A network pharmacological approach to investigate the pharmacological effects of CZ2HF decoction on Alzheimer's disease
Abstract
Background
Alzheimer's disease (AD) is the most common type of dementia, which brings tremendous burden to the sufferers and society. However, ideal tactics are unavailable for AD. Our previous study has shown that CZ2HF, a Chinese herb preparation, mitigates cognitive impairment in AD rats; whereas, its detailed mechanism has not been elucidated.
Methods
Public databases were applied to collect and identify the chemical ingredients of eight herbs in CZ2HF. Criteria of absorption, distribution, metabolism, and excretion was used to screen oral bio-availability and drug-likeness. STITCH database and Therapeutic Target Database were applied to decipher the relationship between compounds and genes related to AD. Kyoto Encyclopedia of Genes and Genomes and Gene Ontology term analyses were used to identify the involved signaling pathways. Cytoscape was adopted to establish the networks The molecular docking was used to validate the interactions between the candidate compounds and their potential targets.
Results
914 compounds were identified in eight herbal medicines of CZ2HF. Among them, 9 compounds and 28 genes were highly involved in the pathologic process of AD. Furthermore, the mechanism of CZ2HF to AD was based on its anti-inflammatory effects mainly through lipopolysaccharide-mediated signaling pathway and TNF signaling pathway. Core genes in this network were TNF, ICAM1, MMP9 and IL-10.
Conclusion
This study predicts the active compounds in CZ2HF and uncovers their protein targets using holistic network pharmacology methods. It will provide a insight into the underlying mechanism of CZ2HF to AD from a multi-scale perspective.
Introduction
Alzheimer's disease (AD) is a progressive neuro-degenerative disorder, which was characterized as learning and memory impairment and disturbance of behavior in clinical due to amyloid beta (Aβ) plaques accumulation and neurofibrillary tangles formation (Voglein J, et al., 2019). Currently, approximately 35 million people are suffering with AD that brings heavy burden to sufferer and society (Molinuevo JL, et al., 2018). Unfortunately, up to now, there are no ideal tactics available and multiple candidate agents fail in late-stage clinical trials owing to the complex aetiology and pathophysiology of AD (Fang J, et al., 2017). Therefore, it is a dire clinical demand to explore valid drug for prophylaxis and treatment of AD.
Traditional Chinese medicine (TCM) has finds substantial application in treating multiple diseases in Asia (Liu L, et al., 2019). TCM formula are mainly composed of various natural herbs, which provide broad prospects for prophylaxis and treatment for diseases such as AD in (Sun YW, et al., 2019; Wang T, et al., 2018). However, the TCM formula are characterized by their multi-elements, multi-targets, multi-signaling pathways and holistic synergistic effects that complete particular preventive and therapeutic effects, thus a great challenge lying ahead of TCM (Chen X, et al., 2013; Yu G, et al., 2018). Therefore, it is of great significance to explore systematically and elucidate the mechanism of TCM formula via novel methods and strategies (Casas AI, et al., 2019). Network pharmacology is exactly a novel approach to investigate active constituents of TCM and their underlying targets (Ding M, et al., 2019). It is able to provide novel probabilities for illuminating multi-scale molecular mechanisms of TCM to treat AD.
Compound Cu-zhi-2-hao-fang (CZ2HF) is a Chinese herb preparation based on TCM prescription, which is of our research team’s patent (No. CN107441468A). This compound preparation is composed of Herba Epimedium, Rhizoma curculiginis, Morinda officinalis, Acorus calamus, Lycium barbarum, Scrophularia ningpoensis, Cinnamomum cassia Presl, Rhizoma zingiberis. Its validity in AD treatment has been verified in clinic. Moreover, in our previous study, CZ2HF also showed an obvious beneficial effects on Aβ25-35-induced learning and memory impairment and hippocampal neuronal injury in rats (Zeng L, et al., 2019); however, its exact molecular mechanism remains still unclear. Network pharmacology approach provides a novel insight into the mechanism of anti-AD formula and its active ingredients as the approach has superiority in the analysis of multi-compounds, multi-targets, and multi-effects. Thus, in this study, network pharmacology was applied to decipher the anti-AD ingredients in CZ2HF and their potential targets. Furthermore, their potential “ingredient-target-pathway” was also explored. The study will not only offer pharmacological basis, but also enhance the progress of TCM as candidate drugs for treating AD.
Materials and methods
Compounds in CZ2HF
To collect the chemical compounds in CZ2HF, we adopted Traditional Chinese Medicine System Pharmacology Database (TCMSP, http://lsp.nwu.edu.cn) (Yi F, et al., 2016) and Traditional Chinese Medicine System Pharmacology Database (TCMID, http://www.megabionet.org/tcmid) (Xue R, et al., 2013) to identify the chemical ingredients of eight herbs in CZ2HF.
Roadmap of the systems pharmacology approach
The roadmap was shown in Figure 1. In brief, the active ingredients of CZ2HF herbs were confirmed through an ADME-screening model, which together with the modules of oral bio-availability (OB) and drug-likeness (DL) screening. Thereafter, the latent targets of the CZ2HF formula were forecasted by target fishing. Furthermore, target-pathway networks were performed for network pharmacology analysis.
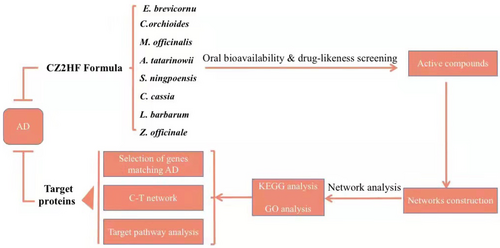
Roadmap of the systems pharmacological approach.
