Structure, dissolution behavior, cytocompatibility, and antibacterial activity of silver-containing calcium phosphate invert glasses
Abstract
Novel CaO-P2O5-Nb2O5-Ag2O invert glasses with substitution Ag2O for Nb2O5 were successfully prepared using a melt-quenching method. Ag2O in the glasses act as a network modifier oxide, playing the same role as Na2O, which breaks the phosphate chains. Analysis of the ultraviolet-visible absorption spectra of the glasses showed that the glass matrix contained ionic silver species and silver nanoparticles. Approximately 0.05 mM of Nb5+ ions released from the glasses, which would be expected to stimulate osteoblast differentiation. A glass containing 1 mol % Ag2O showed a linear increase in the releasing amount of Ag+ ions with increasing soaking time, whereas glasses containing 3–5 mol % Ag2O showed Ag+ ion concentrations of around 13 μM at day 3, and then maintained similar values until day 7. When the solution was replaced with fresh solution every 2 days, the Ag+ ion dissolution amounts indicated almost constantly 13 μM due to AgCl formation. There were no differences in the numbers of primary osteoblast cells on silver-free and silver-containing glasses after cultivation for 1–7 days. The silver-containing calcium phosphate invert glasses showed cytocompatibility with simultaneous antibacterial activity to Escherichia coli and Staphylococcus aureus. © 2017 The Authors. Journal of Biomedical Materials Research Part A published by Wiley Periodicals, Inc. J Biomed Mater Res Part A: 105A: 3127–3135, 2017.
INTRODUCTION
Implant infections are very common and account for a large majority of post-surgical complications. The incidence of biomaterial-centered infections has been reported at 5–10% for dental implants, 5–10% for fixation pins or screws, 2–4% for prosthetic hips, and 3–4% for prosthetic knees.1 These infections are caused by the colonization of bacteria on the biomaterial surfaces.2 Since antimicrobial agents are not effective against bacterial biofilms on material surfaces3; as a result, implant removal often represents the only chance to erode the infection.4 The occurrence of biomaterial-centered infection could potentially be reduced by applying an antibacterial coating on the material surfaces.5 However, the antibacterial activity of the coatings must be maintained for up to 2 to 3 weeks after the implantation to be effective.1
Upon the controlled degradation of the coating, active chemical cues can be released to induce antibacterial activity. Ag+ ions show a wide range antibacterial activities against both gram-positive and gram-negative bacteria, even when applied at low concentrations.6 Consequently, silver is one of the most widely used materials in the development of antibacterial substances5; for instance, Ag+ ions are clinically used for treating severe burns or as an active coating for catheters.7, 8 Feng et al. suggested the bactericidal mechanism of Ag+ ions, which condense the DNA molecules in bacteria so that they lose their replication abilities; the ions also interact with thiol groups in proteins to inactivate the enzyme activity of bacteria.9 However, Ag+ ions also show some toxicity. The half-maximal (50%) inhibitory concentrations of Ag+ ions were reported to be 2.77 × 10−6mol/L and 4.25 × 10−6mol/L for murine osteoblastic (MC3T3-E1) cells and murine fibroblasts (L929), respectively.10 Thus, it is important that the coating material, which carries the Ag+ ions, has excellent chemical durability with controlled degradation behavior.
Such behavior can be achieved with the use of niobium-containing phosphate invert glasses (Nb-PIGs).11, 12 Nb-PIGs consist of short phosphate groups such as ortho- and pyrophosphates (
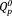 and
and
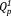 ), which are cross-linked with NbO4 tetrahedra.11, 12 Indeed, the addition of niobia was shown to significantly improve the chemical durability of phosphate invert glasses.11, 13 Specifically, the total amount of ions released from Nb-PIGs on day 7 decreased from 7.5% to 2.5% with an increase of the Nb2O5 content from 3 to 10 mol %.11 In addition, Nb-PIGs could stimulate the differentiation of MC3T3-E1 cells via the niobate ions dissolved from the glasses.14
), which are cross-linked with NbO4 tetrahedra.11, 12 Indeed, the addition of niobia was shown to significantly improve the chemical durability of phosphate invert glasses.11, 13 Specifically, the total amount of ions released from Nb-PIGs on day 7 decreased from 7.5% to 2.5% with an increase of the Nb2O5 content from 3 to 10 mol %.11 In addition, Nb-PIGs could stimulate the differentiation of MC3T3-E1 cells via the niobate ions dissolved from the glasses.14
Radiofrequency magnetron sputtering (RF-sputtering) could be used to coat implants with Nb-PIGs. For example, an amorphous calcium phosphate (ACP) film was successfully deposited onto titanium using RF-sputtering.15 The tensile bonding strength between the ACP film and titanium was >60 MPa when the film thickness was 0.5–1 μm.16, 17 In addition, the ACP film had a smooth surface, which could help to prevent the colonization of bacteria.4 However, the bonding strength of the ACP film on titanium decreased to 30 MPa after immersing in phosphate-buffered saline for 3 days, indicating the low chemical durability of the ACP film.17 Thus, given their superior chemical durability compared to ACP, Nb-PIGs could potentially be excellent candidates for the deposition of amorphous films using an RF-sputtering method. In addition, antibacterial activity can be achieved by incorporation of antibacterial inorganic ions to the Nb-PIGs.
