SNAP25 protects primary cortical neurons from hypoxic-ischemic injury associated with CREB signal
Abstract
Background
Hypoxic-ischemic encephalopathy (HIE) could induce exacerbated changes and unpredictable effects in brain cells, and the mechanism remains unclear.
Methods
HIE model was established in neonatal rats, Zea-Longa score and TTC staining were used to observe the neurobehavior and brain infarct volume in rats subjected to cerebral hypoxia-ischemia (HI). Primary cortical neurons were then cultured in vitro to establish an oxygen and glucose deprivation model. To determine the role of synaptosomal-associated protein-25 (SNAP25) in HIE, PC12 cells were cultured and effective siRNA fragments were screened, and SNAP25 was transfected into primary neurons. Then, quantitative real-time polymerase chain reaction was used to detect the mRNA expression level and immunofluorescence staining was used to observe the morphological changes of neurons before and after the injury. Finally, the abundance values of SNAP25 and its associated genes were filtered using the NCBI and GeneMANIA, respectively.
Results
HI leads to a decrease in neuronal number and an increase in SNAP25 expression. Whereas, the interference of SNAP25 caused marked decrease in neuronal number and the length of neurite. Moreover, the expression levels of CREB and SYP were significantly decreased after interference of SNAP25.
Conclusion
SNAP25 exhibited several neuroprotective effects to neuronal protection in neonatal cerebral HI by regulating CREB and SYP.
Introduction
Hypoxic-ischemic encephalopathy (HIE) is a pathological condition in which the oxygen and glucose levels in brain tissue are reduced due to an impaired blood supply to the entire brain (Martínez-Biarge M, et al., 2014). As an important cause of perinatal brain injury, hypoxia-ischemia (HI) insult of neonatal may cause cerebral palsy, epilepsy, seizures and learning limitations (Li C, et al., 2017). Moreover, the time window between the end of ischemic injury and neuronal death is thought to be related to the activation of competitive programs for gene expression, some of which promote cell survival while others promote neuronal death (Papadopoulos MC, et al., 2000). In addition, HIE is often difficult to diagnose in real time because of convulsions and other non-specific encephalopathy, and is the most common reason of perinatal asphyxia (Zhang Y, et al., 2016). HIE can also lead to severe brain damage and is a common cause of neurological handicaps in adulthood (Kojima T, et al., 2013). Currently, there is no effective clinical treatments to mitigate HI-induced brain injury, due to the lack of understanding of the neural networks associated with HI-induced neurodegeneration and its mechanisms (Liu S, et al., 2015). Thus, it has become an important research direction in the field of neonatal disease treatment in the world to investigate the pathogenesis of HIE and find effective prevention and treatment strategies.
Soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) is widely distributed in the body of almost all of the tissues and cells. Synaptosome associated protein 25 (SNAP25), as a member of the protein family is localized in the plasma membrane of the cytosolic face with the family on the other two members of the syntaxin and synaptobrevin form stable ternary complexes SNAP25 (Bark IC, et al., 1994), which composed of 206 amino acids with molecular weight 25kd, is formed by two α helices coiled structure. As synaptic protein involved in the exocytosis of synaptic vesicles in neurons, SNAP25 plays a crucial role in the regulation of neurotransmitter release and neuronal plasticity (Pevsner J, et al. 1994; Bark IC, et al. 1995; Friedlander RM, et al. 2003). Many results showed that the loss of syntaxin, SNAP25 and genes for synaptotagmin (syt) in SNARE complex can cause cognitive impairment and learning and memory impairment in mice (Pevsner J, et al., 1994). It was also known that cerebral ischemia can activate the Ca2+-dependent synaptic vesicle exocytosis and affects the formation of SNARE complexes and the release of neurotransmitters (Park SJ, et al., 2008), but the concrete role of SNAP25 in ischemia brain, especially in neonatal rats, is not well determined.
This study explored the role of SNAP25 in oxygen and glucose deprivation (OGD) in vitro, and associated mechanism by using SNAP25-mRNA interference, so as to provide the preclinic information for SNAP25 usage and mechanism exploration involving CREB in the treatment of hypoxia and ischemia damage.