Active ingredients screening
To explore the active ingredients of CZ2HF that play an important role in anti-AD, the predicted OB and predicted DL values of them were predicted.
OB, the most important parameter in pharmacokinetic, measures absorption of medicine into blood and pharmacological action. (Zhao M, et al., 2018). In the present study, OBioavail 1.1, a potent internal model, was used to evaluate the OB values according to previous study (Xu X, et al., 2012). The molecules with OB ≥ 30% were selected as active compounds for further analysis.
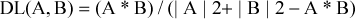
A represents the molecular descriptors of herbal components. B represents the average molecular properties of all compounds in Drug Bank database (http://www.drugbank.ca/). In the present study, compounds without information of ADME were excluded from the list and potential active chemicals were listed only if OB ≥ 30% and DL ≥ 0.18 according to previous study (Lee AY, et al., 2018).
Gene targets for identified compounds in CZ2HF
To identify the target genes related to selected herbal compounds, we applied STITCH DB (http://stitch.embl.de/, ver. 5.0) (Shawky E, 2019) with the set of “Homo sapiens” species, which provided evidence-based target gene names of each compound. Genes were retained only if threshold score ≥ 700 according to the system of STITCH. Furthermore, we adopted Uniprot (http://www.uniprot.org/) (Chen G, et al., 2018) to confirm gene name, gene ID and organism. The information of AD-related targets was obtained from Therapeutic Target Database (TTD, https://db.idrblab.org/ttd/)(Ren G, et al., 2019), a database to provide the potential and known targets of respective targeted disease. We searched TTD with the keyword “Alzheimer’s disease” and obtained AD-associated targets.
Gene ontology (GO) enrichment analysis
How do the dendritic cells function in the pathogenesis of The functional annotation of compounds targeted genes and AD related genes was performed with Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://david.ncifcrf.gov/, ver. 6.8) (Liu JF, et al., 2019), and GO database (http://geneontology.org/) (Menotta M, et al., 2018).
Network construction
Cytoscape (http://www.cytoscape.org/, ver. 3.6.0) (Qin T, et al., 2019) was adopted to establish the connections between herbs and compounds, compounds and targets. We applied the STRING database (http://string-db.org/, ver. 11.0) (Wang W, et al., 2019) to identify the protein-protein interactions (PPI) among selected targeted genes and then visualize them with Cytoscape to construct PPI networks. Thereafter, KEGG pathways (http://www.genome.jp/kegg/pathway.html) (Wan Y, et al., 2019) were used to construct compound-target-pathway networks. Pathways related to biological process (BP), molecular function (MF) and cellular component (CC) were visualized with OmicShare tools (http://www.omicshare.com) (Su M, et al., 2019).
Molecular docking of the compounds and targets interactions
To elucidate the binding modes and provide further penetration into the interactions between the potential compounds and their underlying targets, 3 active compounds and 4 targets interactions were chosen for molecular docking. The binding affinity between selected compounds and targets was carried out with Autodock 4.2 and AutodockTool (ADT). The X-ray crystal structures of inter-cellular adhesion molecule-1 (ICAM-1) (PDB ID:1P53), tumor necrosis factor (TNF) (PDB ID:1TNR), matrix metalloproteinase 9 (MMP9) (PDB ID:6ESM) and interleukin (IL)-10 (PDB ID:2H24) were obtained from Protein Data Bank (PDB) (http://www.rcsb.org) (Wang J, et al., 2017). 3D structures of selected compounds were obtained from PubChem (http://pubchem.ncbi.nlm.nih.gov) (Chamcheu JC, et al., 2017) and were established with ChemBio3D Ultra 14.0 (PerkinElmer Informatics, USA).
Results
Compounds in CZ2HF
Since CZ2HF formula was composed of nine herbs with dozens or indeed hundreds of compounds, it was necessary to establish a compound database for CZ2HF. Therefore, with our greatest efforts, a total of 970 herbal compounds in CZ2HF were mined, including 130 in Herba Epimedium, 78 in Rhizoma curculiginis, 174 in Morinda officinalis, 105 in Acorus gramineus, 188 in Lycium barbarum, 47 in Scrophularia ningpoensis, 100 in Cinnamomum cassia Presl and 148 in Rhizoma zingiberis. Among them, 56 compounds were duplications of the 970 compounds. Therefore, 914 compounds were included in the final list of Table 1.
| Full scientific species name | Abbreviation | Number of compounds |
|---|---|---|
| Epimedium brevicornu Maxim | E. brevicornu | 130 |
| Curculigo orchioides Geartn | C. orchioides | 78 |
| Morinda officinalis F.C.How | M. officinalis | 174 |
| Acorus tatarinowii Schott | A. tatarinowii | 105 |
| Lycium barbarum L. | L. barbarum | 188 |
| Scrophularia ningpoensis Hemsl | S. ningpoensis | 47 |
| Cinnamomum cassia (L.) J. Presl | C. cassia | 100 |
| Zingiber officinale Roscoe | Z. officinale | 148 |
| Total | 8 | 970 |
ADME screening selected compounds
Selection based on pharmacokinetic characteristics is more helpful for comprehending molecular mechanisms and mining potential active compounds. OB shows the ratio of an oral dose of unchanged drug falling into systemic circulation, and indicates convergence in ADME (absorption, distribution, metabolism, excretion) process. DL contributes to optimization of pharmaceutical and pharmacokinetic properties in drug design process (Zhong Y, et al., 2018). Thus, in present study, OB and DL were used to screen the compounds database. The results indicated that all the 914 identified compounds underwent ADME screening. 105 of the 914 compounds, whose OB ≥ 30% and DL ≥ 0.18, were picked out and placed into the candidate ingredient pool. The criteria of OB and DL have been set according to the study (Wang N, et al., 2017). Notably, except that compounds met the criteria, icariside II was included due to its anti-AD effects as reported in our previous study. Taken together, 106 compounds were included in the final list (Table 2).