We predicted that the addition of Ag2O to Nb-PIGs would result in antibacterial activity, and that the amount of Ag+ ion dissolution amount would be easily tunable by modulating the glass composition. In this study, new types of CaO-P2O5-Nb2O5-Ag2O invert glasses were successfully developed by substituting Ag2O for Nb2O5, and their structures and dissolution behaviors were evaluated in a Tris buffer solution (TBS). The cytocompatibilities of the glasses were examined using primary osteoblasts isolated from newborn mouse calvariae, and their antibacterial properties were examined using Escherichia coli and Staphylococcus aureus.
MATERIALS AND METHODS
Preparation of the glasses
Glasses with compositions of 60CaO·30P2O5·(10-x) Nb2O5·xAg2O (mol %, x = 0–5, denoted by xAg) and 60CaO·30P2O5·5Nb2O5·5Na2O (mol %, denoted by 5Na) were prepared with CaCO3 (99.5%, Kishida Chemical), H3PO4 (85% liquid, Kishida Chemical), Nb2O5 (99.9%, Kishida Chemical), Ag2O (99.0%, Wako Pure Chemical), and Na2CO3 (99.5%, Kishida Chemical). The reagents were mixed with distilled water into a slurry, which was dried under an infrared lamp overnight and stored at 140°C. The mixture was melted in a platinum crucible at 1500°C for 30 min and then quenched by pressing between two stainless steel plates. The composition of xAg was determined by energy dispersive X-ray spectroscopy (EDX, JED-2300, JEOL) and is expressed as the average of three samples.
Characterization of the glass structure
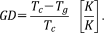 (1)
(1)The glass structures were evaluated by Fourier-transform infrared spectroscopy (FT-IR, FT/IR-4100, JASCO) in the region between 500 and 1400 cm−1, using a KBr pellet method. Solid-state 31P magic-angle spinning nuclear magnetic resonance (MAS-NMR, Varian UNITY Inova 400 plus) spectra were constructed at resonance frequencies of 161.906 MHz with 8-mm rotor spinning at 5 kHz. A single-pulse experiment was performed with a 5-μs pulse width, 60-s recycle delay, and cumulated number of 64. The chemical shift was analyzed in reference to the signal of ammonium dihydrogen phosphate (1.0 ppm). The ultraviolet-visible spectra (UV-Vis, V-550, JASCO) were measured for the silver-doped glasses (1Ag, 3Ag, 5Ag), using the spectrum of non-doped glass (0Ag) as a reference. Polished glasses with a size of 5 × 5 × 0.3 mm3 were scanned in the range of 300–600 nm (scan speed: 100 nm/min). Cross-sectional views of 3Ag were observed with a transmission electron microscope (TEM, JEM-2100 F, JEOL) at an acceleration voltage of 200 kV. The sample was prepared by milling with a focused ion beam (FIB, JEM-9320FIB, JEOL).
Ion dissolution in TBS
Glass powders were obtained by grinding and sieving (125–250 μm). The dissolution behavior of xAg was evaluated by immersing 15 mg of the glass powders into 15 mL of a 50 mM TBS (pH 7.40, 37°C) for 7 days. The concentrations of Ca2+, phosphate, Nb5+, and Ag+ ions in TBS were measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES, ICPS-7000, Shimadzu). The releasing amount of Ag+ ions was additionally measured with TBS replacement; the TBS was removed and replaced with fresh TBS at day 1, 3, and 5, and the ion dissolution amounts in the removed solution were evaluated at day 1, 3, 5, and 7 by ICP-AES. The surface of 5Ag after 7 days of soaking in TBS was observed with scanning electron microscopy (SEM, JCM-6000, JEOL) and EDX (JED-2300, JEOL).
