cdh2 | Cadherin 2, type 1, N-cadherin (neuronal) | –1.57 |
| Wif1 | WNT inhibitory factor 1 | –1.55 |
| Sox17 | SRY (sex determining region Y)-box 17 | –1.38 |
| Ctnnb1 | Catenin (cadherin-associated protein), beta 1, 88 kDa | –1.38 |
| Kremen1 | Kringle containing transmembrane protein 1 | –1.37 |
| Ppp2r5b | Protein phosphatase 2, regulatory subunit B', beta | –1.37 |
| Lrp5 | Low-density lipoprotein receptor-related protein 5 | –1.36 |
| Smo | Smoothened, frizzled family receptor | –1.34 |
| Sfrp1 | Secreted frizzled-related protein 1 | 2.06 |
| Sfrp4 | Secreted frizzled-related protein 4 | 1.80 |
| Dkkl1 | Dickkopf-like 1 | 1.80 |
| Crebbp | CREB binding protein | 1.51 |
| Map4k1 | Mitogen-activated protein kinase kinase kinase kinase 1 | 1.48 |
| Tgfbr1 | Transforming growth factor, beta receptor 1 | 1.43 |
| Pkd1 | Polycystic kidney disease 1 | 1.40 |
| Csnk2b | Casein kinase 2, beta polypeptide | 1.39 |
| Rara | Retinoic acid receptor, alpha | 1.36 |
| Cd44 | CD44 molecule (Indian blood group) | 1.33 |
| Csnk1g1 | Casein kinase 1, gamma 1 | 1.32 |
| Sox12 | SRY (sex determining region Y)-box 12 | 1.31 |
| Tgfb1 | Transforming growth factor, beta 1 | 1.30 |
| Ep300 | E1A binding protein p300 | 1.30 |
| Gene | Description | 1D11 versus PBS (fold) | jck vs WT (fold) |
|---|---|---|---|
| Csnk2b | Casein kinase 2, beta polypeptide | –1.29 | 1.39 |
| Dvl3 | Dishevelled, dsh homolog 3 (Drosophila) | 1.56 | NS |
| Pkd1 | Polycystic kidney disease 1 | 1.41 | –1.40 |
| Smo | Smoothened, frizzled family receptor | 1.34 | –1.34 |
| Ctnnb1 | Catenin (cadherin-associated protein), beta 1, 88 kDa | 1.31 | –1.36 |
| Appl2 | Adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 2 | 1.31 | NS |
| Fzd5 | Frizzled family receptor 5 | 1.26 | NS |
| Dvl1 | Dishevelled, dsh homolog 1 (Drosophila) | 1.20 | –1.16 |
- NS = not significant.
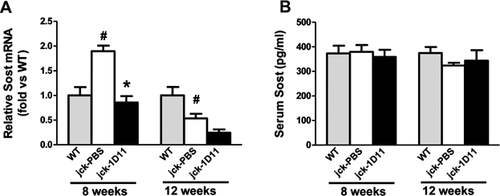
Expression of Sost, a Wnt antagonist, was decreased in jck bones compared with WT mice at 12 weeks of age, most likely owing to significantly elevated serum PTH levels (Table 3, Fig. 11A, and Fig. 1). 1D11 treatment was associated with a strong trend toward a further suppression of Sost mRNA expression in the jck bones at this time point. As we have previously shown, Sost mRNA expression is most highly upregulated at an earlier CKD stage in this model and in human bone biopsies and may contribute to the early repression of osteocyte Wnt/β-catenin signaling. Therefore, to understand the effect of 1D11 on Sost expression, we performed a similar 1 week 1D11 study using 7-week-old mice (Fig. 3). Consistent with our previous observation, Sost mRNA expression was significantly increased in jck bones compared with WT bones at 8 weeks but not at 12 weeks. 1D11 treatment for 1 week completely normalized the increased Sost expression in the 8-week jck bones (Fig. 11A). To further explore changes in sclerostin, serum Sost levels were measured using a commercially available ELISA assay. However, no significant changes were observed between WT and jck mice with or without 1D11 treatment (Fig. 11B).