| No. | Molecule Name | MW | OB (%) | DL |
|---|---|---|---|---|
| 1 | icariside A7 | 462.49 | 31.91 | 0.86 |
| 2 | icariside II | 514.57 | 3.70 | 0.84 |
| 3 | DFV | 256.27 | 32.76 | 0.18 |
| 4 | chrysenol | 300.28 | 35.85 | 0.27 |
| 5 | luteolin | 286.25 | 36.16 | 0.25 |
| 6 | sitosterol | 414.79 | 36.91 | 0.75 |
| 7 | poriferast-5-en-3beta-ol | 414.79 | 36.91 | 0.75 |
| 8 | 24-epicampesterol | 400.76 | 37.58 | 0.71 |
| 9 | 8-Isopentenyl-kaempferol | 354.38 | 38.04 | 0.39 |
| 10 | B602425K094 | 329.48 | 39.14 | 0.49 |
| 11 | anhydroicaritin-3-O-alpha-L-rhamnoside | 676.73 | 41.58 | 0.81 |
| 12 | icariin | 676.73 | 41.58 | 0.61 |
| 13 | kaempferol | 286.25 | 41.88 | 0.24 |
| 14 | linoleyl acetate | 308.56 | 42.1 | 0.2 |
| 15 | anhydroicaritin | 368.41 | 45.41 | 0.44 |
| 16 | yinyanghuo C | 336.36 | 45.67 | 0.5 |
| 17 | yinyanghuo A | 420.49 | 56.96 | 0.77 |
| 18 | yinyanghuo E | 352.36 | 51.63 | 0.55 |
| 19 | 6-hydroxy-11,12-dimethoxy-2,2-dimethyl-1,8-dioxo-2,3,4,8-tetrahydro-1H-isochromeno[3,4-h] isoquinolin-2-ium | 370.41 | 60.64 | 0.66 |
| 20 | olivil | 376.44 | 62.23 | 0.41 |
| 21 | anhydroicaritin | 368.41 | 45.41 | 0.44 |
| 22 | quercetin | 302.25 | 46.43 | 0.28 |
| 23 | magnograndiolide | 266.37 | 63.71 | 0.19 |
| 24 | 1,2-bis(4-hydroxy-3-methoxyphenyl) propan-1,3-diol | 320.37 | 52.31 | 0.22 |
| 25 | 3,2’,4’,6’-Tetrahydroxy-4,3’-dimethoxy chalcone | 332.33 | 52.69 | 0.28 |
| 26 | curculigosaponin C | 769.09 | 39.31 | 0.19 |
| 27 | curculigoside B_qt | 290.29 | 83.36 | 0.19 |
| 28 | beta-sitosterol | 414.79 | 36.91 | 0.75 |
| 29 | ZINC03982454 | 414.79 | 36.91 | 0.76 |
| 30 | stigmasterol | 412.77 | 43.83 | 0.76 |
| 31 | cycloartenol | 426.8 | 38.69 | 0.78 |
| 32 | ethyl oleate | 310.58 | 32.4 | 0.19 |
| 33 | alizarin-2-methylether | 254.25 | 32.81 | 0.21 |
| 34 | supraene | 410.8 | 33.55 | 0.42 |
| 35 | 3beta-24S(R)-butyl-5-alkenyl-cholestol | 456.88 | 35.35 | 0.82 |
| 36 | 3beta,20(R),5-alkenyl-stigmastol | 414.79 | 36.91 | 0.75 |
| 37 | ohioensin-A | 372.39 | 38.13 | 0.76 |
| 38 | diop | 390.62 | 43.59 | 0.39 |
| 39 | asperuloside tetraacetate | 582.56 | 45.47 | 0.82 |
| 40 | americanin A | 328.34 | 46.71 | 0.35 |
| 41 | isoprincepin | 494.53 | 49.12 | 0.77 |
| 42 | 2-hydroxyethyl 5-hydroxy-2-(2-hydroxybenzoyl)-4-(hydroxymethyl)benzoate | 332.33 | 62.32 | 0.26 |
| 43 | (2R,3S)-(+)-3’,5-Dihydroxy-4,7-dimethoxydihydroflavonol | 332.33 | 77.24 | 0.33 |
| 44 | 1,5,7-trihydroxy-6-methoxy-2-methoxymethylanthracenequinone | 330.31 | 80.42 | 0.38 |
| 45 | 1-hydroxy-6-hydroxymethylanthracenequinone | 254.25 | 81.77 | 0.21 |
| 46 | 2-hydroxy-1,5-dimethoxy-6-(methoxymethyl)-9,10-anthraquinone | 328.34 | 95.85 | 0.37 |
| 47 | 1-hydroxy-3-methoxy-9,10-anthraquinone | 254.25 | 104.33 | 0.21 |
| 48 | 1,6-dihydroxy-5-methoxy-2-(methoxymethyl)-9,10-anthraquinone | 314.31 | 104.54 | 0.34 |
| 49 | 2-hydroxy-1,8-dimethoxy-7-methoxymethylanthracenequinone | 328.34 | 112.3 | 0.37 |
| 50 | 8-Isopentenyl-kaempferol | 354.38 | 38.04 | 0.39 |
| 51 | (1R,3aS,4R,6aS)-1,4-bis(3,4-dimethoxyphenyl)-1,3,3a,4,6,6a-hexahydrofuro[4,3-c] furan | 386.48 | 52.35 | 0.62 |
| 52 | sitosterol alpha1 | 426.8 | 43.28 | 0.78 |
| 53 | mandenol | 308.56 | 42 | 0.19 |
| 54 | ethyl linolenate | 306.54 | 46.1 | 0.2 |
| 55 | LAN | 426.8 | 42.12 | 0.75 |
| 56 | atropine | 289.41 | 45.97 | 0.19 |
| 57 | campesterol | 400.76 | 37.58 | 0.71 |
| 58 | cyanin | 411.66 | 47.42 | 0.76 |
| 59 | 24-methylidenelophenol | 412.77 | 44.19 | 0.75 |
| 60 | daucosterol_qt | 414.79 | 36.91 | 0.75 |
| 61 | glycitein | 284.28 | 50.48 | 0.24 |
| 62 | delta-Carotene | 536.96 | 31.8 | 0.55 |
