Safety and Efficacy of S53P4 Bioactive Glass in Osteomyelitis Management: A Systematic Review and Meta-Analysis
Funding: The authors received no specific funding for this work.
ABSTRACT
Osteomyelitis remains a difficult-to-treat bone infection due to its high recurrence risk, complex surgical demands, and rising rates of multidrug-resistant organisms. While conventional treatments rely heavily on antibiotic-loaded materials, bioactive glass S53P4 offers a dual-action alternative, combining bacteriostatic and bactericidal activity with bone regenerative potential. A systematic review and meta-analysis following PRISMA guidelines was conducted to evaluate the clinical efficacy and safety of S53P4 bioactive glass in osteomyelitis treatment. Twenty-eight studies published between 2000 and 2024, encompassing 1122 patients (mean age: 43.6 years), were included. Outcomes analyzed included infection eradication, recurrence, bone healing, functional recovery, and complications. The risk of bias was assessed using ROBINS-I for observational studies and the JBI checklist for case series. A meta-analysis of 10 studies reporting infection eradication at ≥ 12 months was performed using a random-effects model. The pooled infection eradication rate was 88.1% (95% CI: 85.4%–90.4%) with no significant heterogeneity (I2 = 0%). Studies reported consistent efficacy across chronic, diabetic foot, mastoid, and jaw osteomyelitis. S53P4 was effective against polymicrobial and multidrug-resistant infections, including Staphylococcus aureus and Pseudomonas aeruginosa. Healing outcomes were favorable, with high rates of bone integration and return to function. Complications were uncommon and primarily related to soft tissue coverage. Most patients received systemic antibiotics; no studies required local antibiotic-loaded materials alongside S53P4. Bioactive glass S53P4 is a safe and effective adjunct in osteomyelitis management, demonstrating high long-term infection control, robust bone regeneration, and a low complication profile. Its nonantibiotic antimicrobial mechanism makes it particularly suitable in settings of antimicrobial resistance. Future studies should assess its long-term durability and applications in high-risk infections.
1 Introduction
Osteomyelitis, a debilitating bone infection, poses significant challenges in surgical management due to its persistent nature, risk of recurrence, and the increasing prevalence of multidrug-resistant organisms [1-3]. While conventional treatments such as antibiotic-loaded bone substitutes and systemic antimicrobial therapy remain mainstays, their limitations in infection control, tissue compatibility, and bone regeneration have highlighted the need for innovative biomaterials [4, 5].
Bioactive glass, particularly the S53P4 composition, has emerged as a groundbreaking adjunct in the management of osteomyelitis [6]. S53P4 is a silicate-based glass composed of 53% SiO2, 23% Na2O, 20% CaO, and 4% P2O5 by weight, a formulation specifically designed to combine antibacterial properties with osteoconductive and osteostimulative potential [7]. Upon implantation, the glass undergoes a series of ionic exchanges with body fluids, resulting in the localized release of calcium, phosphate, and sodium ions. This ion release creates an alkaline microenvironment (pH ~9–10) and elevates osmotic pressure—conditions that exert bacteriostatic effects by inhibiting bacterial proliferation and, in many cases, bactericidal effects by directly compromising microbial viability, including that of multidrug-resistant organisms. A key step in S53P4's bioactivity is the formation of a silica gel layer on its surface following ion exchange. This layer subsequently facilitates the precipitation of calcium and phosphate ions from the surrounding physiological milieu, which crystallize into natural hydroxyapatite—a bone-like mineral [8]. This hydroxyapatite layer enables bone bonding and provides a scaffold for osteoconduction, while the ionic signaling also promotes osteostimulation by activating osteoblasts and enhancing bone remodeling [8]. As such, S53P4 serves a dual function: it controls infection while supporting structural bone regeneration, making it particularly useful in managing large defects, chronic osteomyelitis, and complex diabetic foot infections (Figure 1).
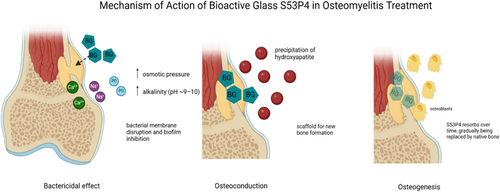
While numerous bioactive glass formulations exist—including borate- and phosphate-based variants—S53P4 is the most extensively studied and clinically adopted for bone infection. Its well-characterized safety profile, robust antibacterial activity, and osteogenic properties distinguish it from newer formulations that lack consistent in vivo validation. Given the heterogeneity of existing literature—with variability in patient populations, infection etiologies (e.g., long bone, mastoid, and diabetic foot), and surgical strategies—this systematic review focuses exclusively on clinical outcomes associated with S53P4 in osteomyelitis.
2 Methods
This systematic review adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42024628351 (Figure 2).
