Application value of molecular imaging technology in epilepsy
Abstract
Epilepsy is a common neurological disease with various seizure types, complicated etiologies, and unclear mechanisms. Its diagnosis mainly relies on clinical history, but an electroencephalogram is also a crucial auxiliary examination. Recently, brain imaging technology has gained increasing attention in the diagnosis of epilepsy, and conventional magnetic resonance imaging can detect epileptic foci in some patients with epilepsy. However, the results of brain magnetic resonance imaging are normal in some patients. New molecular imaging has gradually developed in recent years and has been applied in the diagnosis of epilepsy, leading to enhanced lesion detection rates. However, the application of these technologies in epilepsy patients with negative brain magnetic resonance must be clarified. Thus, we reviewed the relevant literature and summarized the information to improve the understanding of the molecular imaging application value of epilepsy.
Introduction
Epilepsy is a common and recurrent neurological disease, and its cause is complicated (Guerrini R, et al., 2019). If two seizures occur without obvious inducement, the risk of recurrent seizures will be 60%-90% (Fisher RS, et al., 2014). Approximately 70 million people have epilepsy worldwide (Guo K, et al., 2020). Recurrent epilepsy influences patient cognitive function (Lagogianni C, et al., 2020), language (Bartha-Doering L and Trinka E, 2014) and other functions, and some patients even have other diseases, such as depression and anxiety (Yang Y, et al., 2020). According to different forms of seizures, various auxiliary examinations and other underlying etiologies, the International League Against Epilepsy (ILAE) classified epilepsy into different types in 2017 (Figure 1). The description of clinical symptoms is helpful to diagnose epilepsy. In addition to clinical symptom analysis, electroencephalogram (EEG), iconography and cerebrospinal fluid (CSF) analyses are also included (Hussain L, et al., 2020; Luo L, et al., 2020). We summarized the diagnosis of epilepsy in Figure 2. Here, we mainly focused on the application of iconography in epilepsy. Molecular imaging, as an important branch of imaging, has been widely applied in epilepsy. The focus of different imaging techniques differs. Thus, we reviewed relevant molecular imaging studies in recent years and summarized the application value in the field of epilepsy to improve clinical work.
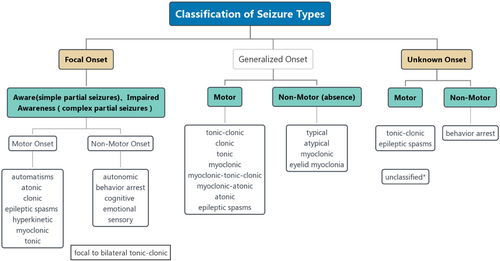
ILAE 2017 Classification of Seizure Types. Unclassified*: the seizure type cannot be placed in other categories because the information is inadequate or the onset is unknown (Fisher RS, et al., 2017).
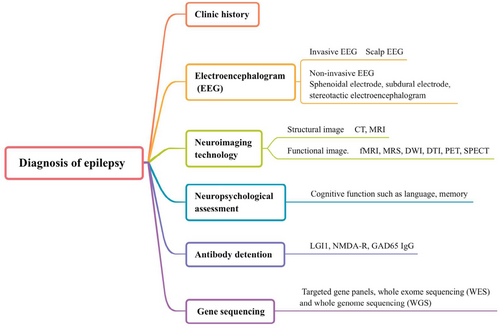
The diagnosis of epilepsy. CT: computerized tomography; MRI: magnetic resonance imaging; DTI: diffusion tensor imaging; DWI: diffusion weighted imaging; fMRI: functional magnetic resonance imaging; MRS: magnetic resonance spectroscopy; SPECT: single-photon emission computerized tomography; LGI1: leucine-rich glioma-inactivated protein 1; NMDA-R: N-methyl-d-aspartate receptor; GAD65: glutamic acid decarboxylase 65.