Cytocompatibility of the glasses
Polished glasses with a size of 7 × 7 × 0.5 mm3 were prepared for cell culture tests and dry-sterilized at 180°C for 90 min. The cells were cultured in alpha-minimum essential medium (α-MEM, with l-glutamine and phenol red, Invitrogen) containing 10% fetal bovine serum (FBS, Invitrogen). The sterilized glass plates were placed into 24-well plates (n = 4), and primary osteoblasts were seeded by adding 1 mL of the culture medium containing cells at a concentration of 4 × 104 cells/mL. Primary osteoblasts were isolated from newborn mouse calvariae as described in our previous report.19 In brief, calvariae from newborn C57BL/6 mice were excised under aseptic conditions, placed in ice-cold α-MEM, and then the fibrous tissues around the bone were gently removed. The calvariae were then subjected to a series of collagenase (Wako Pure Chemical)/trypsin (Nacalai Tesque) digestions at 37°C for 15 min each. The first two digests were discarded, and the supernatants of digests 3–5 were neutralized with α-MEM and pooled. The pooled solutions were filtered using a 100-μm mesh. The filtrates were centrifuged and the resulting pellets were resuspended in α-MEM containing 10% FBS for cell culture at 37°C in a 5% CO2 atmosphere. The culture medium was replaced after 1 day and subsequently after every 2 days of culture. After 1–7 days of culture, the glasses were moved to new 24-well plates and washed twice with α-MEM containing 10% FBS. The cell numbers on the glass plates were determined with a microplate reader (Multiskan FC, Thermo Scientific) using Cell Counting Kit-8 (CCK-8, Dojindo), in accordance with the manufacturer's instructions. The number of cells was calculated by standard curve between the number of cells and the absorbance of the resulting medium at 450 nm.
Evaluation of antibacterial activity
The antibacterial activity of the glasses was evaluated using a shake method (Society of Industrial Technology for Antimicrobial Articles) as described in our previous report.20 Gram-negative and gram-positive bacteria, Escherichia coli (E. coli, strain NBRC3972) and Staphylococcus aureus (S. aureus, strain NBRC12732), respectively, were used in this work. The nutrient broth medium was prepared by dissolving 18 g of “Eiken” nutrient broth (E-MC35, Eiken Chemical) in 1 L of distilled water, followed by autoclaving sterilization at 121°C for 20 min. Powders of 0Ag, 3Ag, and 5Ag (125–250 μm) were dry-sterilized at 180°C for 90 min. Five, ten, and fifty milligrams of the glass powders were soaked in 10 mL of 1/500 nutrient broth (NB) medium with an initial concentration of 4 × 104 colony-forming units (CFU)/mL E. coli or S. aureus. The media were subsequently cultured for 24 h at 37°C in an orbital-shaking incubator (150 rpm). The numbers of colonies after cultivation were counted by a plate-count agar method; agar plates were prepared using a standard agar medium by dissolving yeast extract B2 (2.5 g, Oriental Yeast), tryptone (5.0 g, Becton, Dickinson and Co.), d(+)-glucose (1.0 g, Wako Pure Chemical), and agar (15 g, Wako Pure Chemical) in 1 L of distilled water, followed by autoclaving sterilization at 121°C for 20 min, which were then poured into plastic Petri dishes.
RESULTS
Glass structures
As quenched Ag2O containing glasses were yellowish optically clear and partially crystallized. The crystalline and opaque parts were removed manually by breaking the glasses. Following experiments, optically clear parts were used. The analyzed compositions of xAg are listed in Table 1. The amounts of Ag2O were reasonably close to that of the nominal composition, and the amounts of CaO, P2O5, and Nb2O5 varied by approximately 3.6, 5.6, and 2.5 mol %, respectively. Since the phosphorus Kα (2.013 eV) and niobium Lα (2.166 eV) peaks overlapped, their compositions may cause mismatch from the nominal ones. The powder X-ray diffraction patterns of xAg showed halo peaks (Supporting Information Fig. S1), which indicated that the materials were amorphous.
|
Glass code |
Glass Composition (mol %) | |||
|---|---|---|---|---|
| CaO | P2O5 | Nb2O5 | Ag2O | |
| 0Ag |
60 (61.0 ± 1.8) |
30 (30.0 ± 1.9) |
10 (9.0 ± 0.2) |
- |
| 1Ag |
60 (57.2 ± 2.0) |
30 (35.2 ± 1.1) |
9 (6.5 ± 0.8) |
1 (1.1 ± 0.1) |
| 2Ag |
60 (56.9 ± 0.8) |
30 (35.6 ±0.5) |
8 (5.5 ± 0.5) |
2 (2.0 ± 0.1) |
| 3Ag |
60 (56.4 ± 1.1) |
30 (34.2 ± 0.6) |
7 (5.9 ± 0.7) |
3 (3.5 ± 0.2) |
| 4Ag |
60 (56.9 ± 0.5) |
30 (35.0 ± 0.4) |
6 (4.0 ± 0.1) |
4 (4.0 ± 0.1) |
| 5Ag |
60 (56.8 ± 0.4) |
30 (35.1 ± 0.3) |
5 (3.0 ± 0.1) |
5 (5.1 ± 0.1) |
Figure 1 shows the results Tg, Tc, and GD values for xAg. Tg and Tc decreased linearly with increasing Ag2O content; the Tg and Tc values decreased from 659°C to 589°C and from 749°C to 669°C, respectively. The GD values did not significantly differ with respect to the Ag2O content, with a value of approximately 0.09 across samples. The Tg, Tc, and GD values of 5Na were 614°C, 673°C, and 0.07, respectively.