Discussion
Renal osteodystrophy represents a spectrum of bone diseases from high to low turnover. In CKD, abnormal bone turnover is associated with dysregulated mineral metabolism, increased risk for cardiovascular calcification, left ventricular hypertrophy, and mortality.9, 11, 46 Identification of additional factors regulating bone turnover, especially in the early stages, is important for understanding the pathogenesis of CKD-MBD.
Previously reported longitudinal analysis of jck mice demonstrated that the initial stages of high-turnover disease occurred independently of detectable PTH changes. Increased osteoclast activity was likely caused by induction of sclerostin, decreased β-catenin signaling in osteocytes, and a corresponding increase in the RANKL/OPG ratio.6 Similar changes were found in human bone biopsies, suggesting that alterations in Wnt/β-catenin signaling may be a common feature of ROD. This evidence is supported by clinical epidemiologic studies demonstrating increased serum sclerostin in ROD.28, 29
Jck mice develop CKD as a consequence of activating mutations in Nek8, leading to increased renal expression of polycystin 1 and 2 (PC1 and PC2) with subsequent alterations in cilia structure and function.24 In contrast, loss of function PKD1 mutations induce polycystic kidney disease and skeletal defects.26, 27 Although primary osteoblast cilia are mechanosensors influencing bone remodeling via β-catenin signaling,47 Nek8 mutations did not influence bone disease, presumably because slight increases in PC1 and PC2 expression do not have the same detrimental effects as in the absence or insufficient levels of these proteins.6 In vivo bone parameters and ex vivo bone marrow stromal cell differentiation potential were not different between 6-week-old WT and jck mice before the onset of renal dysfunction. These observations suggest that observed differences in bone are a consequence of renal dysfunction. Coupled to evidence for similar molecular changes in clinical biopsies, our data support the use of jck mouse as a reasonable model that replicates the salient features of human CKD-MBD with associated high-turnover ROD.
Our study focused on TGF-β's potential role on bone in early CKD because this cytokine's positive role in maintaining skeletal health is well known30, 48, 49 and TGF-β1 deletion or transgenic inhibition of the TGF-β pathway in osteoblasts results in decreased bone formation.32, 37 Additionally, TGF-β1 circulating levels and bone expression are upregulated during CKD progression along with increased sclerostin and FGF23.39, 40, 50 Elevated TGF-β activity is also associated with enhanced osteoblast and/or osteoclast activity in genetic bone diseases such as Camurati-Engelmann disease (CED) and osteogenesis imperfecta (OI).31, 32, 51-54 Finally, TGF-β and Wnt/β-catenin cross-talk has been well described.34
The observed elevations of TGF-β1 mRNA, its receptor expression, and TGF-β signaling in jck mice suggest that TGF-β1 may play an important role in the pathogenesis of high-turnover ROD. TGF-β neutralization in jck mice attenuated the elevated levels of bone turnover markers, reduced bone formation rates, decreased osteoclast and osteoblast surface areas, increased trabecular bone volume, and increased cortical thickness with clear reduction in cortical porosity and fibrosis (Figs. 4 and 5). In addition, TGF-β antagonism did not change serum BUN, PTH, Pi, or 1,25(OH)2D3 levels, suggesting a direct effect on bone rather than a systemic effect.
Our findings are consistent with the observations of transgenic mice expressing an osteoblast-specific truncated type II TGF-β receptor, which developed an age-dependent increase in trabecular bone mass attributable to decreased resorption and formation.37 These results coupled with the observations that TGF-β1 expression increases with age raise the possibility that TGF-β1 may contribute to age-associated bone loss. In contrast, our findings are distinct from studies in normal juvenile mice, where TGF-β inhibition was associated with increased osteoblast differentiation and decreased osteoclast differentiation.38, 55 Taken together, our results contribute to the growing evidence that TGF-β's effects on bone are dose and context dependent.