| 63 | CLR | 386.73 | 31.87 | 0.68 |
| 64 | 14b-pregnane | 288.57 | 34.78 | 0.34 |
| 65 | (24R)-4alpha-Methyl-24-ethylcholesta-7,25-dien-3beta-ylacetate | 482.87 | 46.36 | 0.84 |
| 66 | 24-Methylenecycloartan-3beta,21-diol | 456.83 | 37.32 | 0.8 |
| 67 | 24-ethylcholest-22-enol | 414.79 | 37.09 | 0.75 |
| 68 | 24-ethylcholesta-5,22-dienol | 412.77 | 43.83 | 0.76 |
| 69 | 24-methyl-31-norlanost-9(11)-enol | 428.82 | 38 | 0.75 |
| 70 | 24-methylenetanost-8-enol | 440.83 | 42.37 | 0.77 |
| 71 | fucosterol | 412.77 | 43.78 | 0.76 |
| 72 | 31-Norcyclolaudenol | 440.83 | 38.68 | 0.81 |
| 73 | 31-norlanosterol | 412.77 | 42.2 | 0.73 |
| 74 | 31-noelanost-9(11)-enol | 414.79 | 38.35 | 0.72 |
| 75 | 4,24-methyllophenol | 414.79 | 37.83 | 0.75 |
| 76 | lophenol | 400.76 | 38.13 | 0.71 |
| 77 | 4alpha,14alpha,24-trimethylcholesta-8,24-dienol | 426.8 | 38.91 | 0.76 |
| 78 | 4alpha,24-dimethylcholesta-7,24-dienol | 412.77 | 42.65 | 0.75 |
| 79 | 4alpha-methyl-24-ethylcholesta-7,24-dienol | 426.8 | 42.3 | 0.78 |
| 80 | 6-Fluoroindole-7-Dehydrocholesterol | 402.7 | 43.73 | 0.72 |
| 81 | 7-O-Methylluteolin-6-C-beta-glucoside_qt | 318.3 | 40.77 | 0.3 |
| 82 | cryptoxanthin monoepoxide | 568.96 | 46.95 | 0.56 |
| 83 | cycloeucalenol | 426.8 | 39.73 | 0.79 |
| 84 | (E, E)-1-ethyl octadeca-3,13-dienoate | 308.56 | 42 | 0.19 |
| 85 | methyl (1R,4aS,7R,7aS)-4a,7-dihydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl] oxy-1,5,6,7a-tetrahydrocyclopenta[d]pyran-4-carboxylate | 406.43 | 39.43 | 0.47 |
| 86 | lantadene A | 552.87 | 38.68 | 0.57 |
| 87 | physalin A | 526.58 | 91.71 | 0.27 |
| 88 | physcion-8-O-beta-D-gentiobioside | 608.6 | 43.9 | 0.62 |
| 89 | lanost-8-enol | 428.82 | 34.23 | 0.74 |
| 90 | obtusifoliol | 426.8 | 42.55 | 0.76 |
| 91 | lanost-8-en-3beta-ol | 428.82 | 34.23 | 0.74 |
| 92 | paeoniflorin_qt | 318.35 | 68.18 | 0.4 |
| 93 | sugiol | 300.48 | 36.11 | 0.28 |
| 94 | scropolioside A_qt | 590.63 | 38.63 | 0.77 |
| 95 | 14-deoxy-12(R)-sulfoandrographolide | 414.57 | 62.57 | 0.42 |
| 96 | scropolioside D | 772.76 | 36.62 | 0.4 |
| 97 | scropolioside D_qt | 560.6 | 33.17 | 0.82 |
| 98 | harpagoside_qt | 332.38 | 122.87 | 0.32 |
| 99 | (-)-taxifolin | 304.27 | 60.51 | 0.27 |
| 100 | (+)-catechin | 290.29 | 54.83 | 0.24 |
| 101 | ent-Epicatechin | 290.29 | 48.96 | 0.24 |
| 102 | taxifolin | 304.27 | 57.84 | 0.27 |
| 103 | peroxyergosterol | 428.72 | 44.39 | 0.82 |
| 104 | 1-Monolinolein | 354.59 | 37.18 | 0.3 |
| 105 | [(1S)-3-[(E)-but-2-enyl]-2-methyl-4-oxo-1-cyclopent-2-enyl] (1R,3R)-3-[(E)-3-methoxy-2-methyl-3-oxoprop-1-enyl]-2,2-dimethylcyclopropane-1-carboxylate | 360.49 | 62.52 | 0.31 |
| 106 | sexangularetin | 316.28 | 62.86 | 0.3 |
Selected compounds and its target genes
STITCH 5.0 database provided the information about predicted interactions between chemicals and genes. An interaction with a high “combine score” (≥ 700) was considered a strong interaction (Shen X, et al., 2017). Only 22 molecules of 106 compounds possessed potential target genes. Herbs-compounds network was established by Cytoscape 3.6.0 (Figure 2); moreover, 127 target genes of those molecules met the criteria of strong interactions (combine score ≥ 700) (Table 3). Since 29 target genes were duplications of the 127 genes, the compounds-genes network was composed of 98 genes and 22 compounds, which contained 120 nodes and 123 edges (Figure 3). The network of target compounds with target genes were screened based on ADME criteria of eight herbal medicines, which revealed 22 compounds and 98 target genes with 128 nodes and 153 edges (Figure 4).