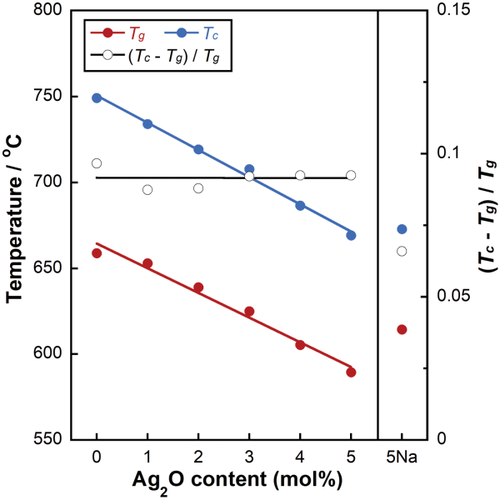
Glass transition temperature (Tg), crystallization temperature (Tc), and glassification degree (GD) for xAg as a function of Ag2O content and that of 5Na.
As shown in Figure 2, the phosphate groups in xAg showed FT-IR bands corresponding to
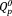 and
and
 groups, including the asymmetric stretching mode of the chain-terminating
groups, including the asymmetric stretching mode of the chain-terminating
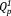 group (υas(PO3)2–, 1105 cm−1),21 the symmetric vibration mode of the
group (υas(PO3)2–, 1105 cm−1),21 the symmetric vibration mode of the
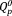 group (υs(PO4)3–, 1050 cm−1),21 the asymmetric stretching vibration of the
group (υs(PO4)3–, 1050 cm−1),21 the asymmetric stretching vibration of the
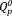 group (υas(PO4)3–, 1000 cm−1),22 the asymmetric stretching vibration of P-O-P bridges of the
group (υas(PO4)3–, 1000 cm−1),22 the asymmetric stretching vibration of P-O-P bridges of the
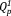 group (υas(P-O-P), 920 cm−1),21, 22 and the symmetric stretching vibrations of P-O-P linkages (υs(P-O-P), 740 cm−1).21 The niobate group showed FT-IR bands corresponding to the asymmetric stretching vibration of the Nb-O-Nb bridges of distorted NbO6 octrahedra (υas(Nb-O), 635 cm−1)22 and the asymmetric stretching vibration of Nb-O-Nb bridges in the 3 D network of regular NbO6 octahedra (υas(Nb-O), 580 cm−1).22 The bands at 550 cm−1 were assigned to the vibrational coupling of [υ(Nb-O) (medium Nb-O distances) + (O-P-O)] stretching with deformation modes.13
group (υas(P-O-P), 920 cm−1),21, 22 and the symmetric stretching vibrations of P-O-P linkages (υs(P-O-P), 740 cm−1).21 The niobate group showed FT-IR bands corresponding to the asymmetric stretching vibration of the Nb-O-Nb bridges of distorted NbO6 octrahedra (υas(Nb-O), 635 cm−1)22 and the asymmetric stretching vibration of Nb-O-Nb bridges in the 3 D network of regular NbO6 octahedra (υas(Nb-O), 580 cm−1).22 The bands at 550 cm−1 were assigned to the vibrational coupling of [υ(Nb-O) (medium Nb-O distances) + (O-P-O)] stretching with deformation modes.13
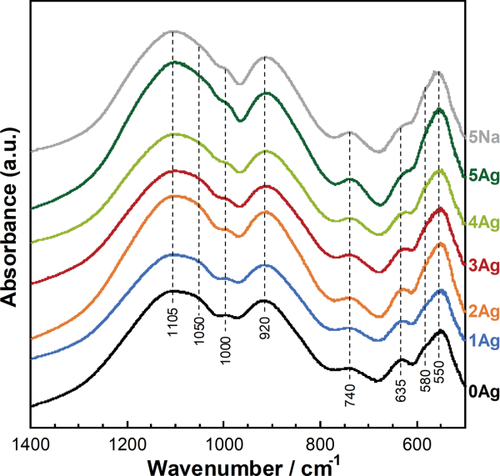
FT-IR spectra for xAg and 5Na.
The 31P MAS-NMR spectra of xAg are shown in Figure 3(a). The center peaks between 15 and −25 ppm were assigned to the
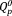 and
and
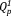 groups, and the remaining peaks observed on both sides of the center peaks were associated with spinning side bands. The experimental spectra were simulated assuming Gaussian lines for the
groups, and the remaining peaks observed on both sides of the center peaks were associated with spinning side bands. The experimental spectra were simulated assuming Gaussian lines for the
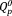 and
and
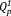 groups. With an increase in the Ag2O content, the content of the
groups. With an increase in the Ag2O content, the content of the
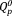 group increased from 43% to 46%, whereas that of the
group increased from 43% to 46%, whereas that of the
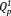 group decreased from 57% to 54% [Fig. 3(b)]. The contents of the
group decreased from 57% to 54% [Fig. 3(b)]. The contents of the
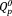 and
and
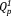 groups of 5Na were 48% and 52%, respectively.
groups of 5Na were 48% and 52%, respectively.