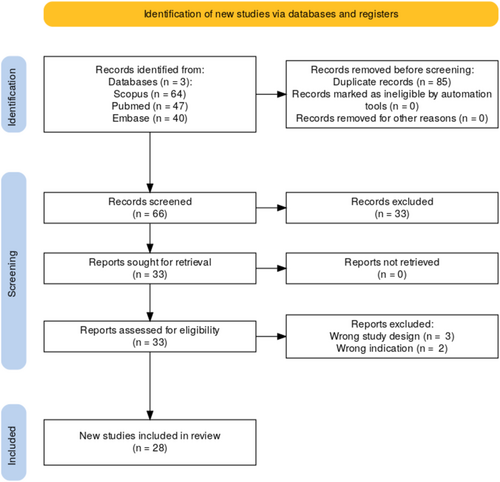
The eligibility criteria for study inclusion were defined using the Population, Intervention, Comparison, Outcome, and Study Design (PICOS) framework. Eligible studies included human participants diagnosed with osteomyelitis of any type—including acute, chronic, posttraumatic, postsurgical, mastoiditis, or diabetic foot-associated osteomyelitis—who were treated with S53P4 bioactive glass. Studies were included regardless of whether S53P4 was used alone or in combination with systemic antibiotics, debridement, or other surgical interventions. The primary outcomes of interest were infection eradication rates and recurrence, while secondary outcomes included wound or bone healing, time to recovery, functional outcomes, and adverse events. Only clinical studies with ≥ 5 patients were included, such as prospective or retrospective cohort studies, controlled trials, and case series. Case reports (n < 5), animal, in vitro, and non-English language studies were excluded. A comprehensive search of PubMed, Embase, and Scopus was performed to identify studies published between January 2000 and October 2024. The search strategy employed a combination of Medical Subject Headings (MeSH), Emtree terms, and free-text keywords. A sample PubMed query was: (“S53P4”[All Fields] OR “S53P4 bioactive glass”[All Fields] OR “bioactive glass S53P4”[All Fields]) AND (“osteomyelitis”[MeSH Terms] OR “bone infection”[All Fields] OR “mastoiditis”[All Fields] OR “diabetic foot”[All Fields] OR “diabetic foot infection”[All Fields] OR “diabetic foot osteomyelitis”[All Fields]) Reference lists of included studies were also screened to capture additional relevant publications in accordance with PRISMA guidelines.
Two reviewers independently screened titles and abstracts to identify studies that met the inclusion criteria. Full-text articles were assessed for eligibility, and disagreements were resolved through consensus or consultation with a third reviewer. Data were extracted using a standardized form, capturing study characteristics (author, year, study design, sample size), patient demographics (age, gender), intervention details, outcomes, and follow-up duration.
The risk of bias was assessed using two tools depending on study design. For non-randomized comparative studies, the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool was applied, and for case series and case reports, the Joanna Briggs Institute (JBI) Critical Appraisal Checklist was used (Table 1).
| Study | Design | Risk of bias tool | Overall risk of bias |
|---|---|---|---|
| Romano et al. (2014) | Retrospective comparative study | ROBINS-I | Moderate |
| Iacopi et al. (2022) | Prospective pilot study | JBI case series | Low |
| Kojima et al. (2021) | Prospective case series | JBI case series | Low |
| Lindfors et al. (2010) | Prospective case series | JBI case series | Low |
| Gatti et al. (2024) | Retrospective observational study | ROBINS-I | Moderate |
| Oosthuysen et al. (2020) | Retrospective case series | JBI case series | Low |
| Van Vugt et al. (2021) | Prospective cohort study | ROBINS-I | Moderate |
| Ferrando et al. (2017) | Retrospective comparative study | ROBINS-I | Moderate |
| Rodriguez et al. (2021) | Prospective case series | JBI case series | Low |
| Kroon et al. (2022) | Retrospective cohort study | ROBINS-I | Moderate |
| Geurts et al. (2019) | Retrospective comparative study | ROBINS-I | Moderate |
| Drago et al. (2013) | Prospective observational study | ROBINS-I | Moderate |
| Al Malat et al. (2018) | Retrospective study | ROBINS-I | Moderate |
| De Giglio et al. (2021) | Retrospective observational study | ROBINS-I | Moderate |
| Stoor et al. (2017) | Prospective case series | JBI case series | Low |
| Kastrin et al. (2021) | Retrospective observational study | ROBINS-I | Moderate |
| De Giglio et al. (2018) | Case series (letter) | JBI case series | Low |
| Venter et al. (2021) | Retrospective cohort study | ROBINS-I | Moderate |
| Korpar and Frangež (2023) | Retrospective case series | JBI case series | Low |
| Auregan et al. (2022) | Retrospective case series | JBI case series | Low |
| Krol et al. (2021) | Prospective case series | JBI case series | Low |
| Lindfors et al. (2017) | Multinational observational study | ROBINS-I | Moderate |
| Ferreira and Epstein (2023) | Retrospective cohort study | ROBINS-I | Moderate |
| Dell'Aquila et al. (2023) | Retrospective observational study | ROBINS-I | Moderate |
| Reis et al. (2023) | Retrospective case series | JBI case series | Low |
| Silvola et al. (2012) | Case series | JBI case series | Low |
| Gaiarsa et al. (2019) | Retrospective case series | JBI case series | Low |
| Ordonez and Molano. (2017) | Case series | JBI case series | Low |
- Note: Green - Low risk of bias; Orange - Moderate risk of bias.
Quantitative data synthesis was performed for studies reporting infection eradication rates, recurrence rates, and complication frequencies. To synthesize infection eradication rates across studies, a meta-analysis was performed using a random-effects model (DerSimonian and Laird method), which accounts for both within-study and between-study variability. This model was selected due to anticipated clinical heterogeneity across studies—such as differences in osteomyelitis etiology, surgical technique, and follow-up duration—thereby providing more conservative and generalizable pooled estimates. Statistical heterogeneity was assessed using the I2 statistic and Cochran's Q test, with τ2 representing between-study variance.