Materials and methods
Animals
Seven-day old neonatal Sprague-Dawley (SD) rats (weight range 12-16 g) were used for in vivo animal experiment, and the primary cortical neuron cultured in vitro were extracted from one-day-old neonatal rats. All of the experimental rats applied in this study were obtained from Experimental Animal Center of Kunming Medical University. All the experiments have been approved by the Animal Care & Welfare Committee of Kunming Medical University. All experimental processes complied with the guidelines for the care and use of laboratory animals published by the National Institutes of Health.
HI model preparation
Animal models were established based on a classical Rice-Vannucci method (Rice JE, et al., 1981).The rats were anesthetized by inhalation of isoflurane (4% for induction and 2% for maintenance), then kept in the supine position. Afterwards, the right common carotid artery was isolated after exposure through a near midline neck incision, then coagulated with an electrocoagulator (Spring Medical Beauty Equipment co., LTD, Wuhan, China). The wound was then sutured and the neonatal rats were returned to their mother for a 1-hour recovery. Subsequently, the nitrogen-oxygen mixed gas bottle was opened, and when the oxygen concentration in the anoxic chamber decreased to less than 8%, the rats in the operation group were immediately put into it for 2 h, and the oxygen concentration in the anoxic chamber was maintained at about 7.5-8.0%. The same dissection was performed on animals in the sham group without ligation of the right carotid artery and subsequent ischemia. Then the rats were taken for Zea-longa score.
Zea-longa score
The success of HI model was evaluated according to Zea-longa's 5-grade scoring criteria. Briefly, Zea-longa score was performed at 0 h, 2 h, 6 h, 12 h, 18 h, 24 h, and 48 h of HI, and the detailed scoring criteria are as follows: scoring 0, the rat was normal with no symptom of neurological impairment; scoring 1, side front paw of the rat could not stretch completely; scoring 2, rotated inwards when walking; scoring 3, tilted inwards when walking; and scoring 4, the rat failed to spontaneously walk and loss of consciousness.
Tissue harvested
For morphological staining, the rats were anesthetized with 3% isoflurane at 24 h after HI, and perfused with 0.9% normal saline until the liver of the rats turned white, then perfused again with 4% paraformaldehyde. Afterward, the brain tissues were acquired and fixed in 4% paraformaldehyde for 72 h, and were then embedded in paraffin for further use. The brain tissues used by qRT-PCR, did not need to be perfused and fixed with 4% paraformaldehyde. Briefly, after perfusion with normal saline, the cortex approximately 3 mm around the infarcted area from the ipsilateral hemisphere and the whole hippocampus were obtained and stored in a 1.5 ml centrifuge tube and put into a refrigerator at -80°C for later use.
TTC staining and infarct volume measurement
TTC staining was conducted 24 h after HI. After anesthesia with isoflurane, the brain was harvested quickly from rats and frozen at -20°C for 10 minutes. The frozen brain was then cut into 5 slices of 2 mm thick and placed in 2% 2, 3, 5-triphenyltetrazolium chloride (TTC) solution culture dish, stained in a 37°C incubator for 10 minutes in dark, and the brain slice was viewed at 5 min intervals to make it evenly colored. The brain slices were successfully stained and fixed with 4% paraformaldehyde for 24 h, and was then photographed to collect images. The infarcted area and hemispheric area of each slice were measured using an image analysis system (ImagePro Plus) to exclude cerebral infarction volume. Percentage of cerebral infarction volume = [left cerebral volume - right cerebral non-infarcted volume)] / left cerebral volume ×100%.
Paraffin section immunofluorescence staining
The paraffin sections of brain tissue were applied to immunofluorescence staining after dewaxing hydration and antigen retrieval treatment. Afterwards, the sections were rinsed three times with 0.01 M PBS and pre-incubated with 30 μL of 5% goat serum for at 37°C 30 min. Then they were incubated for 16-18 h at 4°C with primary antibody of NeuN (mouse, Bioss, bsm-52268R, 1:50), 2% sheep serum instead of primary antibody was added in negative control. Followed by, the sections were rinsed with 0.01 M PBS for three times before being incubated with 30 μL of red-fluorescent-label Alexa Fluor 594 (goat anti-mouse, Abbkine, A23420, 1:100) at 37 °C for 1 h. Finally, 10 μL of DAPI was used to counterstain nuclei. After the coverslip was added, the results of fluorescence staining were observed under a microscope. DAPI stained the nucleus as blue, and the result of NeuN staining was red. Image-Pro Plus 6.0 software was used to automatically calculate the number of NeuN-positive cells.
