Impact of Zn-Modified Hydroxyapatite Whiskers on Physicochemical and Biological Properties of Poly(ε-Caprolactone) Composites Intended for Implantable Medical Devices
Funding: This work was supported by Fundacja na rzecz Nauki Polskiej (Grant No. POIR.04.04.00-00-16D7/18).
ABSTRACT
Poly(ε-caprolactone) (PCL)-hydroxyapatite (HAP) biocomposites were produced by thermal processing to test the impact of HAP addition on the physicochemical and biological properties of PCL. Two different HAPs: zinc-modified and unmodified, were added to the polymer matrix to enhance their biocompatibility, surface properties, and antimicrobial activity. The overall properties of biocomposites were assessed by thermal and mechanical analysis, while their structure and morphology were assessed by electron microscopy and infrared spectroscopy. A short-term degradation process of the composites in terms of their medical application was carried out, and biocompatibility was investigated regarding cytocompatibility, immunocompatibility, and bactericidal activity. PCL/HAP composites with 15 wt.% HAP offer the best-balanced properties with a moderate decrease in mechanical strength, cytocompatibility, and a moderate increase in antimicrobial activity. All the composites show high cytocompatibility with both L929 fibroblasts and hFOB 1.19 human fetal osteoblasts. Zn modification promoted their antimicrobial properties, and they have been proven safe for use in a short degradation test. Therefore, the PCL/HAP and PCL/HAP_Zn biocomposites have potential for medical applications, especially for bone regeneration.
1 Introduction
Regenerative medicine utilizes various materials for restoration and defect replacement. Different materials have already been developed, including bioceramics, bioglasses, polymers, and metals [1-3]. However, specific implantable applications, together with some still existing limitations, require the search for more advanced biomaterials. For example, fixing and stabilizing implants used in the treatment of complicated bone fractures and in bone reconstruction surgery are mainly made of different metal alloys (e.g., titanium, implantable steel, magnesium) [2, 4]. The disadvantages of titanium and implantable steel implants are quite apparent and primarily relate to mechanical stresses at the interface between the implant and the bone. Additionally, there is a risk of inflammatory reactions during a long-term implantation, and patients may also require further surgery to remove the implants after the recovery process [4, 5]. While titanium and stainless steel implants are generally nondegradable and exhibit high mechanical stiffness, Mg-based alloys provide the advantage of biodegradability and mechanical properties closer to natural bone (Young modulus of elasticity) and demonstrate high osteopromotive properties [2, 6, 7]. However, some Mg-based alloys' rapid or even unpredictable degradation remains challenging in the context of simultaneous mechanical integrity and increased corrosion [7].
As a result, another solution may be implants made from biodegradable polymers and their composites, which are receiving increasing scientific attention [8]. Biodegradable and bioactive polymer-based composites designed for bone restoration consider desired structure, biocompatibility, appropriate surface chemistry for cell activity, and adequate mechanical performance [9-11].
One of the most commonly used polymers is PCL, biodegradable polyester, which offers few prominent properties, so it can be dedicated to various applications, for example, as a material for supporting tissues, soft tissues, and drug carriers [12, 13]. In addition to its easy availability and relatively low cost, PCL is noted for its biocompatibility and straightforward processing. These characteristics enable various modifications that can enhance its physical, chemical, mechanical, and biological properties. Due to its relatively low degradation rate and suitable mechanical properties, PCL is considered an excellent choice for long-term bioresorbable implants intended for hard tissues, such as bone, where the healing process typically takes longer [14, 15]. PCL's low melting processing temperature is a key feature that makes it suitable for various processing techniques, such as injection molding, electrospinning, and additive manufacturing [16-18]. This feature allows for the production of implants, such as plates or screws, with excellent mechanical properties [19, 20]. Additionally, numerous studies investigate porous PCL composites prepared through porogen leaching or phase separation techniques [21-24]. Although PCL has advantages, it is bioinert and lacks bioactivity. Therefore, research often explores PCL composites with bioactive fillers like bioglasses or hydroxyapatite (HAP) to improve bioactivity, mechanical properties, and degradation rates [25-29]. However, despite many studies in this field, it is still interesting to systematically compare a selected series of composites with different new types of fillers (especially HAP modified with metal ions with specific biological properties), emphasizing their mechanical, degradation, and bioactive properties in a controlled environment, which is the focus of our research in this paper.
HAP is essential in bio-composites for promoting bone growth. Together with collagen fibers, HAP nanoparticles are key components of bone tissue, and their synergy with the bone's hierarchical structure enhances its mechanical properties. It is important to consider the differences in composition between natural and synthetic HAP when designing new biocomposites. Synthetic HAP often lacks ions like CO32−, Mg2+, and Sr2+, which are present in natural HAP. To mimic the biological effects of natural HAP on bone processes, ionic modification is necessary [30-32]. It has been proven that substitutions in HAP crystal lattice lead to a low ion release rate, essential for long-term bone healing. Regarding the diversity of ions in the natural HAP structure, different species have been investigated, including the most prominent ions Mg2+, Na+, K+, CO32−, F−, as well as lower concentrated Sr2+, Zn2+, and Mn2+ [33-36]. Despite the critical role of different ions, zinc plays a crucial role among the various ions found in natural HAP. It is essential for the normal functioning of several enzymes, particularly those involved in bone transformation. Zinc's activity is linked to its catalytic, structural, and regulatory functions [37]. Zn2+ ions have been confirmed in the calcium-deficient areas of the HAP lattice, leading to reduced crystallinity. Despite this, Zn2+ maintains a positive interaction with microbial membranes, contributing to its antibacterial properties [38, 39].