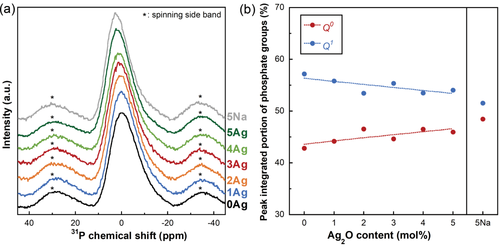
(a) 31P MAS-NMR spectra for xAg, and (b) peak integrated portions of the phosphate groups of xAg as a function of the Ag2O content and that of 5Na.
Figure 4 shows the UV-Vis absorption spectra of xAg. The absorption peaks were detected at around 430 nm and 320 nm. The wavelength below 310 nm indicates a transmittance of 0%. Figure 5 shows the TEM images of 3Ag; the nanoparticles were dispersed in the glass matrix and their average size was 23.8 ± 1.8 nm.
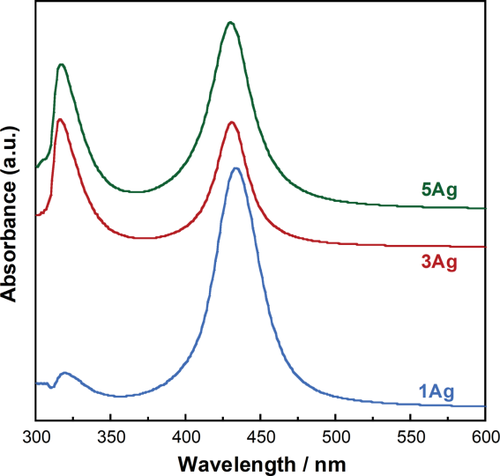
UV-Vis absorption spectra for xAg.
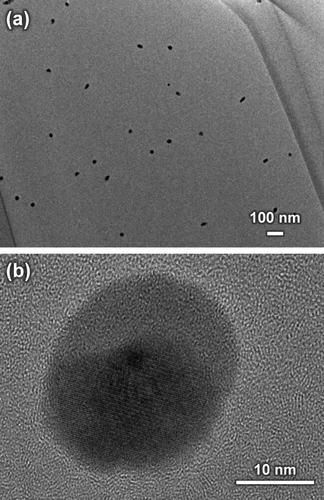
TEM images of 3Ag with (a) low and (b) high magnification views.
Dissolution behaviors of the glasses
Figure 6 shows the Ca2+, phosphate, Nb5+, and Ag+ ion dissolution profiles from xAg into TBS after 1, 3, 5, and 7 days of immersion. The release amounts of Ca2+, phosphate, and Nb5+ ions linearly increased with increasing soaking time. However, the Ag+ ion dissolution amounts of 3–5Ag after day 3 were relatively stable, with values of approximately 12–14 μM. The Ag+ ion dissolution amounts with TBS replacement are shown in Figure 7(a). The Ag+ ion concentration for 3Ag and 5Ag with TBS removed at day 1, 3, 5, and 7 was consistently 13 μM. Figure 7(b–d) shows the SEM image and EDX results of 5Ag after being soaked in TBS for 7 days; the particles composed of Ag and Cl were precipitated on the glass surface. Based on these results, 0Ag, 1Ag, and 3Ag were selected for the cytocompatibility and antibacterial activity tests, since there were no significant differences in the dissolution behaviors between 3Ag and 5Ag.
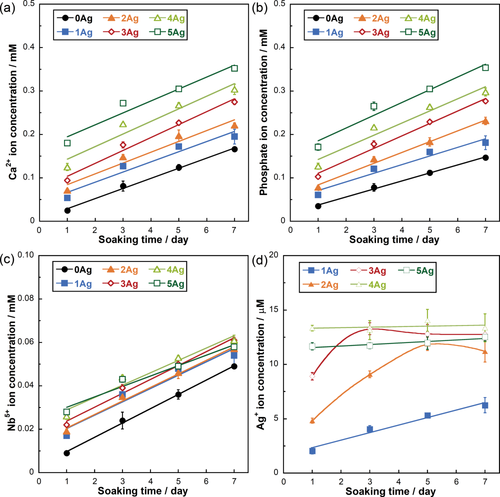
Ion release amount in TBS for (a) Ca2+, (b) phosphate, (c) Nb5+, and (d) Ag+ ions from xAg. Error bars represent standard deviations.