Primary cortical neuron culture
Primary cortical neurons were extracted from one-day-old neonatal rats. Briefly, the cortical tissues were harvested, homogenated and isolated with 0.25% trypsin at 37°C for 10-15 min. Subsequently, samples were eluted with 10% fetal bovine serum and centrifuged under the condition of 1500 rpm for 5 min at room temperature. Next, the supernatant was discarded and a single cell suspension was prepared with new complete culture medium. Afterwards, the cells were seeded at a density of 2 to 5 x 105/ml on poly-L-lysine and laminin-coated coverslips and incubated with 95% air and 5% CO2 at 37°C. The culture medium was changed every 3 days, and the complete medium was replaced by neurobasal medium (neuronal base + 2% B27, serum-free).
Culture of PC12 cells
PC12 cells were placed in warm water for 1 to 2 minutes, and the cells were cultured in Dulbecco’s modifed Eagle’s medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and incubated with 5% CO2 at 37°C. The medium was changed after 12 to 24 h of culture.
Screening the effective siRNA fragments
The SNAP25 gene sequence was accessed in the NCBI, and was sent to the Guangzhou Ruibo company to design the three RNA fragments and a random control fragment (NC). The PC12 cells were inoculated in 6-well plates at 37°C with 5% CO2 for 24 h, the effective interference RNA was screened for cell fusion rate up to 40%. According to the operation instructions of the kit, the cultured PC12 cells were randomly divided into normal group, NC group, Reagent group, SNAP25-F1 group, SNAP25-F2 group, SNAP25-F3 group and CY3 group, 3 wells were set in each group, then interference RNA was centrifuged for 12000 rpm frozen powder 1 min. By using ddH2O to dilute 10 X buffer to 1 X, and according to the instructions of the final system in accordance with the following configuration: each well was added with 60 μl 1 X buffer + 5μl siRNA + 5μl reagent, the cells were cultured for 15-30 min at room temperature.
Target siRNA transfection
After primary cortical neurons were cultured for 5 days, the cells were randomly divided into Normal, NC, Reagent and SNAP25-siRNA groups, with 3 wells set in each group, meanwhile, 1 well was set in CY3 group. The siRNA transfection system was configured as the operation instruction, which each well was added with 92.9 μl cell culture medium, 6 μl 1 X buffer, 0.5 μl siRNA and 0.6 μl Reagent in the 96-well plate, which was then placed in room temperature for 15-30 min. Specifically, 1.25 μl of 20 μM siRNA was diluted with 30 μl of 1 X buffer and gently mixed; then 3 μl of Reagent was added to the diluted siRNA, gently mixed and incubated at room temperature for 10-15 min to prepare transfection complex. Thereafter, the prepared transfection complex was added to an appropriate cell culture medium and gently mixed and the plate were incubated in a CO2 incubator at 37°C for 24 h. Cell morphology was observed on the live cell workstation (Nikon Eclipse Ti-S fluorescence microscope, Nikon) and photographed at 24 h and 48 h with CY3 as a positive control. In order to calculate the number of cells, 5 fields of a view were selected randomly for cell counting (200 X).
Construction of oxygen glucose deprivation (OGD) model
In order to mimic the HI environment in vitro, primary cortical neurons were used to establish OGD model. Specifically, the neurons were incubated with glucose-free medium DMEM (Gibco, USA), in an anoxic chamber containing 0.8% O2, 95% N2, 5% CO2 at 37°C for 2 h. The culture medium was replaced by neuron specific medium (Gibco, USA) after 2 h of hypoxia, the neurons were then incubated with 5% CO2 and 95% air at 37°C for 24 h.