The current study presents systematic studies on PCL filled with HAP and HAP modified with Zn2+ regarding their physicochemical and biological properties. For the first time, the fillers used were selected so that their morphology does not differ from the independently obtained synthetic HAP particles, which is often the case when modifying HAP with other ions. Thanks to this, the obtained results allowed us to describe the effect of Zn modification of HAP on the mechanical, bioactive, and degradation properties of composites, omitting the simultaneous effect of the filler morphology, as is the case in other works on composites with modified HAP particles. Our study provides insight into the interaction between degradation behavior and mechanical stability over time, which has not been comprehensively investigated for such systems in previous studies. The short-term degradation study also provided an initial assessment of PCL/HAP and PCL/HAP_Zn composites' stability and bioactivity, serving as a basis for further evaluation in medical applications.
2 Experimental Section
See Data S1.
3 Results
3.1 HAP and Zn-Modified HAP Characteristics
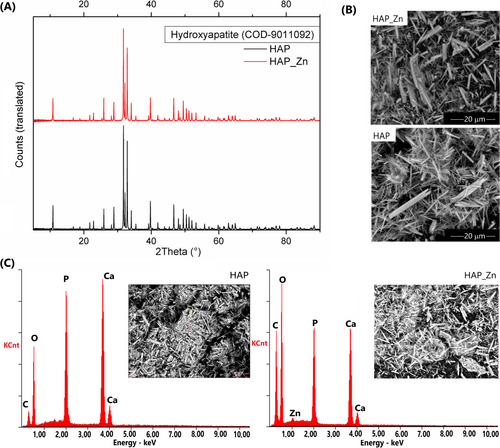
Two types of HAP powders were synthesized using a simple homogenous precipitation method. The resulting HAP fillers demonstrated a single-phase HAP product verified by x-ray diffraction patterns (Figure 1A). These patterns displayed major peaks at 2θ values of 25.8°, 28.9°, 31.8°, 32.9°, and 34.1° corresponding to the (002), (210), (211), (300), and (202) Miller planes. In the case of modified HAP, some peaks shifted slightly from the standard values due to the presence of zinc ions [40]. The chemical composition of HAP and HAP_Zn was confirmed through scanning electron microscopy (SEM)/EDS analysis (Figure 1C), revealing distinct characteristic peaks for phosphorus, calcium, oxygen, and zinc. The morphology of the synthesized particles shows no significant differences between the unmodified material and the Zn-modified version. Both products possess a heterogeneous structure comprised of whisker-like shapes (Figure 1B). The BET surface areas of the unmodified and modified powders were 5.2370 and 2.6488 m2/g, respectively. These values and the heterogeneous nature of the particles suggest that the adhesion between the polymer matrix and HAP is sufficient to create stable composites.
3.2 Structure and Morphology Analysis of PCL-Based Composites (FTIR, SEM, Wettability)
PCL composites with HAP fillers were produced using extrusion and injection molding techniques. The material composition is shown in Table 1.
| Sample name | PCL content [wt.%] | HAP content [wt.%] | HAP_Zn content [wt.%] |
|---|---|---|---|
| PCL | 100 | 0 | 0 |
| PCL/15HAP | 85 | 15 | 0 |
| PCL/15HAP_Zn | 85 | 0 | 15 |
| PCL/30HAP | 70 | 30 | 0 |
| PCL/30HAP_Zn | 70 | 0 | 30 |
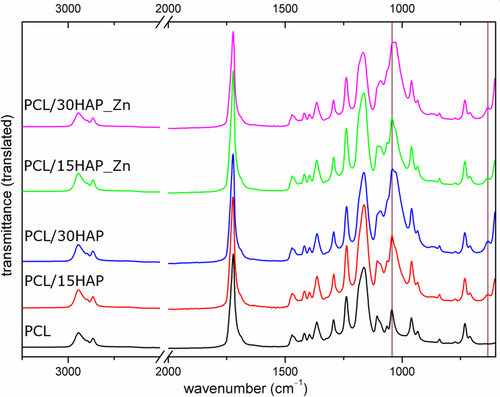
FTIR spectroscopy was used to analyze composites' structure and functional groups (Figure 2). Characteristic PCL bands were present in all samples. The most prominent ester carbonyl (CO) stretching occurs at 1721 cm−1. The CC stretching band was registered at 960 cm−1 while the CO stretching bands are observed in the wavenumber range of 1107–1240 cm−1. The double band at 731/710 cm−1, both with the signal at 1293 cm−1 are assigned to the CC stretching of the polymer's crystalline phase [41], while the band at 1163 cm−1 corresponding to the COC stretching confirms the presence of the amorphous phase. The CH stretching bands of methyl- (CH3) and methylene (CH2) groups appeared at 2895 cm−1, 2944 cm−1, and 2865 cm−1, respectively. For composites, additional signals related to the HAP presence were observed at 1092 cm−1 and 1030 cm−1 (POP), 961 cm−1 (PO43−), and 668–600 cm−1 (OH− and PO43−) [42]. These bands are partially overlapped by the polymer-derived bands, which were proved by comparison with IR of pure polymer and their greater intensity and width. No peak shift was observed that could suggest an internal relation between the filler and the polymeric matrix.
SEM was used to analyze the morphology of the PCL/HAP composites. The analysis revealed almost even filler distribution within the polymer matrix with visibly more homogeneity for composites with higher HAP proportion (Figure 3). A slight influence of material orientation was also observed due to the molding process. Moreover, the molding process influenced the surface morphology of the obtained composites, which showed no increase in roughness after adding HAP particles in the roughness test with the profilometer. The Ra parameter, which defines average surface roughness, was for pure PCL 0.12 μm, and for composites with HAP, it was in the range of 0.07–0.10 μm.