Neuroimaging technology
Among the traditional imaging techniques, computed technology (CT) and magnetic resonance imaging (MRI) are well established (Zeng HM, et al., 2021). CT has substantial diagnostic sensitivity for patients with epilepsy who have brain tumors or head trauma (Kvam KA, et al., 2019). MRI scans use different weighted images and flats to identify white matter and gray matter (Zeng HM, et al., 2021). New neuroimaging techniques, such as functional magnetic resonance imaging (fMRI) and related extension technology, magnetoencephalography (MEG), positron emission tomography (PET), and single-photon emission computed tomography (SPECT), are also commonly utilized methods in epilepsy diagnosis (Goodman AM and Szaflarski JP, 2021; Rowe JB. et al., 1999). Here, we mainly briefly describe the application of magnetic resonance and other new neuroimaging techniques in epilepsy. summarizes the detection indicators and tracers of various imaging techniques in epilepsy diagnosis.
| Type of examination | Content of detection | Tracer | |
|---|---|---|---|
| MRI | Regular MRI Diffusion imaging (DTI and DWI) fMRI |
Abnormal brain structure Diffusion differences of water molecules BLOD changes |
Gadolinium (Enhanced imaging) - - |
| MRS | The changes in NAA, Cr, Cho, ATP, PI and pH | - | |
| PET | Cell metabolism and blood perfusion | TSPO, 18F-FDG, 18F-FMZ | |
| SPECT | Cerebral blood perfusion | 99mTc-ECD 99mTc-HMPAO |
|
- MRI: magnetic resonance imaging; DTI: diffusion weighted imaging; DWI: diffusion weighted imaging; fMRI: functional magnetic resonance imaging; BLOD: blood oxygen level-dependent; MRS: magnetic resonance spectroscopy; NAA: N-acetylaspartate; Cr: creatine; Cho: choline; ATP: adenosine trisphosphate; PI: inorganic phosphorus; pH: pondus hydrogenii; PET: positron emission tomography; TSPO: translocator protein; 18F-FDG: 18-fluorodeoxyglucose positron emission tomography; 18F-FMZ: 18F-Flumazenilflumazenil; SPECT: single-photon emission computerized tomography; 99mTc-ECD: 99mTc-ethylene-cysteine-diethylester; 99mTc-HMPAO: 99mTc-hexamethyl-propylene-amine-oxime
Imaging technology in patients with structural lesions
For brain structural lesions, MRI is usually the first choice (Akkus Z, et al., 2017). It has high spatial resolution and no radiation, and the relationship between the abnormal structure of the brain and surrounding tissue can be detected by weighted imaging (Zucca I, et al., 2018). Conventional MRI includes T1- and T2-weighted images. T1 imaging mainly describes normal anatomy, and T2-weighted images detect changes in brain structure based on the water content (Brown MA, et al., 1999). All MRI images are affected by differences in T1, T2, and proton density in various tissues (Damadian R, 1971). Therefore, brain lesions can be identified using MRI. In addition to nonenhanced sequences, MRI also includes enhanced images. Enhanced MRI requires the injection of a specific contrast agent. Gadolinium is the commonly used contrast agent. Contrast agents disturb the magnetic properties of water protons in the body to diagnose lesions (Ibrahim MA, et al., 2020). Nonenhanced and enhanced MRI sequences are widely used in clinical work.