Immunofluorescence staining
The primary cortical neurons in the groups of Normal, NC, SNAP25-si, OGD, OGD+NC and OGD+SNAP25-siwere cultured on glass cover slips. Briefly, at 24 h after OGD, the cell sections were fixed with 4% formalin and blocked with 5% normal goat serum for 2 h and incubated overnight at 4°C in a humidified chamber with the following primary antibody: Tuj1 (rabbit, Abbkine, 1:100, Polyclonal). The cell sections were then washed 3 times with PBS for 5 min per time and incubated with the appropriate secondary antibody: Alexa Fluor 488 (anti-rabbit, Abbkine, 1:200) for 1 h at 37°C in dark. The cells were washed again as described above. DAPI (Beyotime, 1:100) was used for the nucleus staining. Finally, 200x images of five fields per well were randomly acquired and the number of Tuj1-positive cells was observed under a high-content cell imaging system. The number of Tuj1-positive cells and axon length were measured using Image-Pro Plus 6.0 software.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The total RNAs of the harvested tissues (the cortex approximately 3 mm around the infarcted area from the ipsilateral hemisphere and the whole hippocampus) and cultured neurons were extract by Trizol regent (Takara Bio Inc, Otsu, Japan), and reversely transcribed into cDNA according to the instruction of Revert Aid™ First Strand cDNA Synthesis Kit. The primers were designed by Primer 5.0 software, and the sequences were presented in .
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| SNAP25 | AGGACTTTGGTTATGTTGGAT | GATTTAAGCTTGTTACAGG |
| Bcl-2 | GAGGATTGTGGCCTTCTTTG | GTTCCACAAAGGCATCCCAG |
| NGF | GAAGCCCACTGGACTAAACT | ACAGTGATGTTGCGGGTCTG |
| SNCB | TCCTCTACGTCGGAAGCAAG | CCACTTCCTCTGGCTTCAG |
| SYP | TTCAGGCTGCACCAAGTGT | GCCACGGTGACAAAGAAT |
| VEGF | GATGTGGGGAAGGAGTTTG | CCTCCATGTGTGTCCGTCTAC |
| JAK | TTCACTGGAGTATCTGTTTG | CAACTGCATCTTCTTCATCAT |
| STAT3 | GTGCAGGATCTAGAACAGAA | GACTGGTTGTTTCCATTCAG |
| ERK1 | AACATGAAGGCCCGAAACT | CTCTACTGTGATGCGCTTGTT |
| AKT | GAGAACCTCATGCTGGACAAG | GTCGTTGTCCTCCAGCACCT |
| CREB | CCACATTAGCCCAGGTATCC | TCTGAGTTCCGGAGAAAAGTCT |
| PI3K | ATGGTGAAGGTGGATTGGT | GTAGCGTTGGGTTTGAGAT |
| β-actin | GAAGATCAAGATCATTGCTCCT | TACTCCTGCTTGCTGATCCA |
Afterwards, RT-PCR reaction was carried out in a thermocycler (CFX-96, Bio-Rad, USA), β-actin was used as an internal reference. The reaction condition was: 95°C for 5 min (pre-denaturation), 95°C for 10 s (denaturation), 51°C for 10 s (annealing), and 72°C for 20 s (extension), total for 40 cycles. The data were analyzed by the comparative critical threshold (Ct) method, and the relative expression of the detected genes were normalized by the β-actin values 2-ΔΔCt method.
SNAP25 abundance query and GENEMANIA prediction
The abundance value and chromosomal location of SNAP25 (25 kDa) in rats were searched from the NCBI web site (https://www.ncbi.nlm.nih.Gov/gene/25012). GeneMANIA (http://genemania.org/) is a tool to identify the internal association of gene sets. After the input of SNAP25, the database analyzes the functional correlation and other data recorded in the underlying data to find the functional association between genes.
Statistics analysis
All data were analyzed using SPSS version 20.0 (SPSS Inc, Chicago, USA) and expressed as mean ± standard deviation (SD). Differences between NC and experimental group were compared using One-Way analysis of variance (ANOVA). *p values ≤ 0.05 was considered to be statistically significant.