3 Results
This systematic review includes 28 clinical studies evaluating the efficacy and safety of bioactive glass S53P4 in the treatment of osteomyelitis and related bone infections, encompassing a total of 1122 patients with a mean age of approximately 43.6 years. The included studies span diverse patient populations and infection types, including chronic long bone osteomyelitis, diabetic foot osteomyelitis, septic nonunions, and mastoid infections. For clarity, findings are organized into four main themes: infection eradication and recurrence, healing outcomes and functional recovery, complication profiles, and patterns of antibiotic use (Table 2).
| Title | Author (year) | Study design | Pathology | Number of participants | Mean age ± SD | Antibiotic use | Follow-up duration | Key findings | Summary |
|---|---|---|---|---|---|---|---|---|---|
| A comparative study of the use of bioactive glass S53P4 and antibiotic-loaded calcium-based bone substitutes in the treatment of chronic osteomyelitis: A retrospective comparative study | Romanò CL, Logoluso N, Meani E, et al. (2014) | Retrospective comparative study | Chronic osteomyelitis | 76 (Group A: 27; Group B: 27; Group C: 22) | Not specified | Group A (S53P4) received no local antibiotics. Group B used antibiotic-loaded calcium sulphate, and Group C used calcium phosphate with antibiotics. | ~22 months | Group A (bioactive glass): 92.6% infection control, fewer wound complications compared to Group B (88.9%) and Group C (86.3%); follow-up mean: ~22 months. | Bioactive glass (S53P4) provided similar infection control rates as antibiotic-loaded calcium-based bone substitutes but had fewer wound complications and less drainage. |
| Pilot experience on the use of S53P4 bioactive glass in the surgical management of diabetic foot osteomyelitis | Iacopi E, Pieruzzi L, Goretti C, Piaggesi A (2022) | Pilot study (prospective) | Diabetic foot osteomyelitis | 10 | 56 ± 11 years | All underwent systemic antibiotics. | 6 months | Healing rate: 80%, mean healing time: 34 ± 2 days. No recurrences or adverse events observed. | Application of S53P4 bioactive glass in diabetic foot osteomyelitis resulted in high healing rates and reduced need for secondary surgical procedures, with no adverse effects reported during 6 months of follow-up. |
| Bioactive glass S53P4 to fill-up large cavitary bone defect after acute and chronic osteomyelitis treated with antibiotic-loaded cement beads: A prospective case series with a minimum 2-year follow-up | Kojima KE, Andrade e Silva FB, Leonhardt MdC, et al. (2021) | Prospective case series | Acute and chronic osteomyelitis | 10 | 25.4 years (range: 4–66 years) | Initially treated with antibiotic-loaded cement beads, followed by S53P4; suggests prior local antibiotics were part of the protocol. | 2 years | No reoperations; infection control in all cases; 2-year follow-up. Wound healing: 8 cases excellent, 2 good. Functional evaluation: DASH or Lysholm scores satisfactory in most cases. | Bioactive glass S53P4 combined with prior antibiotic-loaded cement beads effectively treated acute and chronic osteomyelitis, promoting bone regeneration and infection control without reoperations. |
| Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis | Lindfors NC, Hyvönen P, Nyyssönen M, et al. (2010) | Multicenter prospective case series | Chronic osteomyelitis | 11 | 48.4 years (range: 16–84) | Combined systemic antibiotics with S53P4, but S53P4 was used without local antibiotic additives. | Not clearly stated | 9/11 patients had good or excellent outcomes. Two complications (vascular and hematoma-related), no bioactive glass-related adverse effects. | Bioactive glass S53P4 was effective as a bone graft substitute for treating osteomyelitis, demonstrating infection control, bone regeneration, and high tolerance among patients. |
| Clinical outcomes and complications of S53P4 bioactive glass in chronic osteomyelitis and septic non-unions: A retrospective single-center study | Gatti SD, Gaddi D, Turati M, et al. (2024) | Retrospective observational study | Chronic osteomyelitis and septic nonunions | 38 | 45.8 ± 16.1 years | Used systemic antibiotics; S53P4 applied during debridement. | 19.8 ± 7.6 months | Infection eradication rates: 91.7% (osteomyelitis), 78.6% (nonunions); Follow-up: 19.8 ± 7.6 months. | S53P4 bioactive glass effectively managed chronic osteomyelitis and septic nonunions with high infection eradication rates and low complications. |
| Bioactive glass as dead space management following debridement of type 3 chronic osteomyelitis | Oosthuysen W, Venter R, Tanwar Y, Ferreira N (2020) | Retrospective case series | Type 3 chronic osteomyelitis | 24 | 33 years (median; range 16–45) | Not specified. | 13 months | Symptom resolution in 22/24 patients (91.6%); follow-up mean: 13 months. Only one bioactive glass-related complication (wound ooze). | Bioactive glass (Bonalive) as dead space management following debridement of type 3 chronic osteomyelitis was effective, with a high rate of symptom resolution and minimal complications. |
| Mid-term clinical results of chronic cavitary long bone osteomyelitis treatment using S53P4 bioactive glass: a multi-center study | Van Vugt TAG, Heidotting J, Arts JJ, et al. (2021) | Prospective multicenter cohort study | Chronic cavitary long bone osteomyelitis | 78 | 54 ± 18 years | Systemic antibiotic therapy used postoperatively in all patients. | 46 ± 20 months | Infection eradication: 85% overall; 89% infection-free at 1 year; 84% infection-free at 2 years; Follow-up: 46 ± 20 months. | S53P4 bioactive glass effectively eradicated infection in most cases and supported bone healing with a low complication rate. Soft tissue coverage was a major risk factor for treatment failure. |