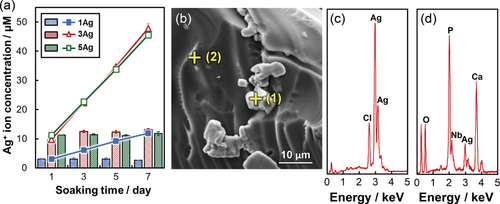
(a) Ion release amount in TBS for Ag+ ions from xAg with TBS replacement; the bars indicates the Ag+ ions concentrations in TBS removed at day 1, 3, 5, and 7, and the solid line indicates the sum of the concentrations. Error bars represent standard deviations. (b) SEM images of 5Ag after 7 days of soaking. (c) EDX spectra of (1, precipitate particles) in (b), and (d) (2, glass surface) in (b).
Cytocompatibility of the glasses
Figure 8 shows the cell numbers on 0Ag, 1Ag, and 3Ag. The number of cells increased with increasing culture period, whereas there were no significant differences in cell numbers among the samples in each culture period.
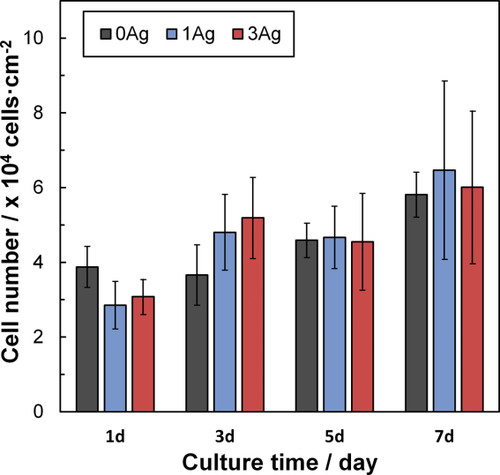
Cell numbers after 1–7 days on xAg. Error bars represent standard deviations.
Antibacterial activity of the glasses
The antibacterial activities of 0Ag, 1Ag, and 3Ag were examined against E. coli and S. aureus as shown in Figure 9. The figure represents the number of CFU after being cultivated with the glass powders for 24 h. Controls (0 and 24 h) mean the initial number of CFU and the number of CFU after being cultivated for 24 h without glass powders, respectively. There was no significant difference in the number of CFU between 0Ag and the control (24 h, without culture with the glass). The numbers of CFU for 1Ag and 3Ag were smaller than those of 0Ag and the control (24 h).
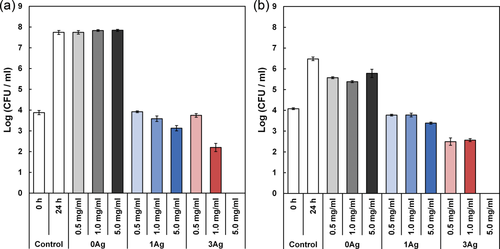
Colony forming units (CFU) of (a) E. coli and (b) S. aureus after cultivation with xAg for 24 h. “0 h control” indicates the seeded number of E. coli and S. aureus, and error bars represent standard deviations.
DISCUSSION
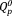 and
and
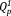 groups without Qp2 group. Thus, xAg consist of
groups without Qp2 group. Thus, xAg consist of
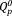 and
and
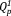 , similar to the phosphate invert glasses. The 31P MAS-NMR results showed that the
, similar to the phosphate invert glasses. The 31P MAS-NMR results showed that the
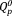 content in xAg increased with increasing Ag2O content. FT-IR band intensities corresponding to
content in xAg increased with increasing Ag2O content. FT-IR band intensities corresponding to
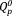 (1000 and 1050 cm−1) increased with increasing Ag2O content. This supports the 31P MAS-NMR results. Whereas, the band intensities of chain-terminating
(1000 and 1050 cm−1) increased with increasing Ag2O content. This supports the 31P MAS-NMR results. Whereas, the band intensities of chain-terminating
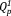 group (1105 cm−1) increased with increasing Ag2O content. The
group (1105 cm−1) increased with increasing Ag2O content. The
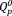 group in the phosphate invert glass was crosslinked by niobate to form O-P-O-Nb-O bond.11, 12 Thus, the band intensity of chain-terminating
group in the phosphate invert glass was crosslinked by niobate to form O-P-O-Nb-O bond.11, 12 Thus, the band intensity of chain-terminating
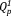 group may be influenced by
group may be influenced by
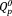 terminal in O-P-O-Nb-O bond. Ahmed et al. reported that the phosphate glass network structure was not changed by substituting Ag2O for Na2O.23 Christie et al. simulated Ag2O/Na2O-containing metaphosphate glasses; the Ag+ or Na+ ions in the glasses were bond to approximately the same number of phosphate chains.24 Ag2O in the phosphate invert glasses would act as a modifier oxide, similar to Na2O. Consequently, the FT-IR spectra of 5Ag and 5Na showed no significant differences.
terminal in O-P-O-Nb-O bond. Ahmed et al. reported that the phosphate glass network structure was not changed by substituting Ag2O for Na2O.23 Christie et al. simulated Ag2O/Na2O-containing metaphosphate glasses; the Ag+ or Na+ ions in the glasses were bond to approximately the same number of phosphate chains.24 Ag2O in the phosphate invert glasses would act as a modifier oxide, similar to Na2O. Consequently, the FT-IR spectra of 5Ag and 5Na showed no significant differences.