The application of MRI in patients with epilepsy includes three aspects: etiological diagnostics, presurgical evaluation and as a control in follow-up (Rüber T, et al., 2018). However, not all lesions detected by MRI will cause seizures (Rüber T, et al., 2018), and not all abnormal brain structures in patients can be found. Refractory epilepsy accounts for 15-30% of epilepsy patients (Minh Xuan N, et al., 2020). Malformations of cortical development (MCD) are significant pathogens that lead to refractory epilepsy (Blumcke I, et al., 2017). An accurate description of the lesion location is essential to preoperatively evaluate patients. T1 and T2 imaging distinguish the cortex and white matter and depend on differences in the myelin sheath. However, it cannot detect abnormal changes in phosphatides in myelin caused by MCD (Lampinen B, et al., 2020). The development of morphometric analysis programs (MAPs), an automated technique derived from T1-maps has helped lesion diagnosis with normal MRI, such as in patients with MCD (González-Ortiz S, et al., 2021). However, we need to be cautious about the analysis of MAP results because there are false positive results in some times (Martin P, et al., 2017). Additionally, MRI is recommended for patients with epilepsy whose EEG results are normal and for those with a first seizure (Blümcke I, et al., 2016; Minh Xuan N, et al., 2020). In addition to conventional diagnostic assistance, MRI guides new therapies for patients (Youngerman BE, et al., 2020), assess hippocampal sclerosis (HS) in patients with temporal lobe epilepsy (TLE) (Dou W, et al., 2020) and detect epileptic foci in patients with drug-refractory neocortical epilepsy (Sierra-Marcos A, et al., 2016). The volume assessment of hippocampal sclerosis is important for the treatment of patients with refractory epilepsy, but the visual assessment is flawed. The automatic segmentation algorithm of the hippocampus has shown good results in recent years (Winston GP, et al., 2013). Furthermore, brain connectivity with MRI techniques has shown promising findings. Lee et al. found differences in brain network connectivity by arterial spin labeling (ASL) MRI in TLE patients with and without HS. The main anomaly indicated that the radius and diameter measured by the global network of patients with TLE combined with HS increased (Lee DA, et al., 2021). For patients with focal epilepsy, alterations were observed in the connectivity of the default mode network (DMN). Additionally, the salience network (SN) was altered (Shu T, et al., 2020). Impaired cognitive function is one of the common comorbidities of patients with epilepsy. Epilepsy and cognitive impairment will affect each other and lead to aggravation of the disease (Helmstaedter C, et al., 2017). Yvonne Höller et al found that cognitive impairment can be predicted in TLE patients by EEG, MRI, and neuropsychology (Höller Y, et al., 2020). Thus, more methods are available to detect comorbidities of epilepsy.
Imaging technology in patients with nonstructural lesions
Structural imaging technology can be used to identify lesions; however, this technique is challenging for patients without nonstructural changes. For lesions without obvious pathologic conditions, many emerging imaging technologies have considerable advantages. However, the principle of each imaging tool differs. They detect abnormal structures according to the microstructure, metabolism, spectrum and hemodynamics (Holdsworth SJ, et al., 2008). A detailed introduction is provided in the following section.
Diffusion tensor imaging (DTI) and diffusion-weighted imaging (DWI)
DTI and DWI are new noninvasive weighted MR imaging techniques. The fractional anisotropy (FA), apparent diffusion coefficient (ADC) and mean diffusivity (MD) are the main parameters of DTI that reflect the water molecule diffusion rate (Bao Y, et al., 2018; Rodrigues NB, et al., 2018). We evaluated microstructural changes in various brain regions using DTI. More importantly, DTI shows higher sensitivity and can better identify microstructural abnormalities than conventional MRI (Bao Y, et al., 2018). FA and MD are the mostly used indicators. The values of FA and MD represent structural changes and the integrity of white matter (Carlson HL, et al., 2014; Gross DW, 2011). However, some studies have suggested that FA changes can be caused by axonal degeneration, demyelination, an increased myelin barrier and an increased cross fiber ratio (Gross DW, 2011). Therefore, the cause of the abnormal results for epilepsy patients should be carefully investigated using DTI technology.