| Treatment of cavitary bone defects in chronic osteomyelitis: bioactive glass S53P4 vs. calcium sulphate antibiotic beads | Ferrando A, Part J, Baeza J (2017) | Retrospective comparative study | Chronic osteomyelitis | 25 (Group 1: 12; Group 2: 13) | 50 ± 18 years (Group 1); 48 ± 17 years (Group 2) | Compared S53P4 to calcium sulphate antibiotic beads. Antibiotics used locally in control group. | ~23 months | Infection recurrence: 8.3% (bioactive glass) vs. 7.7% (calcium sulphate); Follow-up: ~23 months; Complication rates similar (8%). | Bioactive glass S53P4 and calcium sulphate antibiotic beads were equally effective for managing cavitary bone defects in chronic osteomyelitis, showing similar infection control and complication rates. |
| Bioactive glass, a new tool for the treatment in the diabetic foot recalcitrant osteomyelitis: A case series with 24-month follow-up | Rodríguez Á, Parra G, Cuervas-Mons M (2021) | Prospective case series | Diabetic foot recalcitrant osteomyelitis | 6 | Median: 56.5 years (range 49–63) | Patients received systemic antibiotics pre- and postsurgery. No local antibiotic delivery with S53P4. | 24 months | Healing rate: 66.6% (4/6); no infection recurrence; follow-up: 24 months; postsurgical complications: 50%. | BAG-S53P4 was effective for infection eradication and osseointegration in diabetic foot osteomyelitis, but complications related to skin coverage occurred in some cases. |
| Mastoid obliteration using S53P4 bioactive glass in cholesteatoma surgery: A 10-year single-center experience in 173 adult patients with long-term MRI controlled follow-up | Kroon VJ et al. (2022) | Retrospective cohort study | Cholesteatoma with mastoid obliteration | 173 | 42 ± 16 years | Not specified. | Mean 53 months | 10% recidivism at mean 53-month follow-up; 12% estimated 5-year recidivism; 95% Merchant grade 0–1; minor complications only. | Long-term follow-up of 173 adult patients showed that mastoid obliteration using S53P4 is safe and effective, with low recidivism, minimal complications, and good infection control. |
| Cost-effectiveness study of one-stage treatment of chronic osteomyelitis with bioactive glass S53P4 | Geurts J, van Vugt T, Thijssen E, Arts JJ (2019) | Retrospective comparative cost-effectiveness study | Chronic osteomyelitis | 50 (25 S53P4, 25 PMMA) | 52.2 ± 17.3 years | S53P4 group treated without local antibiotics; PMMA group had antibiotic-loaded beads. | Not specified | S53P4 one-stage treatment had higher eradication (92% vs. 80%), significantly lower cost (€6573), fewer surgeries, and shorter hospital stays. | One-stage treatment with S53P4 bioactive glass was both more effective and more cost-efficient than traditional two-stage PMMA treatment for chronic osteomyelitis. |
| Bioactive glass BAG-S53P4 for the adjunctive treatment of chronic osteomyelitis of the long bones | Drago et al. (2013) | Prospective observational study | Chronic osteomyelitis of the long bones | 27 | 44 ± 14 years | S53P4 used without local antibiotics. Patients received systemic antibiotics based on cultures. | 17.8 months | 88.9% infection eradication rate at mean 17.8-month follow-up; effective against multi-resistant pathogens; no local or systemic side effects observed. | BAG-S53P4 showed strong in vitro bactericidal effects and high clinical efficacy in long bone osteomyelitis without local antibiotics. |
| The use of bioactive glass S53P4 as bone graft substitute in the treatment of chronic osteomyelitis and infected non-unions—A retrospective study of 50 patients | Al Malat T, Glombitza M, Dahmen J, Hax P-M, Steinhausen E (2018) | Retrospective study | Chronic osteomyelitis and infected nonunions | 50 | Not specified | All patients received systemic antibiotics, but BAG used alone locally. | 12.3 months | At 6 months, 70.3% of bone defects healed; at 12 months, 83.3% healed. 80% of patients achieved full weight bearing within 4 months. No BAG-related complications; reinfection in 7 patients. | BAG-S53P4 was well tolerated and effective for dead space management in COM and infected nonunions. Radiographic healing observed, with no biomaterial-related complications. |
| Efficacy and safety of bioactive glass S53P4 as a treatment for diabetic foot osteomyelitis | De Giglio R, Di Vieste G, Mondello T, et al. (2021) | Retrospective observational study with control group | Diabetic foot osteomyelitis | 44 (22 BG group, 22 control) | 68.0 ± 10.2 years | All patients initially received parenteral antibiotics, followed by targeted therapy for ~2 weeks. BG group had significantly lower need for additional antibiotic cycles (13.6% vs. 45%). | 12 months | BG group had significantly higher osteomyelitis resolution (90% vs. 61.9%, p = 0.03) and reduced need for additional antibiotics (13.6% vs. 45%). No BG-related complications. | S53P4 significantly improved infection resolution in diabetic foot osteomyelitis compared to standard debridement alone and reduced the need for further antibiotic therapy, with no reported complications. |
| Regeneration of cystic bone cavities and bone defects with bioactive glass S53P4 in the upper and lower jaws | Stoor P, Apajalahti S, Kontio R (2017) | Prospective case series | Jaw cysts, benign tumors, odontogenic infections | 20 patients (21 defects) | Mean: 44 years (range: 19–69) | Not specified. | Within 2 years | 95% of defects showed successful healing with no biomaterial-associated infections despite 65% of cases having infection at time of surgery; bone regeneration observed within 2 years, with good support for adjacent teeth. | BAG S53P4 granules enabled reliable bone regeneration in infected cystic jaw defects, improving outcomes and preserving dental structures; no permanent nerve damage or infections were reported. |