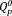 group in 5Ag indicated slightly smaller amount than that of 5Na. This may indicate 5Ag contains a smaller amount of network modifier than that of 5Na. That is, Ag+ ions in 5Ag show smaller amount than Na+ ions in 5Na, since silver nanoparticles were formed in 5Ag glass matrix. The
group in 5Ag indicated slightly smaller amount than that of 5Na. This may indicate 5Ag contains a smaller amount of network modifier than that of 5Na. That is, Ag+ ions in 5Ag show smaller amount than Na+ ions in 5Na, since silver nanoparticles were formed in 5Ag glass matrix. The
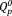 group increased slightly with increasing Ag2O content in xAg (x = 0–5), due to increase of network modifier in xAg (that is, increasing Ag+ ions in the glass network). The field strength (F) is defined as follows25:
group increased slightly with increasing Ag2O content in xAg (x = 0–5), due to increase of network modifier in xAg (that is, increasing Ag+ ions in the glass network). The field strength (F) is defined as follows25:
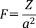 (2)
(2)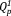 , compared with 5Na, since it contains a smaller amount of network modifier as discussed above. The GD of 5Ag (0.09) was larger than that of 5Na (0.07), since the field strength of Ag is larger than that of Na, and the amount of
, compared with 5Na, since it contains a smaller amount of network modifier as discussed above. The GD of 5Ag (0.09) was larger than that of 5Na (0.07), since the field strength of Ag is larger than that of Na, and the amount of
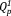 in 5Ag is slightly larger than that of 5Na. The GDs of xAg were found to be approximately 0.09, which is close to values of the previously reported phosphate invert glasses containing TiO2 (0.08) or Nb2O5 (0.09).11, 12 In addition, xAg showed comparably good glass-forming ability, even though Ag2O was substituted for Nb2O5.
in 5Ag is slightly larger than that of 5Na. The GDs of xAg were found to be approximately 0.09, which is close to values of the previously reported phosphate invert glasses containing TiO2 (0.08) or Nb2O5 (0.09).11, 12 In addition, xAg showed comparably good glass-forming ability, even though Ag2O was substituted for Nb2O5.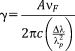 (3)
(3)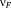 is the Fermi velocity of the free electrons (1.4 × 106 m/s for silver), c is the velocity of light (3.0 × 108 m/s),
is the Fermi velocity of the free electrons (1.4 × 106 m/s for silver), c is the velocity of light (3.0 × 108 m/s),
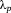 is the SPR wavelength, and Δλ is the full width at half maximum of the SPR peak. The calculated radius of 1Ag, 3Ag, and 5Ag was 4.8, 6.7, and 5.1 nm, respectively (that is, diameter of 9.6, 13.4, and 10.2 nm, respectively). The average silver nanoparticle size in 3Ag measured from the TEM images was 23.8 ± 1.8 nm. The difference between the calculated and measured diameters of the silver nanoparticles may be underestimated, since the broadening of the plasmon absorption bands can be attributed to not only the particle size but also to the size distribution and interactions between the particles.28 There was no significant difference in the silver SPR peak width and calculated particle size among the samples; this may indicate that the distribution of silver particle size was similar. The absorption peak detected around 320 nm is attributed to the presence of ionic silver species associated with aggregates (Ag0),27, 29 which may coordinate with the phosphate glass network structure. The absorption under 310 nm was constant (that is, transmittance 0%) due to the threshold absorption of the phosphate glass network matrix.21, 27
is the SPR wavelength, and Δλ is the full width at half maximum of the SPR peak. The calculated radius of 1Ag, 3Ag, and 5Ag was 4.8, 6.7, and 5.1 nm, respectively (that is, diameter of 9.6, 13.4, and 10.2 nm, respectively). The average silver nanoparticle size in 3Ag measured from the TEM images was 23.8 ± 1.8 nm. The difference between the calculated and measured diameters of the silver nanoparticles may be underestimated, since the broadening of the plasmon absorption bands can be attributed to not only the particle size but also to the size distribution and interactions between the particles.28 There was no significant difference in the silver SPR peak width and calculated particle size among the samples; this may indicate that the distribution of silver particle size was similar. The absorption peak detected around 320 nm is attributed to the presence of ionic silver species associated with aggregates (Ag0),27, 29 which may coordinate with the phosphate glass network structure. The absorption under 310 nm was constant (that is, transmittance 0%) due to the threshold absorption of the phosphate glass network matrix.21, 27It may be difficult to evaluate dissolution behavior of xAg using glass