As the addition of HAP filler could affect the hydrophilicity of the composites, the water contact angle of the obtained composites was also determined. It can be observed (Table 2) that the water contact angles are similar for all composites and remain in the range of 68.3° ± 1.6° to 72.4° ± 2.0°. For composites with the addition of Zn-modified HAP: PCL/15HAP_Zn and PCL/30HAP_Zn, the water contact angle increased slightly compared to the surface of pure PCL. For composites with the addition of unmodified HAP, the contact angle increased only for the PCL/15HAP composite, while for the PCL/30HAP composite, it decreased slightly.
| Sample | Water contact angle, θ (°) |
|---|---|
| PCL | 69.7 ± 0.7 |
| PCL/15HAP | 72.1 ± 0.9 |
| PCL/15HAP_Zn | 72.4 ± 2.0 |
| PCL/30HAP | 68.3 ± 1.6 |
| PCL/30HAP_Zn | 72.0 ± 0.8 |
3.3 Thermal Properties of PCL and Its Composites
Thermal behavior under elevated temperatures of PCL-based materials was assessed by TG and DSC analysis—the results are presented in Figure 4A and Table S1. Thermal degradation for polymeric PCL matrix occurs practically in one step between 30°C and 485°C with a mass loss in the 70–100 wt.% range. The inconsiderable mass loss of about 1–2 wt.% is observed for all extruded/injected materials above 480°C, and this is related to the final decomposition of polymeric material and dehydroxylation of HAP in composites. It was observed that introducing HAP increases the temperature at 5 wt.% of mass loss, which was a useful parameter for the materials comparison. The only exception, in this case, is composite with 30 wt.% of modified HAP for which temperature at 5 wt.% of mass loss was slightly lower than for PCL. The solid residue values determined for PCL/HAP composites correspond to the filler’ content inside the polymeric matrix, around 14 wt.% for composites with 15 wt.% of HAP addition and around 26.5 wt.% for the material containing 30% of HAP.
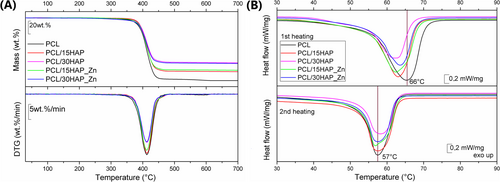
DSC curves (Figure 4B) showed a broad signal representing melting of the polymer in the temperature range of 12°C–76°C and weak ‘shoulders’ suggesting that different types of crystallites were created. The decreased crystallinity for PCL/HAP composites was observed compared to unfilled PCL. For comparative purposes, values of both heating scans were presented—the first includes the “thermal history effect,” while the second shows the behavior of material manufactured under controlled conditions during DSC measurement. The values after 1st melting and controlled cooling are significantly lower regarding both the melting temperature and corresponding enthalpy, which may come not only from different response of the material to the DSC sensor, but also it may suggest partial degradation during another thermal processing of sensitive PCL polymer. The change in polymer thermal characteristics after extrusion and followed injection is confirmed by TG and DSC parameters for virgin PCL and PCL pellets from the extrusion process. All mentioned parameters showed higher values than those for processed PCL and its composites.
3.4 Processing Parameters of PCL-Based Materials (Melt Flow Index, Vicat Softening Temperatures, Density)
In the context of the processing properties of the considered composite materials and the possibility of various shapes manufacturing, including stabilizing medical implants, a parameter determining the rheological properties of the materials—the melt flow index (MFI) (Table 3)—was investigated. Comparative studies were carried out for both unprocessed polymer and polymer subjected to the first processing step—extrusion; and for polymer and composites obtained by the double processing step—extrusion and injection into a mold. The MFI values clearly show that, for pure PCL, both the first and second heat treatment steps in the molding process lead to a slight increase in MFI. The results also show that the addition of HAP filler to the polymer matrix results in a decrease in MFI in the range of 10.3%–29.0%. The MFI values for composites containing HAP are similar to the MFI for composites containing HAP_Zn, and no consistent differences between the MFI values were observed when a modified and unmodified Zn filler was used in the composite.
| Sample | MFI (g·10 min−1) | TVicat (°C) | Density, ρbulk (g·cm−3) |
|---|---|---|---|
| PCL virgin | 14.66 ± 0.57 | — | — |
| PCL extruded | 15.48 ± 0.87 | — | — |
| PCL | 15.59 ± 1.00 | 59.1 ± 0.4 | 1.14 ± 0.02 |
| PCL/15HAP | 13.89 ± 0.50 | 58.7 ± 0.2 | 1.26 ± 0.02 |
| PCL/15HAP_Zn | 13.43 ± 0.41 | 58.9 ± 0.9 | 1.25 ± 0.03 |
| PCL/30HAP | 10.68 ± 0.24 | 58.8 ± 0.4 | 1.40 ± 0.01 |
| PCL/30HAP_Zn | 10.97 ± 0.13 | 58.3 ± 0.3 | 1.39 ± 0.02 |
A Vicat softening temperature was determined (Table 3) for the polymer and composite samples to compare the thermal properties of materials. The Vicat values stay in the range of 58.3°C–59.1°C, and composites showed slightly lower values than PCL polymer.
The determined density was also present, and as might be expected, the obtained results show an increase in the material density in proportion to the HAP addition (Table 3).