| NO. | Targets name | Molecule name | NO. | Targets name | Molecule name |
|---|---|---|---|---|---|
| 1 | SREBF2 | beta-sitosterol | 64 | NSDHL | lophenol |
| 2 | ABCG8 | beta-sitosterol | 65 | EBP | lophenol |
| 3 | ABCG5 | beta-sitosterol | 66 | MSMO1 | lophenol |
| 4 | APOE | beta-sitosterol | 67 | CYP51A1 | obtusifoliol |
| 5 | DHCR24 | beta-sitosterol | 68 | MSMO1 | Sitosterol alpha1 |
| 6 | CASP3 | beta-sitosterol | 69 | C5orf4 | Sitosterol alpha1 |
| 7 | SREBF1 | beta-sitosterol | 70 | ABCA1 | stigmasterol |
| 8 | ABCB11 | beta-sitosterol | 71 | ABCG8 | stigmasterol |
| 9 | ICAM1 | beta-sitosterol | 72 | ABCG5 | stigmasterol |
| 10 | CYP7A1 | beta-sitosterol | 73 | TNF | stigmasterol |
| 11 | SREBF2 | sitosterol | 74 | IL8 | stigmasterol |
| 12 | ABCG8 | sitosterol | 75 | SLCO1B1 | stigmasterol |
| 13 | ABCG5 | sitosterol | 76 | IL10 | stigmasterol |
| 14 | APOE | sitosterol | 77 | APOB | taxifolin |
| 15 | DHCR24 | sitosterol | 78 | TNFSF11 | taxifolin |
| 16 | CASP3 | sitosterol | 79 | NQO1 | taxifolin |
| 17 | SREBF1 | sitosterol | 80 | ABCC1 | taxifolin |
| 18 | ABCB11 | sitosterol | 81 | MTTP | taxifolin |
| 19 | ICAM1 | sitosterol | 82 | ASP3 | peroxyergosterol |
| 20 | CYP7A1 | sitosterol | 83 | SP7 | icaritin |
| 21 | FDFT1 | squalene | 84 | EIF2AK3 | icaritin |
| 22 | LSS | squalene | 85 | BAK1 | icaritin |
| 23 | DHCR24 | squalene | 86 | CCND1 | icaritin |
| 24 | CCL4 | squalene | 87 | ESR1 | icaritin |
| 25 | CCL5 | squalene | 88 | ESR2 | icaritin |
| 26 | CCL2 | squalene | 89 | ABCC1 | chrysoeriol |
| 27 | CCL3 | squalene | 90 | NOTCH2 | liquiritigenin |
| 28 | IL1RN | squalene | 91 | ESR2 | liquiritigenin |
| 29 | IL8 | squalene | 92 | PDE5A | icariin |
| 30 | NR1I2 | kaempferol | 93 | ESR1 | icariin |
| 31 | CDK1 | kaempferol | 94 | NOS3 | icariin |
| 32 | UGT3A1 | kaempferol | 95 | PNPLA2 | icariin |
| 33 | CYP1B1 | kaempferol | 96 | SCARB1 | icariin |
| 34 | RPS6KA3 | kaempferol | 97 | JUN | icariin |
| 35 | AHR | kaempferol | 98 | SLCO2B1 | icariin |
| 36 | UGT1A9 | kaempferol | 99 | SLCO1B3 | icariin |
| 37 | UGT1A3 | kaempferol | 100 | RELA | icariin |
| 38 | UGT1A8 | kaempferol | 101 | MAPK8 | luteolin |
| 39 | UGT1A7 | kaempferol | 102 | MMP9 | luteolin |
| 40 | CHRM1 | atropine | 103 | CASP3 | luteolin |
| 41 | CHRM2 | atropine | 104 | JUN | luteolin |
| 42 | CHRM4 | atropine | 105 | FOS | luteolin |
| 43 | CHRM3 | atropine | 106 | CDK2 | luteolin |
| 44 | CHRM5 | atropine | 107 | EGFR | luteolin |
| 45 | BCHE | atropine | 108 | SMAD2 | luteolin |