plates, due to their high chemical durability. Thereby, glass powders were used for the present test to easily estimate their dissolution behavior. The Ca2+ and phosphate ion dissolution amounts were increased with increasing Ag2O content, that is, with decreasing Nb2O5 content, in xAg; niobia significantly improves the chemical durability of the phosphate glasses.11, 13 The Nb5+ ion dissolution amount of 1–5Ag at day 7 was approximately 0.05 mM; this is expected to enhance the differentiation of osteoblasts.14, 30 The Ag+ ion concentrations of 3–5Ag after day 3 were stable with a value of 13 μM [Fig. 6(d)]. The Ag+ ion dissolution amount of 3Ag and 5Ag with TBS replacement was also 13 μM for every soaking period, and the EDX spectrum showed the peaks of Ag and Cl [Fig. 7(c)]. Saravanapavan et al. reported silver-containing sol-gel glass foams, and Ag+ ion dissolution behaviors in simulated body fluids (SBF) was maintained in equilibrium with silver chloride (AgCl) precipitation.31 Silver-doped bioactive glass also showed AgCl precipitation after being soaked in SBF.32 Thus, the Ag+ ion dissolution amount of 3–5Ag increased until 13 μM and then kept constant due to AgCl precipitation. However, the Ag+ ion dissolution amount of 1Ag was lower than 13 μM after being soaked for 7 days; this may show no AgCl precipitation. Bellantone et al. reported that Ag2O-doped bioactive glasses showed broad-spectrum bactericidal activity against E. coli, Pseudomonas aeruginosa, and S. aureus with an Ag+ ion concentration of 1–10 μM.33 The total Ag+ dissolution amount for 1Ag until day 7 was 11 μM in the TBS exchange condition, which may be sufficient to show antibacterial activity. 3–5Ag had an Ag+ ion dissolution amount of 13 μM with AgCl precipitation after day 3, which could also show antibacterial activity. However, dissolved Ag+ ions can be toxic to cells,10 and precipitated AgCl on the surface plays a substantial role in cytotoxicity.32 Hence, it is necessary for Ag2O-containing biomaterials to confirm their cytocompatibility.
The glass plates were used for the cytocompatibility test, since the application for xAg will be expected to be coating materials on the metal. There were no significant differences in the cell numbers among the 0Ag, 1Ag, and 3Ag surfaces after cultivation for 1, 3, 5, and 7 days (Fig. 8). Thus, 1Ag and 3Ag showed cytocompatibility, and the dissolved Ag+ ions from 1Ag and 3Ag showed no cytotoxicity. In our previous work, Nb-PIG containing 3 and 7 mol % of Na2O improved alkaline phosphatase (ALP) activity.14 The dissolution behavior of xAg are comparable with those of Nb-PIGs,11 since xAg and Nb-PIGs are comprised of almost similar glass structure. Accordingly, xAg were expected to enhance differentiation of osteoblast cells by dissolved Nb5+ ions from the glasses.
The antibacterial activity of the glasses was tested against E. coli and S. aureus, using shake method. This method was used as a fundamental investigation tool to monitor the effect of the glass compositions. The pH of Nb-PIGs in α-MEM after 24 h showed no significant differences between Nb-PIG containing 0, 3, and 7 mol % of Na2O.14 When phosphate invert glasses containing TiO2, which acts a similar role as Nb2O5 in the phosphate invert glasses,12 were soaked in TBS, they showed almost no pH change after 7 days, due to their high chemical durability.34 Consequently, 0Ag, 1Ag, and 3Ag in 1/500 NB media can show no significant pH change during the antibacterial test; the pH of media is considered not to be dominant source of antibacterial activity for xAg. The E. coli and S. aureus CFU of 1Ag and 3Ag after 24-h culture showed almost the same levels as the initial values. Consequently, 1Ag and 3Ag showed bacteriostatic activity. However, there were almost no bacteria left after culture with 3Ag containing a larger amount of glass powder (5 mg/mL), which indicated bactericidal activity. The Ag+ ion dissolution amounts of 1Ag and 3Ag after 1 day were 2.05 and 8.90 μM, respectively: the amount can indicate antibacterial activity.33 Consequently, dissolved Ag+ ions are dominant source for exhibiting antibacterial activity. Therefore, substituting even a small amount of Ag2O, such as 1–3 mol %, can confer a calcium phosphate invert glass with antibacterial activity without cytotoxicity.
CONCLUSIONS
In this work, Ag2O-containing calcium phosphate invert glasses were successfully developed, and their structure, dissolution behavior, cytocompatibility, and antibacterial activity were evaluated. Ag2O in phosphate invert glasses acted as a modifier of oxides, playing a similar role to Na2O. The silver in xAg existed as ionic silver species, coordinated with the phosphate network structure, and silver nanoparticles dispersed in the glass matrix. The Ag+ ion dissolution amount of 1Ag was 11 μM, and that of 3–5Ag was 13 μM after day 3 due to AgCl precipitation. Ag2O-containing phosphate invert glasses showed cytocompatibility and antibacterial activity against E. coli and S. aureus.