3.5 Rheological Properties
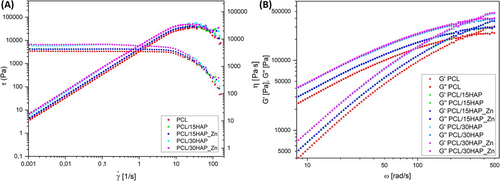
The detailed effect of HAP and HAP_Zn whiskers concentration on the viscosity of composites was determined using rotation and oscillatory tests. Figure 5A illustrates the flow characteristics of PCL composites. All samples exhibit an increase in shear stress with shear rate, which is a defining characteristic of non-Newtonian shear-thinning flow behavior. The effect of shear on apparent viscosity can be observed in the decrease of the flow curves that occur with an increase in shear strain. Doping the polymer matrix with HAP and zinc results in an increase in composite viscosity. In the case of PCL, the viscosity increases from 3350 Pa·s to a maximum of 6550 Pa·s as the HAP content rises from 0% to 30% (Table 4). With regard to the PCL/30HAP and PCL/30HAP_Zn samples, a slight increase and then a decrease in viscosity within the range of low shear rates was observed.
| Sample | η0 [Pa·s] |
|---|---|
| PCL | 3350 |
| PCL/15HAP | 4236 |
| PCL/15HAP_Zn | 4154 |
| PCL/30HAP | 5941 |
| PCL/30HAP_Zn | 6550 |
The results of the frequency sweep oscillatory shear tests carried out in the linear viscoelastic range showed an increase in the G′ and G″ moduli as the amount of filler in the composite increased (Figure 5B). Furthermore, in the low and mid frequency range, the loss modulus (G″) is the dominant parameter in all samples, defining the region of viscous behavior of the sample in the plastic state. Beyond the yield point, that is, the frequency at which the two moduli are in equilibrium (G′ = G″), the character of the sample changes and exhibits elastic behavior. The results of the determined yield stress are presented in Table 5 for ease of comparison, and the determined values are comparable for the PCL and composites. The addition of HAP and Zn fillers does not show significant differences in the flow region. Only a slight shift of the cut-off towards higher frequencies was observed for the composite samples.
| Samples | G′ = G″ | ω (rad/s) |
|---|---|---|
| PCL | 218,200 | 268.9 |
| PCL/15HAP | 260,200 | 267.6 |
| PCL/15HAP_Zn | 271,900 | 289.9 |
| PCL/30HAP | 345,200 | 277.5 |
| PCL/30HAP_Zn | 357,300 | 277.2 |
3.6 Mechanical Properties
The mechanical properties of PCL/HAP and PCL/HAP_Zn composites were examined through the static tension test and dynamic Charpy impact tests. The results, including tensile strength, Young's modulus, and Charpy impact strength, are summarized in Table S2 and Figure 6.

The tensile strength values (Figure 6A) decreased from 39.29 MPa for pure PCL to a range of 22.6–30.4 MPa for the composites, depending on the filler type and concentration. The lowest tensile strength was observed in the PCL/30HAP_Zn material, indicating that the Zn modification of HAP significantly affects the mechanical performance of PCL composites. Interestingly, in our research, the addition of 15 wt.% of Zn-modified HAP leads to a more significant decrease in tensile strength than unmodified HAP. For composites with 30 wt.% of ceramic particles, the reduction in tensile strength is similar for both types of HAP.
The results in Figure 6B indicate that the addition of apatite particles enhances the Young's modulus of the composites. Combining polycaprolactone with HAP and zinc-modified HAP leads to stiffer composites. Composites with Zn-modified HAP show a smaller increase in Young's modulus (2.13 GPa for 30% Zn-modified apatite addition) compared to those with unmodified HAP (2.43 GPa for 30% unmodified apatite).
Our results in the Charpy impact test indicate that higher HAP content weakens the material (Figure 6C). Composites with 15% filler show Charpy impact values of 6.51–6.69 kJ/m3, while those with 30% filler range from 4.73 to 4.83 kJ/m3. Additionally, composites with Zn-modified HAP exhibit slightly lower impact strength than those with unmodified HAP, confirming a minor decrease in mechanical strength for composites with Zn-modified HAP.
3.7 Short Time Degradation Process—Changes of Molecular Weight, Sample Weight, Mechanical Strength, and pH
The short degradation process was carried out for 12 weeks, and during this time, the changes in molecular weight, sample weight, mechanical strength, and pH of incubating solutions were determined. To obtain reliable results that reflect natural conditions, a short degradation process was conducted in a human body-like environment (37.5°C, PBS).
During a 12-week degradation, PCL composites with HAP or HAP_Zn in whisker form showed no significant decrease in molecular weight (Table 6). After 6 weeks, both the weight average molecular weight (Mw) and the number average molecular weight (Mn) increased slightly, with only a minor decrease observed at 12 weeks for one composite of each type.
| Sample name/degradation time [weeks] | Mw [kDa] | Mn [kDa] | Polydispersity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 0 | 6 | 12 | 0 | 6 | 12 | |
| PCL | 147.9 | 167.0 | 174.3 | 71.6 | 77.2 | 86.2 | 2.1 | 2.2 | 2.0 |
| PCL/15HAP | 158.9 | 174.9 | 171.2 | 76.2 | 90.7 | 84.2 | 2.1 | 1.9 | 2.0 |
| PCL/15HAP_Zn | 157.4 | 170.1 | 173.5 | 76.0 | 83.0 | 85.5 | 2.1 | 2.1 | 2.0 |
| PCL/30HAP | 157.4 | 172.3 | 176.1 | 77.0 | 85.5 | 86.3 | 2.0 | 2.0 | 2.0 |
| PCL/30HAP_Zn | 157.1 | 172.4 | 174.8 | 77.8 | 85.0 | 82.8 | 2.0 | 2.0 | 2.1 |
PCL composites with HAP and Zn-modified HAP also showed a slight weight loss of 0.24%–0.31%, while pure PCL had a weight loss of 0.47% during the 12-week incubation (Figure 7A, Table S3). These results indicate that the addition of fillers reduces the weight loss during degradation, suggesting that the ceramic particles do not degrade. No clear trend in weight loss was observed between composites with 15% and 30% of ceramic particles after 12 weeks of incubation, nor between the composites with unmodified HAP and Zn-modified HAP.