DTI can be used to investigate microstructural abnormalities, predict postsurgical tissue function, and explore changes in brain connectivity. In a clinical study of patients with and without HS, the FA value of all patients was decreased in the ipsilateral cingulate gyrus (CG) and fornix (FORX), but that of the ipsilateral hippocampus was decreased only in patients with HS. Additionally, the changes in FA in GC and FORX were related to the frequency of seizures. In addition, the MD in the hippocampus was increased in all patients (Bao Y, et al., 2018). The outcomes were consistent with those of a previous study (Liu M, et al., 2012). White matter in the corpus callosum also significantly decreased in photosensitive epilepsy (PSE) (Du H, et al., 2014). Additionally, a change in FA value correlated with postoperative verbal fluency in TLE (Yogarajah M, et al., 2010). Carlson HL et al. found that bilateral thalamus cortical pathways in epilepsy patients were abnormal, and damage was associated with the disease course (Carlson HL, et al., 2014). DTI helps locate epileptic foci and recognizes cognitive changes between epilepsy patients and healthy individuals by analyzing these indices (A Yassine I, et al., 2018). Luis et al. found that the FA value is decreased and the relevant cognitive score is related to the FA value. Furthermore, TLE patients with mesial temporal sclerosis (MTS) may have worsened cognitive impairment (Rodríguez-Cruces NB, et al., 2018). FA and MD within the superficial white matter in the left medial temporal lobe/posterior cingulate (LMT/PC) have a strong predictive effect on verbal memory function (Chang YA, et al., 2019). In one study, the path length and connection density of 20 TLE patients were detected by DTI. The path length and connection density were found to be decreased in some brain regions (Liao W, et al., 2011). In another study, DTI and graph theoretical analysis were performed in 30 nonlesional TLE patients and the global network efficiency of white matter was decreased (Yu Y, et al., 2019).
DWI reflects the diffusion of water molecules by evaluating ADC at the microscopic level to locate the areas of cellular edema (Kanner AM, 2006; Wang R, et al., 2008). Na+/K+-ATPase activity increases in ictal epilepsy, Na+ enters cells, and K+ flows out of cells. Na+/K+-ATPase energy will decrease postseizure, leading to ion imbalance and cell edema and reduces the extracellular space. DWI shows high signal and ADC decreases in brains, representing irreversible neuronal damage (Helpern JA, et al., 1995; Mendes A, et al., 2016; Wang Y, et al., 1996). However, a study reported that ADC was increased in mesial temporal lobe epilepsy (MTLE) (Kanner AM, 2006). Thus, the value of ADC in specific situations must be analyzed. More interestingly, abnormal ADC values may not be caused by multiple seizures because a retrospective study suggested that DWI changes may also occur in a single seizure with structural abnormalities (Hübers A, et al., 2018). In a prospective study of 22 children with febrile status epilepticus (FSE), DWI was used to evaluate hippocampal signal changes in the acute phase after FSE. Twenty-seven percent of the patients had an abnormal hippocampal signal, and some patients with hyperintensity in the hippocampus developed focal epilepsy (Yokoi S, et al., 2019). In children with focal epilepsy (FE), DWI strongly predicted language function and seizure outcome after surgery (Banerjee S, et al., 2021). The performance of diffusion-weighted imaging maximum a posteriori probability (DWI-MAP) analysis can guide the surgical region to decrease postoperative neurological deficits (Lee, et al., 2019).