| 46 | ACHE | atropine | 109 | CCNA2 | luteolin |
| 47 | TAC1 | atropine | 110 | AKT1 | luteolin |
| 48 | GAST | atropine | 111 | MCL1 | quercetin |
| 49 | DHCR24 | campesterol | 112 | CYP1B1 | quercetin |
| 50 | HSD3B2 | campesterol | 113 | PIM1 | quercetin |
| 51 | ABCG8 | campesterol | 114 | HCK | quercetin |
| 52 | MSMO1 | cycloeucalenol | 115 | SLC2A2 | quercetin |
| 53 | C5orf4 | cycloeucalenol | 116 | CYP2C8 | quercetin |
| 54 | AKR1B1 | fucosterol | 117 | CYP1A1 | quercetin |
| 55 | PTPN1 | fucosterol | 118 | ATP5B | quercetin |
| 56 | MMP13 | glycitein | 119 | HIBCH | quercetin |
| 57 | MAPK1 | glycitein | 120 | STK17B | quercetin |
| 58 | MAPK3 | glycitein | 121 | STAT3 | icariside II |
| 59 | JUN | glycitein | 122 | EGF | icariside II |
| 60 | SP1 | glycitein | 123 | PARP1 | icariside II |
| 61 | CDK4 | glycitein | 124 | UGT1A10 | icariside II |
| 62 | HSD17B7 | lophenol | 125 | UGT1A1 | icariside II |
| 63 | C5orf4 | lophenol | 126 | UGT1A7 | icariside II |
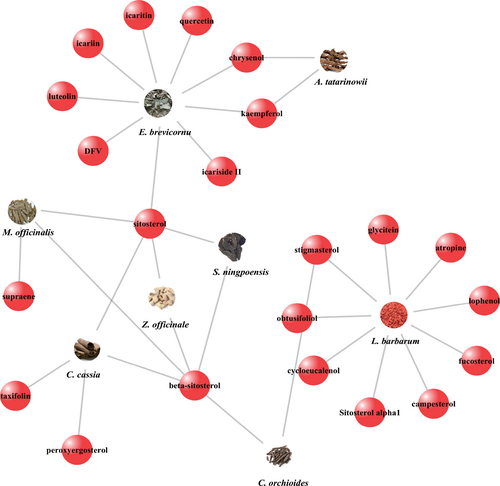
Only 23 molecules of 106 compounds were selected from CZ2HF. Compounds were indicated by red spheres.
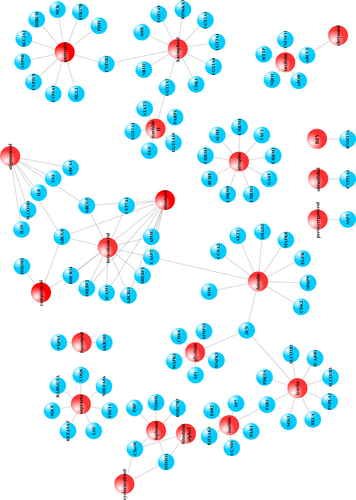
23 compounds and 96 target genes were identified by ADME screening and compound-target gene interactions for CZ2HF were constructed. Compounds were indicated by red spheres and target genes and represented by blue spheres.
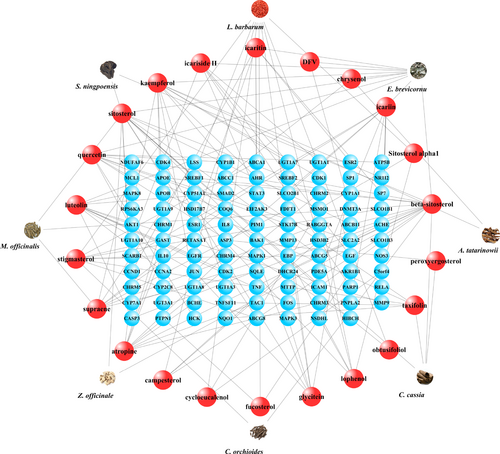
Herbal medicine-compound-target gene interactions were elucidated by merging together data into a network. 23 compounds were represented by red spheres and 96 target genes were represented by blue spheres.
Potential target genes and network analysis related to AD
The PPI network of the 98 target genes was created by Cytoscape, which included 98 nodes and 829 edges. Moreover, the size of each node is proportional to its degree of being involved in this network. Notably, AKT1 and TNF owned the first two numbers of degrees. The AD-associated genes were collected from the TTD. 28 repeating genes were selected through matching above-mentioned 98 genes with AD-related genes, which were labeled purple (Figure 5). Moreover, the network of target compounds with AD-related target genes, which were screened with ADME criteria of seven herbal medicines, revealed 9 compounds and 28 target genes with 44 nodes and 43 edges (Figure 6).
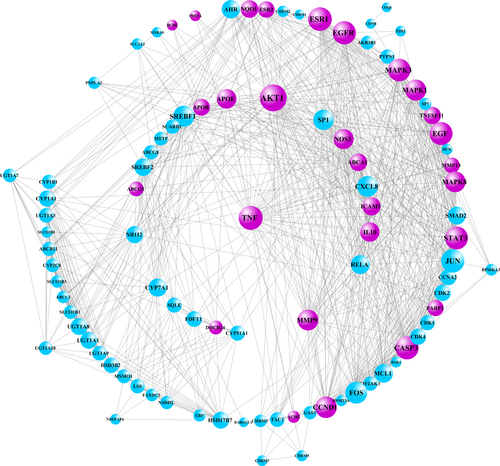
Potential target genes and network analysis related to AD. The PPI network of the 98 target genes was constructed, which includes 98 nodes and 829 edges.