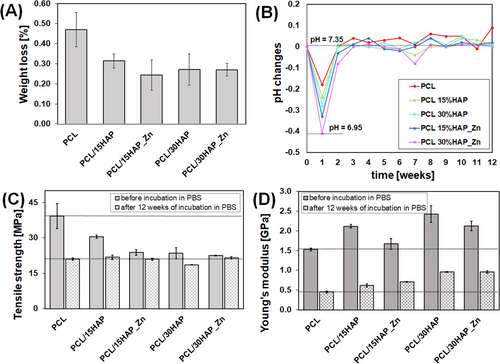
Although the weight loss of the obtained composites was small after 12 weeks of degradation, a significant reduction in mechanical strength was evident. Tensile strength significantly decreases primarily in pure PCL, and for the obtained composites, the higher filler addition causes a smaller decrease in tensile strength. In the case of composites with HAP_Zn, the changes in tensile strength are smaller both for 15% and 30% of filler addition (Figure 7C, Table S3). After 12 weeks of degradation, the tensile strength of PCL with and without fillers was nearly equal. Young's modulus after 12-week degradation process decreased by 55%–71% (Figure 7D, Table S3). The trend of higher filler content corresponding to increased Young's modulus remained consistent, with no differences between unmodified and Zn-modified HAP samples.
The short degradation process was also monitored by measuring changes in the pH of the incubating solutions. As shown in Figure 7B, the pH of the PBS incubation solution (pH = 7.35) decreased slightly at the beginning (after 1 week of incubation) but then rose back to nearly its initial state, maintaining a range between 7.28 and 7.38 for the entire 12 weeks of the study. A similar trend in pH changes was observed across all samples, with the most significant drop occurring in the composite containing 30% HAP_Zn, which decreased to a pH of 6.95 after 1 week.
3.8 Biological Properties
3.8.1 Cell Viability
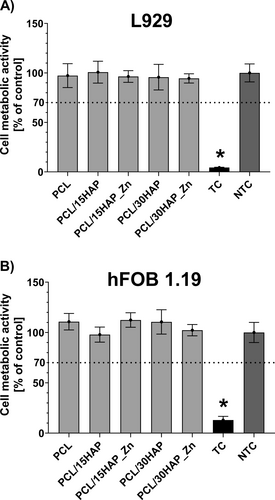
Cytosafety of tested materials was investigated using an MTT reduction assay on hFOB 1.19 and on L929 according to ISO standards—a component is considered safe if cell viability is 70% or higher compared to cell control (100%) (Figure 8). After 24 h of exposure to all investigated materials, the cell viability of L929 did not decrease below 90% (PCL 97.3% ± 12.0%; PCL/15HAP 100.8% ± 11.0%; PCL/15HAP_Zn 96.4% ± 5.9%; PCL/30HAP 95.7% ± 12.9%; PCL/30HAP_Zn 94.4% ± 4.7%). All of the investigated materials achieved cell viability hFOB 1.19 between 89% and 111% after 24 h (PCL 89.7% ± 1.34%; PCL/15HAP 97.0% ± 3.0%; PCL/15HAP_Zn 111.8% ± 0.6%; PCL/30HAP 103.5% ± 1.7%; PCL/30HAP_Zn 101.1% ± 4.3%). Based on our results, it can be concluded that PCL/HAP/Zn composites exhibit high cytocompatibility with both L929 and hFOB 1.19.
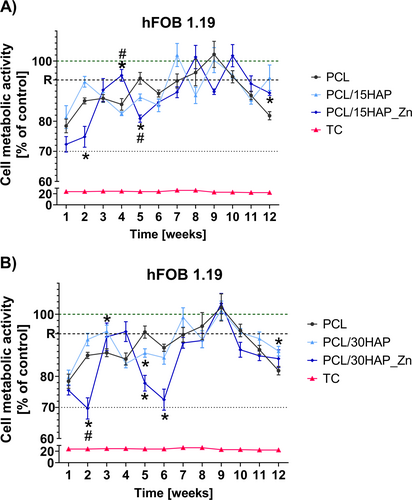
The study also aimed to assess the effect of incubating solutions on the survival of hFOB 1.19 cells during the degradation process of composites for up to 12 weeks. After 24 h of incubation, the results demonstrate that all tested post-incubation solutions were cytocompatible with hFOB 1.19 osteoblasts. However, PCL/15HAP_Zn and PCL/30HAP_Zn led to a significant reduction in cell metabolic activity, although there was no consistent trend. The greatest decline in hFOB 1.19 cell viability was observed for PCL/15HAP_Zn after incubation with 1-, 2-, and 5-week post-intubation fluids (72.3% ± 2.6%, 74.8% ± 3.5%, and 80.9% ± 1.3%, respectively) (Figure 9A) and for PCL/30HAP_Zn after incubation with 1-, 2-, 5-, and 6-week post-intubation fluids (75.5% ± 1.5%, 70.0% ± 3.4%, 77.8% ± 2.5%, and 72.5% ± 3.4%, respectively) (Figure 9B).