| Possible advantages of S53P4 bioactive glass in the treatment of septic osteoarthritis of the first metatarsophalangeal joint in the diabetic foot | Kastrin M, Urbančič Rovan V, Frangež I (2021) | Retrospective observational study | Septic osteoarthritis in diabetic foot (first MTP joint) | 22 (S53P4: 10, control: 12) | S53P4 group: 62.1 ± 13.88; control: 57.0 ± 14.69 | Group A (S53P4) received 14 days iv antibiotics (amoxicillin-clavulanate), followed by 4 weeks oral antibiotics. | 12 months | 100% healing in S53P4 group; 75% in control group; no reinterventions or added antibiotics in S53P4 group over 1-year follow-up. | Segmental resection with S53P4 showed superior outcomes compared to standard care in diabetic foot septic osteoarthritis, with fewer complications and better joint preservation. |
| Bioactive glass S53P4: A new opportunity for the treatment in the diabetic foot osteomyelitis | De Giglio R, Stefania I, Mondello T, et al. (2018) | Case series (letter to the editor) | Diabetic foot osteomyelitis | 25 | Not specified | Systemic antibiotics used before and after surgery. No additional antibiotics in S53P4 group during follow-up. | 12 months | At a mean follow-up of 12 months, no signs of infection recurrence were observed. Partial incorporation of BAG noted on X-rays, with no osteolysis or periosteal reactions. BAG was well tolerated with no complications reported. | BAG-S53P4 was applied after debridement and antibiotic therapy in 25 patients with diabetic foot osteomyelitis, achieving promising infection control with radiographic evidence of healing and no recurrence or biomaterial-related complications. |
| The management of chronic osteomyelitis in adults: Outcomes of an integrated approach | Venter RG, Tanwar YS, Grey JP, Ferreira N (2021) | Retrospective cohort study | Chronic osteomyelitis | 80 | 36.25 ± 13.39 years | One-stage S53P4 approach with systemic antibiotics; local antibiotic not mentioned. | Not specified | 94% infection resolution, 6% complication and recurrence rate; S53P4 used for dead space management in type III Cierny–Mader cases. | Demonstrated high infection resolution and low recurrence using a single-stage protocol with S53P4 in chronic osteomyelitis patients. |
| Preservation surgery of septic osteoarthritis and osteomyelitis in the diabetic foot using S53P4 bioactive glass—A case series | Korpar I, and Frangež I (2023) | Retrospective case series | Diabetic foot osteomyelitis and septic osteoarthritis (MTP, midfoot, ankle); 10 with charcot neuroarthropathy | 16 | 61.0 ± 8.85 years | All patients received systemic antibiotics. | Not specified | 1 recurrence; 0 early complications; 1 late amputation; majority showed successful healing and limb preservation. | S53P4 was used for joint/bone preservation in the diabetic foot. No 30-day readmissions or complications; 1 late recurrence leading to amputation. Suggests safety and potential of S53P4 in tissue-sparing diabetic foot surgery. |
| Utilization of bioactive glass S53P4 inside an induced membrane for severe bone defect with high risk of infection: A multi-center preliminary experience | Aurégan JC, Villain B, Glombitza M, et al. (2022) | Retrospective multicenter case series | Critical-sized bone defects with high infection risk (e.g., chronic osteomyelitis, nonunion, open fractures) | 8 | 43 ± 23 years | Patients received systemic antibiotics tailored to cultures. | ~17 months | All patients achieved bone union (87.5%) or near-union; no recurrence of infection; no BAG-related complications over ~17 months follow-up. | S53P4 bioactive glass, used alone or with autograft, was effective and safe in induced membrane procedures for patients at high risk of infection. |
| Mastoid obliteration with S53P4 bioactive glass after canal wall down mastoidectomy: Preliminary results | Król B, Cywka KB, Skarżyńska MB, Skarżyński PH (2021) | Prospective Case Series | Chronic otitis media with cholesteatoma, post-canal wall down mastoidectomy | 11 | 50.6 years | Not specified. | 6 months | No recurrence of cholesteatoma; full healing at 6 months; reduction in cavity volume; stable audiometric results; no major complications | S53P4 bioactive glass was safe and effective for mastoid obliteration post-CWD mastoidectomy, with positive healing outcomes, no regrowth of infection, and improved anatomical reconstruction. |
| Antibacterial bioactive glass, S53P4, for chronic bone infections—A multinational study | Lindfors N, Geurts J, Drago L, et al. (2017) | Multinational observational study | Chronic bone infections | 116 | Not specified | 85% of patients were treated using one-stage surgery without local antibiotics, while 15% received local antibiotics with a two-stage protocol. | Not reported | Infection eradication was achieved in 90.4% of cases, with no bioactive glass-related adverse effects reported. | This multinational study of 116 patients with chronic bone infections demonstrated that S53P4 bioactive glass is safe and effective, achieving high rates of infection control without adverse effects. |