Magnetic Resonance Spectroscopy (MRS)
The recently described MRS technique, is used to diagnose many diseases, including brain neoplasms, multiple sclerosis (MS), epilepsy, and infectious brain lesions (Gonen OM, et al., 2020; Oz G, et al., 2014). In contrast to MRI, MRS reflects lesions through changes in the spectrum and can display changes in relevant indices in cells (Oz G, et al., 2014). Thus, MRS has been a complementary tool to diagnose disease in clinical work. MRS has two models, H-MRS and P-MRS. H-MRS tests the changes in N-acetylaspartate (NAA), creatine (Cr) and choline (CGO) metabolites (Mory SB, et al., 2003). P-MRS mainly detects phosphocreatine (PCR), ATP, inorganic phosphorus (PI) and pH (Holloway CJ, et al., 2011). Because of the physiological relationship between brain function and its metabolism, MRS is widely used in epilepsy diagnosis (Englot DJ, et al., 2012). It can detect metabolic changes in epileptic foci (Pardoe H, et al., 2014). A study found that NAA/Cr was lower in the thalamus of juvenile myoclonic epilepsy (Mory SB, et al., 2003). This result indicated that epilepsy discharges affect the function of thalamus neurons. Xu MY et al suggested that metabolic changes are negatively correlated with the duration and frequency of seizures (Xu MY, et al., 2015). In patients with epilepsy, NAA/Cr also decreased in the front of the cingulate gyrus, and the higher is the frequency of seizures, the lower is the NAA/Cr (Naldi I, et al., 2017). Overventilation causes alkaline poisoning to induce epilepsy. A previous study found that pH and PI are increased in epileptic foci (Laxer KD, et al., 1992). Additionally, the relevant MRS score can predict language, memory and cognitive damage after surgery in epilepsy patients (Hanoğlu L, et al., 2004). If the NAA/Cho+Cr ratio of the right hemisphere is higher than that of the left hemisphere preoperatively, the language will worsen after the operation. If the left hemisphere score is low, the patient's language and cognitive function will deteriorate, likely because of the brain structure (Hanoğlu L, et al., 2004).
Functional magnetic resonance imaging (fMRI)
fMRI detects deoxyhemoglobin in venous blood and depends on blood oxygen level-dependent (BLOD) changes. Oxyhemoglobin is diamagnetic, and deoxyhemoglobin is paramagnetic, leading to magnetic properties that differ in deoxygenated blood compared with those in surrounding tissues (Kwon H, 2019; Ogawa S, et al., 1990). fMRI has been applied in clinical and basic neuroscience studies. Clinically, it is used to evaluate brain function before various treatments, such as surgery. In neuroscience applications, increased understanding of neuron degeneration disorders can be acquired (Kwon H, 2019). Abnormal neuron discharge leads to changes in brain blood flow. The location of epileptic foci using fMRI is based on changes in the deoxygenated hemoglobin concentration (Moeller F, et al., 2013). Additionally, fMRI is used to locate the dominant side for memory, language and cognitive function before surgery (Sidhu MK, et al., 2018; Szaflarski JP, 2020). However, a meta-analysis suggested that compared with the intracarotid amobarbital procedure (IAP), a traditional method to identify functional areas, the consistency of memory location is inferior by fMRI (Massot-Tarrús A, et al., 2020). In 2017, the American Academy of Neurology highlighted that patients with MTLE can undergo fMRI to replace IAP for lateral memory function (Szaflarski JP, et al., 2017). A large-sample study indicated the consistency of up to 91% of fMRI and IAP tests for locating language and predicting language function postoperatively (Janecek JK, et al., 2013). The 2017 guidelines of the American Academy of Neurology also suggested that individuals with MTLE, generalized epilepsy, or extratemporal epilepsy can consider fMRI as a substitute for IAP to test language function and predict language deficit (Szaflarski JP, et al., 2017). A study based on fMRI found that the brain connectivity of the lesion region is weakened and that cognitive function is damaged in patients with TLE (Guo L, et al., 2017). Thus, cognitive disorders in epilepsy patients are associated with brain connectivity and activation disorders. As mentioned previously, some authors suggest that fMRI can be used instead of the IAP test. However, some scholars believe that many studies comprised small samples or had a single-center design, lacking sufficient evidence to prove the advantages and disadvantages of the two methods. Thus, they believe that IAP remains the main technical means of preoperative evaluation (Szaflarski JP, 2020). However, fMRI may warrant further future development because it is noninvasive and sensitive. Additionally, fMRI can be applied in studies on network connectivity. In a study of patients with intractable mesial temporal lobe epilepsy (MTLE) and benign epilepsy with centrotemporal spikes (BECT), fMRI was used to analyze functional network connectivity patterns of MTLE and BECT. Different brain network patterns were found between MTLE and BECT. The main difference was cortical-subcortical hypoconnectivity in MTLE, but BECT was normal, and the authors hypothesized that the different outcomes of these two diseases might be attributed to different brain network patterns (Fu C, et al., 2021). In epilepsy patients with depressive dysfunction, Chen et al observed changes in the amplitude of low-frequency fluctuations (ALFFs) and regional homogeneity (ReHo), reflecting alterations in brain activity. Alterations in brain activity and dysfunction of the DMN have been demonstrated (Zhu X, et al., 2018).