3.8.2 Immunocompatibility of Biomaterials With Human Monocytes
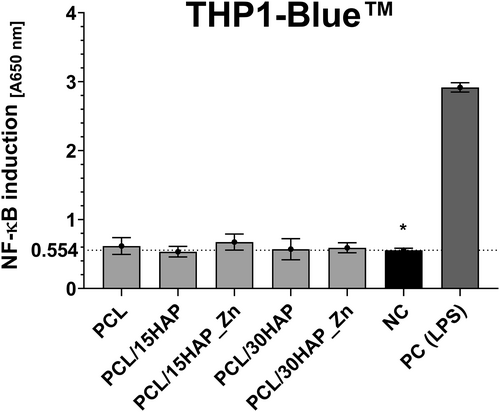
THP1-Blue NF-κB monocyte model was used to assess the in vitro immunocompatibility of PCL-based biomaterials containing zinc-modified HAP. The physiological level of NF-κB induction remained within the range of 0.554 ± 0.029 and was significantly upregulated in monocytes stimulated with E. coli LPS to 2.917 ± 0.068. None of the tested biomaterials (PCL/15HAP_Zn: 0.674 ± 0.117 and PCL/30HAP_Zn: 0.590 ± 0.072) and their reference samples (PCL, PCL/15HAP, and PCL/30HAP: 0.615 ± 0.122, 0.533 ± 0.077, and 0.569 ± 0.153, respectively) showed significant stimulation of NF-κB transcription in comparison to cells not treated with biocomposites (Figure 10). Immunocompatibility results indicate no significant proinflammatory response.
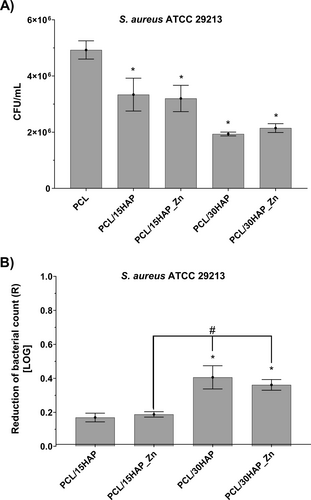
3.8.3 Comparative Analysis of CFU/mL
The antimicrobial activity was assessed by counting the reduction in the number of S. aureus after 24 h of exposure to the control and tested biomaterials (Figure 11). The results were compared with reference PCL. A reduction of bacteria by 90%, 99%, and 99.9% corresponds to a logarithmic scale decrease of 1, 2, and 3, respectively. The samples PCL/15HAP and PCL/15HAP_Zn displayed a decrease of 0.17 ± 0.03 and 0.19 ± 0.02 on the logarithmic scale in the number of S. aureus cells compared to the control PCL, resulting in a reduction of 37.8% and 40.3%, respectively. PCL/30HAP and PCL/30HAP_Zn demonstrated reductions of 0.41 ± 0.07 and 0.36 ± 0.03 on the logarithmic scale, leading to a reduction of 58.8% and 56.0% in the number of bacteria studied, respectively. As previously described, PCL shows no antimicrobial activity [43, 44]. Overall, the studies revealed that incorporating HAP and zinc into the polymer resulted in a decrease in the number of bacteria compared to the pure polymer. None of the tested samples failed to exhibit significant antimicrobial activity in the experiment.
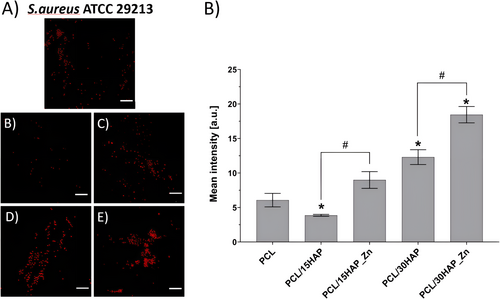
3.8.4 Confocal Imaging
To evaluate the antibacterial properties of the studied biomaterials and their modifications, confocal microscope imaging was used after prior staining of S. aureus cells with propidium iodide (Figure 12). The results have shown that biomaterials containing HAP_Zn statistically significantly increased the fluorescence intensity of S. aureus cells corresponding to the number of dead bacteria in comparison to the unmodified HAP composites. The addition of Zn caused an increase in fluorescence intensity of S. aureus cells to about 150% for PCL/15HAP_Zn (8.98 ± 1.20) and PCL/30HAP_Zn (18.45 ± 1.18) over 300% compared to PCL (6.06 ± 0.98) material. The modest antibacterial activity of PCL/HAP/Zn composites against S. aureus is enhanced by zinc incorporation.
4 Discussion
4.1 Impact of Unmodified and Zn-Modified Hydroxyapatite Whiskers on Processing Possibility and Physicochemical and Mechanical Properties of PCL Composites
The processing possibility of the obtained composites was determined by research on a parameter determining the rheological properties of materials—MFI. The result indicated that for pure PCL, a higher MFI is noted in each subsequent processing stage. This observation is most likely due to the fact that at each stage of processing, there are high thermomechanical stresses that can cause a decrease in viscosity caused by the disruption or reorganization of polymer chains. On the other hand, the MFI values were lower as HAP loading increased, regardless of whether modified or unmodified HAP was used. These results stay in agreement with literature data [45], and simultaneously proved that the use of both HAP and HAP_Zn particles makes it possible to obtain materials in which the flow parameters are at a level that still allows processing. The results obtained may be relevant for the large-scale melt mixing process of polymer composites, where the key issue is to maintain adequate melt flow to ensure good filler dispersion in the polymer matrix.
The processing possibility is confirmed by the rheological characteristics of the obtained PCL/HAP and PCL/HAP_Zn composites. The observed flow behavior (decrease of flow curves with an increase in shear strain) can be attributed to the fact that, during the shear process, the molecules are oriented more in the direction of shear, and the chains disentangle to a certain extent, thereby reducing their flow resistance and viscosity. The effect of a slight increase in the viscosity of composites with the addition of 30% fillers and subsequent decrease in their viscosity within the range of low shear rates is undoubtedly related to the strong anisotropy of the HAP whiskers, which undergo reorientation in the direction of flow.
Apart from the clearly visible orientation of HAP particles that appears during processing, an important feature of the obtained composites is the almost uniform distribution of HAP fillers within the PCL matrix, indicating good dispersion of filler throughout the entire volume of the composite. The observed slightly higher homogeneity for composites with higher filler content (30%) is probably the result of better fragmentation of filler whiskers during the processing of more filler-loaded composites, resulting in a more homogeneous polymer-ceramic mass.