The complexity of TGF-β's regulation on bone turnover is illustrated by its influence on osteoblast/osteocyte differentiation, osteoclastogenesis, and the coupling between bone formation and resorption. Our findings suggest that blocking TGFβ's actions primarily lead to attenuation of osteoblast activity in the high-turnover ROD setting. We observed increased pSMAD2/SMAD2 expression only in protein extracted from jck bone shafts but not bone marrow. It is possible that a small cell subpopulation within the bone marrow may also respond to TGF-β because the significant increase in osteoblast precursors derived from jck bone marrow is attenuated by TGF-β neutralization (Fig. 10A). Because immunostaining in jck bones clearly demonstrated that pSMAD2/3 was induced primarily in osteoblasts and early osteocytes but not osteoclasts (Fig. 2), this observation together with the immunoblot data (Fig. 9) supports a direct role on osteoblast lineage cells. Indeed, the reduced pSMAD2 in osteoblasts and osteocytes was expected because TGF-β's influence on osteoblast lineage is well described both in vitro and in vivo. TGF-β1 has been shown to temporally influence the differentiation state of osteoblasts through promotion of proliferation and differentiation including increased matrix/osteoid deposition necessary for normal mineralization. Although observed signaling changes were limited to the osteoblast lineage, osteoclast number and activity were significantly suppressed by long-term TGF-β antagonism (Figs. 4, 5, and 7).
As several in vitro studies have demonstrated cell- and dose-dependent effects of TGF-β on osteoclasts via modulation of MAP kinase pathways, we cannot rule out direct effects based on the absence of pSMAD2/3 modulation.56-60 However, it should be noted that in vivo evidence for a direct role of TGF-β on osteoclasts is lacking. Alternatively, TGF-β may modulate osteoclast activity indirectly through the relative ratio of osteoblast/osteocyte-expressed RANKL/OPG. Regulation of osteoclast proliferation and/or differentiation by osteoblasts/osteocytes is not a novel mechanism. For example, continuous elevation of PTH leads to increased bone resorption by stimulating RANKL expression in osteoblasts.61
TGF-β1 is the key factor responsible for recruitment of osteoblast precursor cells to resorption sites thereby coupling osteoblast and osteoclast differentiation/activity both temporally and spatially.32, 62 Costaining of TRAP and osterix confirmed increased numbers of remodeling units containing colocalized osteoclasts and osteoblasts with no evidence of resorption/formation decoupling. Nonetheless, the antibody's effect on bone formation could be at least partially owing to the neutralization of osteoclast-released TGF-β and subsequent inhibition of osteoblast precursor migration.
All major regulators of bone metabolism contribute to the regulation of osteoblast and osteocyte life span by modulating apoptosis.63 TGF-β is known to induce apoptosis in a variety of cell types; however, data on its role in osteoblastic or osteocytic apoptosis are contradictory. TGF-β inhibits osteoblast and osteocyte apoptosis in vitro,64, 65 but mice lacking Smad3 exhibit decreased bone mass associated with increased osteoclast apoptosis.66 Our data demonstrated that inhibiting TGF-β could attenuate bone disease in CKD with corresponding restoration of Wnt/β-catenin signaling, an important regulator of osteocyte apoptosis. TGF-β could contribute to disease pathogenesis via effects on apoptosis and/or indirectly antagonizing Wnt/β-catenin promotion of survival. However, repression of TGF-β signaling did not have a significant effect on osteocyte apoptosis in jck mice as measured by TUNEL staining of bone sections (Fig. 10).