| The role of bioceramics in the management of osteomyelitic voids | Ferreira N, and Epstein GZ (2023) | Retrospective cohort study | Chronic Osteomyelitis (appendicular skeleton) | 41 (S53P4 group) | 30.5 ± 16.0 years | S53P4 used without local antibiotics. | Not specified | 2% recurrence rate for S53P4 group; 98% remission; well tolerated; no major S53P4-related complications | S53P4 bioactive glass demonstrated a high remission rate and low complication profile in managing osteomyelitic voids. Compared to other bioceramics (cerament, osteoset), S53P4 performed well in infection resolution and was particularly favorable for single-stage procedures. |
| Outcome and predictors of treatment failure in chronic osteomyelitis using bioactive glass granules and putty formulations | Dell'Aquila AM, Reis GNB, Cuba GT, et al. (2023) | Multicenter retrospective observational study | Chronic cavitary osteomyelitis | 92 (6 months), 78 (12 months) | 49.8 ± 21.1 years | Nearly all patients received systemic antibiotics (e.g., teicoplanin, meropenem, daptomycin); only 2/92 patients did not receive systemic antibiotics. | 6 and 12 months | Infection eradication at 6 and 12 months: 85.9% and 87.2%. Smoking and non-fermenting Gram-negative bacilli were independent predictors of recurrence. Granule-only formulation had higher recurrence. | BAG-S53P4 is effective for treating chronic cavitary osteomyelitis. Putty or combined formulations had lower failure rates. Smoking and specific bacteria were risk factors for recurrence. |
| S53P4 bioactive glass putty in the local treatment of cavitary chronic osteomyelitis | Reis GNB, Cuba GT, Targa WHC, et al. (2023) | Retrospective observational case series | Cavitary chronic osteomyelitis | 31 | 53.6 ± 24.26 years | Systemic antibiotics used in nearly all patients; 6.5% had no antibiotics, 29% monotherapy, 64.5% combination therapy. Most common: teicoplanin (41.9%), meropenem (29%), daptomycin (22.6%). | 22 months | 90.3% achieved disease-free survival; 9.7% indefinite outcomes; follow-up mean: 22 months; no heterotopic ossification or glass-related complications. | S53P4 bioactive glass putty was safe and effective in treating cavitary chronic osteomyelitis, including resistant infections. Most patients received short-course antibiotic therapy, and favorable outcomes were observed even in complex cases. |
| Mastoidectomy cavity obliteration with bioactive glass: A pilot study | Silvola JT (2012) | Case series with planned data collection | Chronic mastoid cavity infections | 14 patients, 16 ears | Not specified | Not specified. | 2.2 years | All ears became dry postoperatively. One case required reoperation due to BG leak; no chronic infections postsurgery. Mean follow-up: 2.2 years. | This pilot study showed that bioactive glass can effectively obliterate chronically infected mastoid cavities, achieving a dry and stable ear environment with minimal complications. |
| A retrospective case-series on the use of S53P4 bioactive glass for the adjunctive treatment of septic diaphyseal non-union | Gaiarsa GP, Reis PR, Kojima KE, Silva JS, Lima ALLM (2019) | Retrospective case series | Septic diaphyseal nonunion | 18 | 33.6 ± 12.6 years | Postop systemic antibiotics were administered in all cases. | Not specified | Radiological healing achieved in 17 of 18 patients (94.4%) with a mean modified RUST score of 12.3. No BAG-associated complications. | S53P4 bioactive glass was successfully used alongside internal/external fixation to manage septic nonunions, demonstrating high healing rates and effective infection control with minimal complications. |
| Bioactive glass S53P4 in treatment of osteomyelitis: A report of 6 clinical cases | Ordonez S and Molano J (2017) | Case series | Chronic osteomyelitis | 6 | Not specified | Not specified. | Not specified | All patients showed good clinical recovery without BAG-related adverse effects. Cavities were adequately filled and tolerated. | S53P4 bioactive glass demonstrated safety and effectiveness as a bone graft substitute in 6 patients with osteomyelitis, showing good radiologic and clinical outcomes without complications. |
3.1 Infection Eradication and Recurrence
S53P4 demonstrated consistently high infection eradication rates across a range of clinical settings. In chronic osteomyelitis, Romanò et al. reported a 92.6% eradication rate in patients treated with S53P4 compared to 88.9% and 86.3% in groups treated with antibiotic-loaded calcium sulphate and phosphate, respectively, over a mean 22-month follow-up [9]. Gatti et al. confirmed 91.7% eradication for osteomyelitis and 78.6% for septic nonunions after 19.8 months [10]. Van Vugt et al. reported 85% eradication in chronic cavitary osteomyelitis, with 89% of patients infection-free at 1 year and 84% at 2 years [11].
Ferrando et al. found eradication rates of 91.7% for S53P4 versus 92.3% for calcium sulphate beads over ~23 months [12]. In diabetic foot osteomyelitis, Rodríguez et al. achieved 100% infection control over 24 months, while De Giglio et al. showed 90% eradication versus 61.9% in a control group at 12 months [13, 14]. Drago et al. reported 88.9% infection control at 17.8 months in long bone infections, and Dell'Aquila et al. confirmed 87.2% eradication at 12 months across 78 patients [15, 16].
Al Malat et al. documented 83.3% healing of bone defects at 12 months with S53P4 in chronic osteomyelitis and infected nonunions [17]. Lindfors et al. (2010) reported infection control in 9 of 11 patients, while Reis et al. found a 90.3% disease-free survival rate over 22 months [18, 19]. Gaiarsa et al. (2019) demonstrated 94.4% radiographic healing in septic diaphyseal nonunions [20, 19]. Finally, the large multinational study by Lindfors et al. (2017) confirmed a 90.4% eradication rate in 116 patients with chronic bone infections [21].