Due to the potential application in implant medicine, where maintaining the interaction between tissue and implant materials is crucial, the surface of the obtained materials was characterized in roughness and wettability. According to some literature data, hydrophobic or rough surfaces can hinder cell adhesion processes, such as contact, attachment, and proliferation [46, 47]. Others indicate that different grades of surface roughness have different effects on the cells [48]. While there are various methods of designing biomaterial surfaces to control cell behavior [49], our results are consistent with previous observations and indicate that the processing method may play a significant role in the surface topography [50]. In the applied injection molding technique, the surface roughness of the obtained samples is a reproduction of the roughness of the injection mold, and HAP particles almost completely embedded in the polymer matrix do not affect the change in roughness. The influence of the applied processing method on the surface morphology of the obtained samples is also confirmed by the water contact angle results. As a rule, this parameter can depend on two factors: the hydrophilicity of the system and the roughness [51-53]. However, when access to hydrophilic HAP particles is limited by their embedding in the polymer during the processing, there is no visible change in the wettability of the composite surface.
The addition of HAP and HAP_Zn fillers into PCL also affects the mechanical properties of composites. The apatite particles act as stress concentration points, reducing impact strength [50]. The tendency of decreasing mechanical strength with an increase in the filler amount is observed for composites, and it also results from the mutual immiscibility and lack of interfacial adhesion between the polymer matrix and the filler [54, 55]. Moreover, it was already observed for other bioactive PCL/HAP composites that the presence of HAP particles induces the reduction in crystallites' size, leading to decreased tensile strength [56]. This observation was also confirmed by our DSC test results. It was shown that as the HAP content increases, the crystallinity for PCL/HAP composites decreases compared to unfilled PCL, while solid filler limits the macromolecules movement possibility and thus leads to less intensive crystallites formation.
4.2 Impact of Short Time Degradation Process of PCL/HAP and PCL/HAP_Zn on Properties of the Composites
The degradation rate of biocomposites intended for orthopedics is crucial because it must align with bone regeneration. Additionally, monitoring changes in mechanical properties, molecular weight loss, the release of low-molecular-weight degradation products, and cellular responses is essential for safety purposes [57]. PCL complete degradation may take up to 4 years, depending on molecular mass, crystallinity, and wettability [58]. However, in the initial stages of degradation, some PCL properties change and are worth noting, while others may stay unaffected.
During a 12-week degradation study involving incubation in PBS of the obtained composites, no significant changes in molecular weight were observed. This is likely due to a two-stage mechanism in the hydrolytic degradation of PCL. Water initially diffuses into the amorphous regions of the polymer due to their loose packing, leading to random chain scission of the ester groups. This scission shortens chain lengths, but it can also cause rearrangement, ordered packing, and crystallization [59, 60]. In the second step, hydrolytic attack targets the crystalline domains, leading to molecular weight changes over time. The results obtained are consistent with other research indicating no decrease in molecular weight under similar degradation conditions [61-63]. Furthermore, the slight decrease in molecular weight of our PCL composites aligns with research indicating that HAP accelerates degradation [64]. This may be due to the hydrophilic nature of HAP fillers, which enhance water absorption in the hydrophobic PCL [54]. Another explanation could involve a change in the surface area available to the solvent. When HAP particles detach from the polymer matrix after immersion in PBS, they create empty spaces, making the material more vulnerable to hydrolytic attack [65].
During a 12-week incubation in PBS, a slight weight loss of composites was observed, and the addition of fillers reduced the weight loss during degradation, suggesting that the ceramic particles did not degrade. Besides that, the small mass loss may be influenced by the fact that the susceptibility of polymer chains to breaking doesn't occur in the short incubation time. Additionally, it is well established that the structure's geometry significantly impacts the weight loss rate during in vitro incubation. Thin and porous structures tend to degrade more quickly due to the improved diffusion of degradation products, resulting in greater weight loss than thicker, solid structures [62, 66, 67].
The parameter that changes visibly during the short-time degradation process is mechanical strength. After 12 weeks of degradation, a decrease in both the tensile strength and Young's modulus was observed. Additionally, based on the observation that after 12 weeks of degradation, the tensile strength of PCL with and without fillers was nearly equal, it can be concluded that HAP and HAP_Zn whiskers certainly enhance the material's strength under degradation conditions.
During a 12-week incubation in PBS, slight changes in the pH of the incubation solution were also observed, which can be attributed to some acidic degradation byproducts. During PCL degradation, ester bonds are cleaved from the polymer backbone, shortening the chain lengths and forming capronic acid, which occurs after 1–2 weeks of degradation [59]. The local appearance of these acidic by-products can increase the acidity of the surroundings (especially if they are not removed), promoting autocatalysis, which then accelerates the degradation rate [59]. From other literature reports and our previous studies, we know that HAP particles can release alkaline ions (especially Ca2+), which can neutralize the acidic products of polymer chains' decomposition to some extent [36, 68]. Notably, in our research, degradation is very slow, and the alkaline ions released from HAP help neutralize the acidic byproducts in the second week of incubation. Based on these results, it can be concluded that the composites remain safe regarding environmental pH changes throughout the 12 weeks and that the environmental conditions will not accelerate the degradation process.
4.3 Impact of Unmodified and Zn-Modified HAP Whiskers on Biological Properties of PCL Composites
According to ISO standards—a component is considered safe if cell viability is 70% or higher compared to cell control (100%). As we considered our composites as implantable material for bone regeneration, we used the MTT reduction assay on hFOB 1.19 and on L929 to investigate the cytosafety of materials. The achieved cell viability of L929 is over 90% and hFOB 1.19 is between 89% and 111% for the tested PCL/HAP/Zn composites, indicating their cytocompatibility and potential for bone regeneration. Cell viability of over 90% after 24 h aligns with previous studies demonstrating enhanced cellular responses from HAP and zinc ions [69, 70].