Positron emission tomography (PET)
PET is a quantitative and qualitative method that detects cell metabolism and blood perfusion using tracers (Spencer SS, 1994). It is widely used to diagnose various diseases, including epilepsy (Jones T, et al., 2012). Its images mainly rely on the intravenous injection of contrast agent to measure changes in cerebral blood flow and metabolism (Sarikaya I, 2015). Tracers such as those detecting the expression of translocator protein (TSPO), 18-fluorodeoxyglucose positron emission tomography (18F-FDG), and 18F-flumazenil (18F-FMZ) are commonly used. PET plays a vital role to diagnose epilepsy. TSPO, a biomarker of neuroinflammation, is mainly expressed on microglia and astrocytes (Li F, et al., 2016). To date, TSPO has two different radioligands, first-generation ([11C]PK11195) and second-generation ([18F]FEPPA, [18F]PBR-111, [11C]PBR28, and [11C]DPA-713) (Scott G, et al., 2017). William H. Theodore and his team found that TSPO increased on both ipsilesional sides (Gershen LP, et al., 2015). Another study in 2019 showed that TSPO has higher binding with radioligands ([11C]PBR28, DPA-713) in neocortical epilepsy (Dickstein LP, et al., 2019). These studies suggested that neuroinflammation may be a mechanism of seizures. However, TSPO will combine with benzodiazepine drugs, which interfere with TSPO sensitivity. Thus, patients who will undergo PET must discontinue using relevant drugs (Kalk NJ, et al., 2013).
18-FDG-PET is commonly used clinically (Spencer SS, 1994). Brain blood perfusion and metabolism are enhanced in ictal epilepsy. PET scan shows high metabolism. During an interictal attack, large neurons are inactivated, cerebral blood flow perfusion is reduced, and the lesion shows low metabolism (Schur S, et al., 2018). Zeenat Jaisani et al showed that the low metabolic area is consistent with the abnormal parts of MRI pathological structure and that the severity of metabolic changes is affected by the pathological structure of MRI. Additionally, PET has significant value for the prognosis of epilepsy operations. Functional structural changes caused by TLE will further lead to abnormalities of the thalamus, and abnormal metabolism of these extratemporal parts can be used as a predictor of a poor prognosis after surgery (Jaisani Z, et al., 2020). A meta-analysis showed that refractory TLE patients with ipsilateral hypometabolism had better postoperative outcomes (Willmann O, et al., 2007). Furthermore, PET showed hypermetabolism interictal refractory epilepsy, which is related to the peak frequency of EEG (Bansal L, et al., 2016). Another study also found that interictal PET shows low or high metabolism, and high metabolism during interictal PET is helpful to locate epileptic foci (Schur S, et al., 2018). 18-FDG is widely used in imaging technology. However, 18F-FDG is not the best tracer for patients with epilepsy. As observed for MRI or histopathology, changes in glucose metabolism and impaired hippocampal sclerosis re inconsistent (Hodolic M, et al., 2016). Thus, further study of 18-FDG-PET is warranted.