A meta-analysis of 10 clinical studies involving 434 patients was performed to assess the infection eradication rates associated with bioactive glass S53P4 in the treatment of osteomyelitis. The pooled infection eradication rate, calculated using a random-effects model, was 88.1% (95% CI: 85.4%–90.4%), demonstrating consistently high effectiveness across a broad range of osteomyelitis etiologies. The common-effects model produced an identical estimate (88.1%, 95% CI: 84.6%–90.9%), with negligible between-study heterogeneity (I2 = 0.0%, τ2 = 0), as supported by the nonsignificant Q-test (Q = 4.41, p = 0.8827) (Figures 3 and 4).
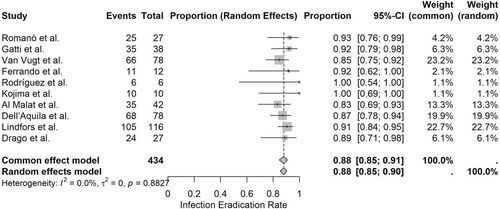
3.2 Healing Outcomes and Functional Recovery
S53P4 was associated with strong bone healing and functional recovery across multiple studies. Kojima et al. reported excellent wound healing and no reoperations over 2 years, with favorable DASH and Lysholm scores [22]. Iacopi et al. achieved an 80% healing rate in diabetic foot infections, with a mean healing time of 34 days and no complications [23]. Lindfors et al. (2010) observed good-to-excellent outcomes in 9 of 11 cases, and Rodríguez et al. documented complete healing in all patients, albeit with soft tissue complications in 50% [13, 18].
Stoor et al. noted 95% bone regeneration success in jaw cysts within 2 years [24]. Al Malat et al. showed that 80% of patients reached full weight-bearing within 4 months [17]. Król et al. observed full healing and anatomical reconstruction in 11 patients after mastoid obliteration [25]. Kastrin et al. achieved 100% healing in diabetic foot septic osteoarthritis, while Korpar and Frangež demonstrated successful limb preservation in most cases despite the presence of Charcot neuroarthropathy [26, 27].
3.3 Complications
The safety profile of S53P4 was favorable, with low complication rates across nearly all studies. Romanò et al. (2014) reported fewer wound complications in the S53P4 group than in comparator groups [13, 9]. Oosthuysen et al. observed only one case of transient wound ooze out of 24 patients [28]. Kojima et al. [22] and Reis et al. [19] reported no S53P4-related adverse events. Venter et al. recorded a low 6% recurrence rate with no major complications [29] Similarly, Silvola et al. noted only one reoperation among 14 patients treated for chronic mastoid cavity infections, with the remaining ears staying dry [30].
Some studies highlighted potential soft tissue challenges. Rodríguez et al. [13] reported a 50% complication rate due to coverage issues, while Al Malat et al. [17] noted seven reinfections in their 50-patient cohort. Nevertheless, most complications were unrelated to the bioactive material itself and were manageable with standard care.
3.4 Antibiotic Use
Antibiotic strategies varied across studies but generally involved systemic therapy in combination with S53P4 implantation. Several studies, including Drago et al. [15], Lindfors et al. [18], and Dell'Aquila et al. [16], reported using systemic antibiotics tailored to microbial culture results without local antibiotic supplementation. Romanò et al. [9] explicitly avoided local antibiotics in the S53P4 arm, while other groups like Kojima [22] used prior local antibiotic-loaded beads followed by S53P4. De Giglio et al. demonstrated that S53P4 reduced the need for additional antibiotic courses compared to controls [31].
Korpar and Frangež [27], Kastrin et al. [26], and Gaiarsa et al. [20], all used systemic antibiotics postoperatively. Reis et al. [19] found that 93.5% of their cohort received antibiotics, predominantly teicoplanin, meropenem, and daptomycin, with combination therapy in nearly two-thirds of patients. This consistent reliance on systemic antibiotics underscores the complementary role of S53P4 in infection control rather than a complete replacement for antimicrobial therapy.
Kroon et al., in their large mastoid obliteration study, did not explicitly detail antibiotic protocols but emphasized the infection-free outcomes over a mean follow-up of 53 months, suggesting effective infection management with or without systemic antibiotics [32]. In the multicenter study by Aurégan et al., systemic antibiotics were administered according to intraoperative cultures, although S53P4 was used without additional local antimicrobials, supporting its adjunctive value in high-risk infections [33]. Ferreira and Epstein similarly noted high infection resolution using S53P4 without the need for local antibiotics, instead focusing on single-stage protocols complemented by systemic therapy [34]. Ordonez and Molano did not detail antibiotic usage but reported favorable infection outcomes and radiographic healing, implying the likely integration of systemic therapy in line with standard osteomyelitis care [35].
4 Discussion
The findings of this systematic review underscore the transformative potential of bioactive glass S53P4 in osteomyelitis treatment, reinforcing its role as both an infection-fighting agent and a bone-regenerating scaffold. Unlike conventional therapies that address infection and bone loss separately, S53P4's dual mechanism allows for simultaneous bacterial eradication and structural repair, making it particularly valuable in cases complicated by multidrug-resistant pathogens or extensive skeletal defects. These results suggest that S53P4 is more than just an alternative to antibiotic-loaded materials—it represents a shift toward biomaterials that actively participate in healing rather than serving as passive fillers. By integrating antimicrobial activity with osteoconductive properties, S53P4 has the potential to simplify treatment protocols, reduce surgical interventions, and improve long-term patient outcomes in osteomyelitis management [36-45].