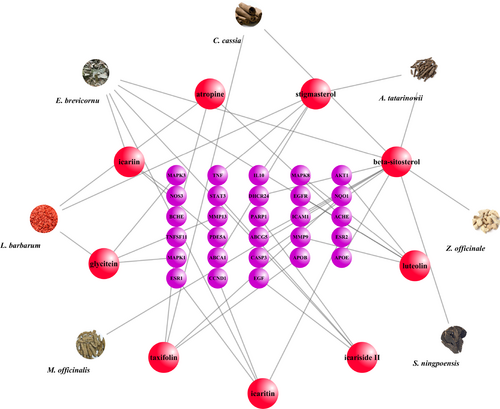
CZ2HF network with 44 nodes and 43 edges linking 9 compounds and 28 target genes related to AD. Compounds were represented by red spheres, target genes were showed as red spheres.
Pathway analysis
To identify the function and related signaling pathways of these target genes, we applied KEGG pathways and GO term to conduct functional enrichment analysis. We listed top 20 signaling pathways which related to aforementioned 98 genes (Figure 7). The results showed that the top 10 pathways were related to the process of BP, MF, CC (Figure 7). Top 20 signaling pathways related to AD-associated genes were also identified (Figure 7). Ultimately, herbs-compounds-genes network related to AD was created by Cytoscape (Figure 8).
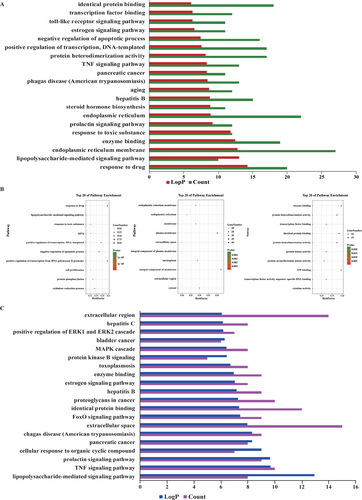
GO analysis of the target genes. Y-axis represented the dramatically enriched ‘Biological Process’ categories in GO to the relative target genes, and x-axis represented the enrichment scores of these targets. Bubble plot demonstrated the fold enrichment values of the top 10 most significantly enriched terms were analyzed by significant GO terms using P values.
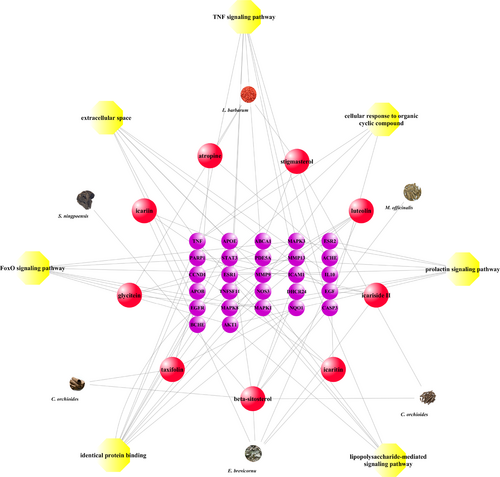
The herbal medicine-target-pathway network for CZ2HF. Herbs are indicated by according represented images; compounds were represented by red spheres; target genes were showed as red spheres; pathways were labelled with yellow spheres.
Molecular docking
The results further revealed that the binding energy between TNF and stigmasterol, ICAM1 and sitosterol, ICAM1 and beta-sitosterol, MMP9 and luteolin, IL-10 and stigmasterol were -9.14 kcal/mol, -6.4 kcal/mol, -6.59 kcal/mol, -9.99 kcal/mol, -8.7 kcal/mol, respectively (Figure 9).
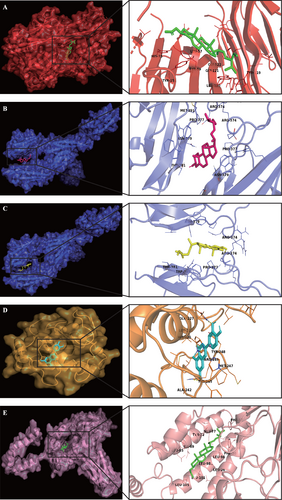
Binding detections of selected compound-target interactions were showed by the substrate binding surface and binding sites. (A) stigmasterol-TNF; (B) sitosterol-ICAM1; (C) β-sitosterol-ICAM1; (D) luteolin-MMP9; (E) stigmasterol-IL-10. The molecules were displayed as ball and stick models.
Discussion
Emerging evidence shows that TCM exerts excellent neuroprotective effects on AD due to its multiple active ingredients and targets. Although our previous study has revealed the therapeutic effects of CZ2HF formula on Aβ25-35-induced AD in rats (Zeng L et al., 2019), its therapeutic mechanisms remains still a mystery. Therefore, we applied a network pharmacology with large-scale text-mining, ADME screening, target predicting to decipher the active compounds, potential targets and possible mechanisms of CZ2HF formula for treatment of AD.
In this study, the results demonstrated that 22 compounds of eight herbs from CZ2HF were screened by ADME and 98 potential targets were identified among them as evidenced by STITCH DB database. Notably, sitosterol was detected in five different herbs of CZ2HF, as well as beta-sitosterol. Accordingly, it is reported that beta-sitosterol was considered as a potential anti-AD drug due to its beneficial effects on memory deficits (Adebiyi OE, et al., 2019). Furthermore, 98 targets were selected from the 22 compounds, of which there were 28 AD-related genes. Beta-sitosterol and sitosterol commonly aim 10 targets of 98 genes respectively; among them, APOE, ICAM1, DHCR24 and CASP3 were related to AD. Moreover, recent report indicated that luteolin, a natural flavonoids compound, exerted neuroprotective effects on AD by suppressing neuroinflammation (Contarini G, et al., 2019). Interestingly, our results showed that 9 potential targets of luteolin were confirmed by network pharmacology analysis. Among them, MAPK8, AKT1, EGFR and MMP9 were related to AD. Icariin and icariside II are the main pharmacological compounds of Herba Epimedium. More importantly, our previous studies have demonstrated that both icariin and icariside II could halt the disease progress of AD by targeting Aβ formation and neuroinflammation (Yan L, et al., 2017; Zong N, et al., 2016). In this study, network analysis successfully identified 6 AD-associated targets that belonged to icariin and icariside II. Among them, PDE5A was considered as a significant target because after inhibition of PDE5 by icariin or icariside II, the effects of anti-AD could be achieved (Jin F, et al., 2014; Yin C, et al., 2016). Evidences have demonstrated that PPI network could be useful in discovering the multiple interactions of numerous proteins in some complicated diseases including AD, and identifying potential therapeutic targets (Zhang JY, et al., 2019). In this study, AKT1, TNF, ERS1, EGFR, JUN, CASP3, EGF, STAT3, MAPK3 and FOS were considered as key target genes as evidence of its high degree.