PET is also used for reoperation evaluation. One study showed that when MRI-negative, PET-positive patients receive operative treatment, their seizure-free rate is similar to that of MTLE during the follow-up (Capraz IY, et al., 2015). John S. Duncan et al. found that FDG-PET could identify 53% of epileptic foci in drug-refractory epilepsy patients who were MRI-negative (Rathore C, et al., 2014). Another study also suggested that the surgical effect is satisfactory for TLE patients with a negative MRI and a positive PET (Yang PF, et al., 2014). These results prove that MRI-negative structural pathological changes cannot be used as an evaluation criterion to exclude surgery. PET can be used as an evaluation method for surgery. However, John S. Duncan et al believed that no technology provides accurate data to evaluate the further treatment of patients. Normal or altered PET metabolism cannot be used as an absolute standard for surgery in patients with focal epilepsy (Rathore C, et al., 2014). Recently, PET-related fusion technology has improved the diagnostic rate of lesions. A previous study on extratemporal epilepsy showed that PET-MRI increased the detection rate of epileptic foci from 68.6% to 94% (Ding Y, et al., 2018). The application of PET-MRI will improve the spatial resolution of PET and diagnostic accuracy of epileptic foci. Additionally, PET imaging observes the therapeutic effect and mechanism of epilepsy. Du Ruili et al used PET to observe the metabolic changes of TLE rats transplanted with human neural stem cells (NSCs) and GABA precursor cells (GPCs). The two methods decreased seizures. The interictal metabolism of epileptic foci in the NSC group was significantly improved, while no significant change was found in the GPC group (Du R, et al., 2019). This finding implies that both cell types can be used to treat epilepsy, but the intervention mechanisms differ. The current evaluation methods of PET mainly rely on visual evaluation by professional imaging doctors and are easily affected by the subjectivity of observers. Therefore, some scholars have combined the standardized uptake value (SUV) and partial volume correction (PVC) to quantitatively evaluate PET results and reflect them more rigorously and objectively (Peter J, et al., 2016).
Single-Photon emission computed tomography (SPECT)
With the development of molecular imaging, the epileptic foci detection rate has increased; however, their inspection principles differ. MRI mainly shows the abnormal structure of brain tissue, PET detects changes in brain tissue metabolism, and SPECT detects changes in cerebral blood perfusion (Spencer SS, et al., 1995). SPECT is the only technique that detects cerebral blood perfusion changes in interictal epilepsy. SPECT injects radioactive developer through the vein, and developer quickly passes the blood-brain barrier (Ergün EL, et al., 2016). Imaging shows cerebral blood flow perfusion, and the distribution of the developer is proportional to the cerebral blood flow (Ergün EL, et al., 2016). Abnormal neuronal discharge in ictal epilepsy leads to increased cerebral blood flow, and SPECT scanning shows high perfusion. By contrast, perfusion will be reduced during interictal epilepsy (Setoain X, et al., 2014).