The pooled infection eradication rate at 12 months or longer was 88.1% (95% CI: 85.4%–90.4%) based on a meta-analysis of 10 studies, underscoring the sustained efficacy of S53P4. Follow-up durations in these studies ranged from 12 to 46 months, and eradication was maintained across this spectrum. For example, Van Vugt et al. reported an 85% eradication rate with a mean follow-up of 46 ± 20 months, while Dell'Aquila et al. [16] showed 87.2% success at 12 months in a multicenter cohort of 78 patients.
Importantly, the antimicrobial effect of S53P4 is both bacteriostatic and bactericidal, achieved not through direct antibiotic loading but via a physicochemical mechanism: upon implantation, the material releases sodium, calcium, and phosphate ions, raising local pH and osmotic pressure to levels hostile to pathogens. This effect halts bacterial proliferation (bacteriostatic) and leads to direct bacterial death (bactericidal), as supported by in vitro data and observed eradication in clinical settings. This broad-spectrum activity is especially valuable in treating multidrug-resistant organisms such as MRSA, Pseudomonas, and mixed infections. For instance, Drago et al. [15] and Dell'Aquila et al. [16] both noted high eradication rates despite polymicrobial and resistant infections, with no reported failures linked to specific pathogen types.
While many included studies support these findings, clinical success can depend on anatomical and procedural factors. The Lindfors et al. [21] multinational study, for instance, suggested that outcomes may be less favorable in cases requiring complex flap coverage. This aligns with Rodríguez et al. [13], where 50% of patients experienced soft tissue-related complications despite infection resolution. These findings emphasize the importance of integrating S53P4 within a multidisciplinary surgical plan that prioritizes adequate debridement, vascularized coverage, and possibly negative pressure wound therapy.
S53P4 also contributes to structural recovery through osteoconduction and osteostimulation. The formation of a silica-rich layer upon implantation provides a scaffold on which calcium and phosphate ions precipitate and eventually crystallize into hydroxyapatite—a critical step in bone bonding. This was evident in studies by Kojima et al. [22], Lindfors et al. [18, 21], and Stoor et al. [24], where radiographic bone formation and defect healing were consistently observed within 1–2 years.
The cost-effectiveness of S53P4 is another compelling factor in its adoption. Geurts et al. reported a €6573 cost savings per patient when using S53P4 in a one-stage protocol compared to antibiotic-loaded PMMA, primarily due to reduced need for reoperations, shorter hospital stays, and fewer systemic antibiotic cycles [7]. Notably, such analyses are currently lacking for calcium-based substitutes like calcium phosphate or sulphate, which limits head-to-head comparisons. However, Romanò et al. [9] and Ferrando et al. [12] showed similar infection control with fewer wound complications in S53P4-treated groups, indirectly supporting its clinical efficiency.
While modifying the composition of S53P4 is cautioned against—due to risks of altering its biological and mechanical properties—future research may benefit from exploring new formulations such as injectable putties, sprays, or creams. These alternatives, as discussed by Thijssen et al., could extend S53P4's utility in anatomically constrained or poorly vascularized regions, without compromising its bioactive performance [46].
There were some limitations to this review. This review focuses solely on S53P4, due to it being the most extensively studied and clinically applied bioactive glass formulation. While this provides a robust analysis of its efficacy, it may not fully capture the potential advantages or limitations of emerging bioactive glass compositions with differing resorption rates, mechanical properties, or antimicrobial mechanisms. Furthermore, the findings also reveal areas where S53P4's use may be optimized. The complications associated with soft tissue coverage, reported in 50% of cases in Rodríguez et al. [13], suggest that S53P4's success is partially dependent on the surrounding tissue environment. Future studies should explore adjunctive strategies to enhance soft tissue healing and mitigate these challenges. Additionally, while the short- to mid-term outcomes are well-documented, long-term data on the durability of S53P4's effects are limited. Understanding its performance in terms of bone remodeling, mechanical strength, and resistance to reinfection over time will be critical for its broader adoption.
In summary, S53P4 demonstrates high efficacy in infection control, promotes bone regeneration, and has a strong safety and cost-effectiveness profile. However, its optimal use requires attention to soft tissue management and anatomical context. Additional high-quality randomized trials and longer-term studies are needed to confirm its durability and broaden its indications, particularly in polymicrobial infections, diabetic foot osteomyelitis, and skull base applications.
5 Conclusion
Bioactive glass S53P4 represents a major step forward in osteomyelitis treatment, demonstrating not only high infection eradication rates but also the ability to actively support bone regeneration. Its dual antimicrobial and osteoconductive properties make it a powerful tool in managing complex cases, particularly those complicated by multidrug-resistant bacteria or significant bone loss. As a biomaterial that both eliminates infection and promotes healing, S53P4 offers a promising alternative to traditional treatments, potentially reducing the need for prolonged antibiotic use and staged surgeries. By consolidating current clinical evidence, this review provides a foundation for refining the clinical use of S53P4 and guiding future research to broaden its role in osteomyelitis management.
Author Contributions
Antoinette T. Nguyen: conceptualization, methodology, investigation, formal analysis, writing – original draft, visualization. Rena A. Li: investigation, resources, writing – review and editing. Robert D. Galiano: writing – review and editing, supervision. All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
The authors have nothing to report.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data analyzed in this study is publicly available on PubMed, Embase, and Scopus.