The findings also revealed that 98-gene-related 20 top pathways were annotated by KEGG pathway and GO term. Among them, the molecular biology of specific pathways including lipopolysaccharide-mediated and TNF signaling pathways, which were pivotal signaling pathways in the network and the potential therapeutic targets in AD. Furthermore, the results demonstrated that the major target genes in the network including TNF, ICAM1, MMP9 and IL-10, which were involved in the inflammatory-related pathways. TNF is a pivotal pro-inflammatory cytokine in inflammatory responses, which plays a crucial role in normal brain function at physiological conditions (Clark IA, et al., 2018); however, excessive TNF contributes to the pathological process of neuroinflammatory disease like AD. That is link to neuroinflammation characterized by expression of TNF (Wu Y, et al., 2019). ICAM-1 is low at physiological level. However, it is quickly induced by pro-inflammatory cytokines. ICAM-1 is closely associated with neuroinflammation in AD (Walker DG, et al., 2017). Moreover, MMP9 is an crucial inflammatory component. Its activation has demonstrated to be involved in AD pathogenesis and is considered as a feature of AD due to its neurotoxic effect (Caldeira C, et al., 2017). Notably, IL-10 is an important anti-inflammatory regulator, which exerts against both central and peripheral inflammatory diseases (Guillot-Sestier MV, et al., 2015). Intriguingly, our results suggested that stigmasterol directly were bonded to TNF and IL-10, luteolin directly bonded to MMP9, and sitosterol directly bonded to ICAM1 with evidence collected from molecular docking method. Our findings demonstrated that these pathways were involved in the inhibitory effect on AD of CZ2HF. However, further research was dire to systematically decipher the other biological activities linked with CZ2HF, and elucidate its pivotal mechanisms. Among the target ingredients associated with the network pharmacology analysis, stigmasterol, sitosterol, luteolin were identified both as major active anti-inflammatory and anti-AD compounds of CZ2HF with evidence collected from compound-target-pathway networks. Stigmasterol is detected both in Lycium barbarum and Rhizoma curculiginis, which has been proved to be able to markedly attenuate cognitive impairment in vanadium-induced neurotoxicity(Adebiyi OE, et al., 2018); while, sitosterol is detected both in Morinda officinalis and Scrophularia ningpoensis, which has been regarded as a potential agent for the treatment of memory deficit disorders such as AD (Ayaz M, et al., 2017). Furthermore, luteolin, an active compound derives form Herba Epimedium also exerts therapeutic effects on Aβ1–40-induced injury in rats through mediation of NF-κB signaling pathways (Zhang JX, et al., 2017). Interestingly, further evidence showed that stigmasterol directly were bonded to TNF and IL-10, sitosterol and beta-sitosterol directly bonded to ICAM1, luteolin directly bonded to MMP9, which were consistent with the results of previous study as well as network analysis. Taken together, these results indicated that the major anti-inflammatory ingredients of CZ2HF were effective for the treatment of AD.
Conclusion
In summary, network pharmacological analysis of CZ2HF predicts that 22 compounds and 98 target genes are identified in the eight herbal medicines of CZ2HF with evidence collected from Network pharmacological analysis. In particular, 28 AD-related genes are further identified. The major compounds are sitosterol, beta-sitosterol, stigmasterol, and luteolin, which are associated with the core genes including TNF, ICAM1, MMP9 and IL-10. Our findings offer a further insight into the underlying mechanism of CZ2HF on treating AD from a multi-scale perspective.
Ethical statement
Not applicable.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of China (Grant No. 81760727), Science and Technology Support Plan of Guizhou Province (Grant No. Supporting Science and Technology Cooperation of Guizhou Province [2020]1Y010), Innovative Research Team of comprehensive utilization with Lithocarpus polystachyus Rehd, sweet tea in Zunyi City (Grant No. Technology Talents [2021]4), National key R & D plan for Research on modernization of Traditional Chinese Medicine (Grant No. 2017YFC1702005), Post subsidy project of State key R & D plan in social development field (Grant No. SQ2017YFC170204-05), Program for Changjiang Scholars and Innovative Research Team in University, China (Grant No. IRT-17R113), Program for Outstanding Youth of Zunyi Medical University (Grant No. 15zy-002).
Transparency statement
All the authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Authors' contribution
Yu Wei, Qi-Hai Gong and Chang-Yin Yu contributed the central idea, analysed most of the data, made figures & tables and wrote the initial draft of the paper. Jian-Mei Gao, Fan Xu and Jing-Shan Shi contributed to refining the ideas, carrying out additional analyses and finalizing this paper.