The sensitivity of SPECT is well established. The sensitivities of interictal and ictal epilepsy are 66% and 90%, respectively, for TLE. The interictal and ictal sensitivities are 60% and 81%, respectively, in extratemporal lobe epilepsy (Spencer SS, et al., 1995). Another SPECT analysis showed that the diagnostic accuracy of TLE is 90% and that of extratemporal epilepsy is 70% - 83% (Ergün EL, et al., 2016). One meta-analysis showed that SPECT sensitivity is 97% in the seizure phase and 44% in the interictal phase (Sr DMD, et al., 1998). The diagnostic rate of the interictal phase is lower than that of the episodic phase. Thus, the interictal phase is usually used as a basic control to evaluate the imaging results of the episodic phase (Ergün EL, et al., 2016). The different sensitivities and specificities of SPECT for epileptic foci detection may be related to blood perfusion, blood flow distribution, attack time and imaging technologies (Tepmongkol S, et al., 2015). A study suggested that the correct location of epileptic foci is related to the injection time of the tracer. If the injection time surpasses 35 seconds, epileptic foci will migrate, and the detection accuracy will decrease (Ramchuankiat S, et al., 2017). If the time can be controlled to within 15 seconds after seizures, the accuracy will be improved (Theodore WH, 2017). Some scholars advocate using an automatic injection system for developers to shorten the injection time, reduce the measurement error and improve the detection rate (Setoain X, et al., 2012). The commonly used tracers for SPECT include I-123 and 99mTc-labeled developers. I-123 has poor stability and cannot display the brain condition during perfusion. Therefore, Tc-99m is more commonly used and becomes the preferred choice for SPECT (la Fougère C, et al., 2009). Tc-99m-labeled developers include HMPAO and ECD. In vitro, the stability of 99mTc-ECD is better than that of 99mTc-HMPAO. Thus, 99mTc-ECD is commonly used in interictal epilepsy (Setoain X, et al., 2014). However, some studies have shown that the perfusion of Tc-99mHMPAO is better than that of 99mTc-ECD during seizures in patients with TLE or cortical epilepsy (Lee DS, et al., 2002). Clinicians should choose suitable tracers according to actual needs. Additionally, the development of SPECT fusion technology has significantly improved the detection of lesions and plays a crucial role in more fields.
Conclusion and future perspective
Presently, the number of patients with epilepsy is gradually increasing. The timely diagnosis of epilepsy is critical for patient treatment. Molecular imaging technology plays a vital role in the diagnosis of epilepsy and its comorbidities. Each imaging technology has advantages, but they also have shortcomings. MRI mainly detects brain structure and is easily performed and widely used clinically; however, identifying abnormal tissue without structural lesions is challenging. The structure of white matter and integrity of fiber bundles are detection indicators of DTI, while the sensitivity to gray matter is not good. DWI mainly focuses on the diffusion movement of water molecules. The shortcoming is the presence of a substantial number of artifacts during DWI with slight motion of the patients (Holdsworth SJ, et al., 2008). MRS assesses the characteristics of metabolites in the brain. Homogenizing the magnetic field and suppressing water and fat are necessary for MRS scans (Saindane AM, 2015). fMRI mainly observes changes in BLOD in the brain, but its temporal resolution is worse than that of neuroelectrophysiology (Goodman AM, et al., 2021). PET and SPECT focus on metabolism and cerebral blood flow. These two techniques may be useful to detect seizure zones in ictal and interictal phases; however, their price make them an unsatisfactory choice for patients (Tsougos I, et al., 2019; Zeng HM, et al., 2021). Epilepsy is a common disease and has a long treatment period. Although modern imaging techniques have been widely used clinically and experimentally, new methods are warranted in the future, not only the analysis of traditional indexes. At the same time, the comorbidities of epilepsy must be clarified, such as depression, anxiety and cognitive dysfunction, which are adverse for patients. Molecular imaging technology is expected to analyze these abnormalities in a timely manner in the future. Epilepsy is a network disease. Allone et al. analyzed the cognitive function of patients with TLE and found that some patients have changes in the network structure (Allone C, et al., 2017). Although brain network changes in epilepsy patients with or without comorbidities have been proposed in the past few years, but most studies did not conduct dynamic observations. We believe that observing network changes with functional imaging technology during the formation of epilepsy comorbidities and whether active treatment can reverse the changes is more conducive to understanding the pathogenesis and achieving better treatment.
Ethical statement
Not applicable.
Acknowledgments
Not applicable.
Conflict of interest
No conflicts of interest were declared.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81660227) and Guizhou Provincial Graduate Workstation (No. GZZ2017004).
Transparency statement
All the authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Authors' contribution
Rong Yan, Hai-Qing Zhang designed and written this article, Rong Yan, Hai-Qing Zhang, Jing Wang, Yong-Su Zheng, Zhong Luo, Xia Zhang, Zu-Cai Xu contributed to collection and analysis of literature. Zu-Cai Xu revised the manuscript and approved the final version.