In relation to the results of research on the effect of incubating solutions on the survival of hFOB 1.19 cells during the degradation process, the post-incubation fluids after 1 and 2 weeks of PCL, PCL/HAP, and PCL/HAP_Zn degradation might cause a slight decrease in the metabolic activity of cells due to lower pH levels or Zn2+ release. As has been pointed out by Kurzyk et al. [36], the viability of hFOB 1.19 was found to be dependent on Zn2+ release. It is worth noting that while there is a decrease in cell metabolic activity over time, particularly with composites containing zinc, the degradation products over 12 weeks remain within the cytocompatible range. As mentioned previously, the observed decline in cell viability is likely due to pH fluctuations and/or Zn2+ ion release, emphasizing the need for optimization to minimize potential cytotoxicity. This finding is consistent with studies indicating the importance of controlled Zn release for long-term biocompatibility [69]. Controlled Zn release is critical for the sustainable delivery of Zn2+ ions, preventing sudden spikes and associated cytotoxic effects [70]. Literature suggests that controlled release mechanisms modulate the local microenvironment, maintaining pH balance and reducing potential inflammatory responses [71]. Additionally, well-regulated Zn release is vital for promoting cellular functions like proliferation and differentiation without inducing toxicity, thus supporting tissue regeneration and healing processes [72].
Considering the interaction of the monocytes with biomaterial dedicated to the medical application which would result in NF-ĸB pathway activation and thus local inflammation or bone tissue damage consequences, it is crucial to detect probable proinflammatory effects. The obtained immunocompatibility results, which indicated no significant proinflammatory response, are crucial in the aspect of preventing implant rejection. Activation of the NF-κB pathway remains within physiological levels, consistent with findings on PCL biomaterials not significantly triggering immune responses, making them suitable for medical implants [73].
The evaluation of antibacterial properties using confocal microscope imaging indicated that the modest antibacterial activity of PCL/HAP/Zn composites against S. aureus is enhanced by zinc incorporation. This effect is well documented, as zinc ions possess intrinsic antibacterial properties, reducing bacterial viability significantly [72, 74]. Confocal imaging supports these findings, showing increased fluorescence intensity of dead bacterial cells, indicating enhanced antibacterial efficacy of PCL/HAP/Zn composites. This enhancement is crucial for preventing infections in bone implants, aligning with studies demonstrating that zinc doping significantly improves the antibacterial properties of HAP composites [69, 72, 75].
5 Conclusions
The paper presents a comparative analysis of the physicochemical, mechanical, and biological properties of PCL composites produced by mechanical processing with the addition of two types of micrometric HAPs. Fillers with a similar whiskers-like morphology differed in the presence of Zn ions in the crystal lattice, which allowed us to determine the effect of zinc doping without taking into account the aspect of filler morphology. Considering the use of this material for medical applications, the investigation on the processing parameters and the results of a short degradation test were also presented.
It was proved that mechanical processing may lead to composites with a uniform distribution of the HAP filler in the polymer mass, regardless of whether it is modified with zinc ions or not, and the addition of ceramic particles improves the wettability and roughness of the composites' surface.
In the context of processing properties, the addition of HAP particles reduces the MFI, slightly reduces the softening point according to the Vicat scale, and increases viscosity; however, for the tested material compositions (15 and 30 wt.% addition of ceramic particles to PCL) it is still possible to process them to obtain the planned shape.
DSC tests have shown that as the HAP content increases, the amount of PCL crystallites decreases, which translates into further material properties. In the static tension test and dynamic Charpy impact test, it was observed that the addition of whiskers-like apatite particles to PCL causes a decrease in mechanical strength, with a slight decrease in the mechanical strength of composites with the addition of Zn-modified HAP.
The short time degradation process of composites showed that the molecular weight of the polymer does not change significantly in a short time, the same as the weight loss of the composites. Despite this, changes in mechanical strength were clearly noticeable during the short degradation process, as a decrease in Young's modulus in the range of 55%–71% and a decrease of tensile strength, with the biggest for pure PCL. After 12 weeks of degradation, the tensile strength of PCL containing fillers and PCL without fillers was almost equal, demonstrating that under real degradation-promoting conditions, HAP and HAP_Zn whiskers have the ability to strengthen the material. Tests of pH changes of PBS incubation solutions confirmed that the degradation of the obtained PCL composites is very slow, and these tests showed that during 12 weeks of incubation, the obtained composites are safe in the context of environmental pH changes. The safety was confirmed by tests of incubating solutions containing degradation products regarding cytocompatibility with hFOB 1.19 osteoblasts.
In terms of biological response, all PCL/HAP/Zn composites show high cytocompatibility with both fibroblasts and human fetal osteoblasts, indicating their potential for bone regeneration. Immunocompatibility results indicate no significant proinflammatory response. None of the tested biomaterials showed significant stimulation of NF-ĸB transcription in comparison to cells not treated with biocomposites. Antimicrobial activity tests proved that the obtained composites caused a reduction in the number of Gram-positive S. aureus bacteria after 24 h of exposure compared to pure PCL, with the reduction of these bacteria being greater for composites with a higher HAP content. Additionally, confocal imaging showed increased fluorescence intensity of dead bacterial cells on composites containing Zn-modified HAP, indicating increased antibacterial effectiveness of PCL/HAP/Zn composites, which is crucial for preventing infections of bone implants.
Acknowledgments
This research was funded by the Foundation for Polish Science, grant number POIR.04.04.00-00-16D7/18.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data are available from the corresponding author upon reasonable request.