Our whole genome RNA sequencing data from 12-week-old mice further validated our previously reported repression of osteocyte Wnt/β-catenin signaling (Tables 3 and 4).6 Wnts (Wnt16, Wnt10b, and Wnt5b), Wnt receptors (Frzb and Lrp5), and β-catenin are all significantly suppressed, whereas negative regulators of Wnts (Sfrp1, Sfrp4, and Dkk1) are significantly elevated. In addition, Pkd1, a known downstream gene for β-catenin, is also significantly upregulated.26 More importantly, 1-week antagonism of TGF-β normalized expression of β-catenin, casein kinase β2, and Pkd1 expression and modulated other genes associated with stimulation of the Wnt/β-catenin pathway.
Sclerostin is significantly increased in jck bones and human biopsies from the early stages of CKD. This change is associated with an expected repression of β-catenin signaling and increased osteoclast activity.6 Consistent with this finding, our whole genome RNA data from 8-week-old mice demonstrated that Sost expression was increased 2.3-fold in jck bones compared with WT bones (p < 0.05). After 1 week of anti-TGF-β administration, Sost expression in the jck bones was normalized to levels similar to that in age-matched WT mice (p < 0.05 versus jck). At 12 weeks of age, elevated SOST could no longer account for changes in β-catenin signaling, presumably because PTH, a negative regulator of Sost expression, is elevated.67-69 To further confirm our observation, we measured serum Sost levels with an ELISA assay. However, significant changes were not observed between WT and jck, or jck treated with vehicle or 1D11 at 8 weeks of age. Previously, we demonstrated that mRNA levels as detected by real-time RT-PCR were consistent with changes in sclerostin protein levels as assessed by immunostaining.6 Taken together, these data raise the possibility that local Sost's effect on bone may predominate. Indeed, Jastrzebsk and colleagues have recently reported a similar discrepancy between Sost bone mRNA levels and serum protein levels in serum from an ovariectomy model.70
The current studies focused solely on initial mechanisms associated with onset of high bone turnover and did not utilize experimental conditions to fully address the potential influence of TGF-β on vascular calcification. Although we have previously demonstrated that jck mice do exhibit vascular calcification and early signs of left ventricular hypertrophy, these events occur in significantly older mice than analyzed in the current studies. Similarly, rats treated with high doses of adenine, vitamin D, calcium, and phosphorus can also exhibit significant vascular calcification. However, these conditions induce very severe changes in bone disease that do not reflect changes typically observed in clinical biopsies. The adenine model described in our studies exhibits a high-turnover bone disease similar to jck mice without the treatments previously described to drive vascular calcification. Additional studies will be required to assess the role of TGF-β on vascular calcification.
In summary, our data demonstrate that increased TGF-β1 expression and signaling is associated with the suppression of Wnt/β-catenin signaling in bone during CKD progression and may contribute to the pathogenesis of high-turnover ROD. TGF-β neutralization attenuates increased bone turnover and improved bone quality via a SMAD-dependent restoration of Wnt/β-catenin signaling in osteoblasts/osteocytes. Our findings also provide important evidence that TGF-β contributes to the pathogenesis of high-turnover ROD independent of serum PTH level.
Disclosures
SLiu, WS, JHB, WT, YS, BK, RG, SR, LP, KM, SC, THX, HL, SL, and SCS are employees of Genzyme, a Sanofi company. All other authors state that they have no conflicts of interest.
Acknowledgments
The authors thank, from Genzyme, Kuber Sampath for discussions and data interpretation; William Weber for imaging analysis; Leah Curtin for colony management; and Michael Phipps, Matthew DeRiso, Patrick DeCourcy, and Timothy Devlin for genotyping. The authors also thank Keith Condon, Nicoletta Bivi, and David Southern from Indiana University School of Medicine for technical help in quantification of apoptosis.
Authors' roles: SLiu and SCS contributed to project concept and strategy development. SLiu, WS, JHB, WT, YS, BK, RG, SR, LP, KM, and GZ contributed to specific study design, data acquisition, and analysis. SLiu, YS, LP, CX, TB, SL, and SCS contributed to data interpretation. All authors participated in revising the manuscript and approved manuscript submission.