Results
HIE is related to brain edema and cerebral infarction, and causes neurological dysfunction
IIn oder to elucidate the regulatory role of SNAP25 after HI, we successfully built the HI model. Compared with the sham group, we observed the right side of rat brain was swollen and the meninges were pale at 24 h after HI (Figure 1), and the HI group had an obvious infarct area in the right cerebral cortex by using TTC staining (Figure 1). Before HI modeling, the behaviors of rats were normal and Zea-longa score was 0, however, the score of the HI group was significantly increased. The peak score in the HI group rapidly reached greater than 3 and gradually declined, meanwhile, the Zea-longa score in the HI group was still significantly higher than that in the sham group at 48 h after HI (Figure 1, p < 0.05). The volume percentage of the right cerebral infarction area was measured. When compared to sham group, the infarct volume of HI group reached 20.3% (Figure 1, p < 0.05). Paraffin sections of the right cerebral cortex at 24 h after HI were subjected to immunofluorescence NeuN staining, and NeuN was a specific neuronal marker. When compared to sham group, the number of NeuN-positive cells and the fluorescence intensity in the HI group were significantly decreased (p < 0.05), which indicated the right cortical neurons of the rats were reduced at 24 h after HI (Figure 1).
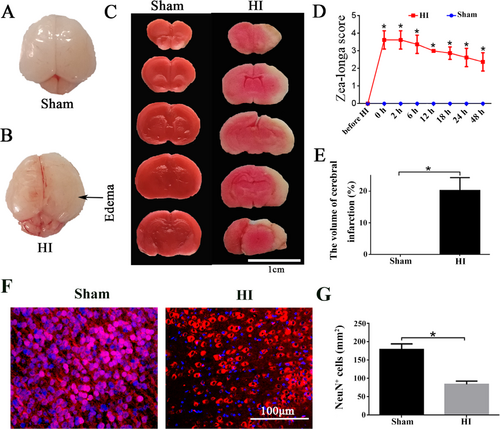
Decreased infarct state of cerebral cortex and the number of neurons in right cerebral cortex after HI. (A, B) The sham group and HI group showed a general view of the brain, and the arrow pointed to the right brain swelling area. Scale bar =1 cm. (C) The results of TTC staining in sham and HI groups. (D) The results of the Zea-longa score. (E) The percentage of the volume of the brain infarct. (F) NeuN fluorescent staining in sham and HI groups, Blue stained for nuclei and red stained for NeuN neurons. Scale bar =100 μm. (G) The number of NeuN positive cells in the sham and HI groups/mm2. *p values ≤ 0.05 vs sham group (n=6).
HI induced increased expression of SNAP25-mRNA in vivo and in vitro models
After we successfully built HI model in the right side of the rat brain, an OGD model of cortical neurons was also established in vitro. The relative expression of SNAP25-mRNA in the left and right cortex, left and right hippocampus, and cortical neurons at 24 h after HI and 2 h after OGD were detected by qRT-PCR. The results suggested the relative expression of SNAP25-mRNA in the right cortex (Figure 2) and in cortical neurons after 2 hours of OGD were remarkably increased (Figure 2, p < 0.05), while, there were no obvious difference in left cortex, left hippocampus and right hippocampus (Figure 2).
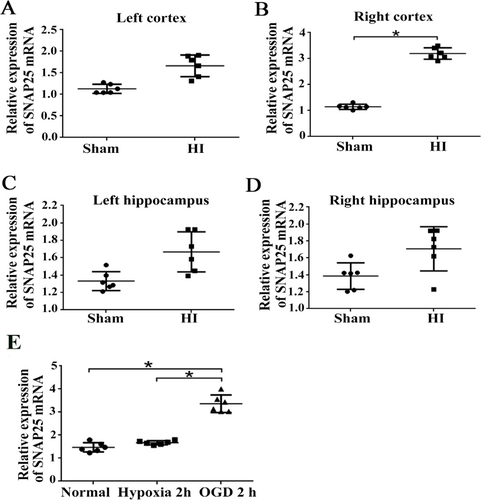
The relative expression of SNAP25-mRNA increased in vivo and vitro models at 24h after HI. (A) Compared to sham group, the relative expression of SNAP25 mRNA were upgraded in HI groups including left cortex, (B) right cortex, (C) left hippocampus and (D) right hippocampus at 24 h after HI. (E) The relative expression of SNAP25 mRNA increased significantly at 2 h after OGD group compared with the normal group and pure hypoxia group. *p values ≤ 0.05 vs. OGD group (n=6).
Successful screening and verification of SNAP25 interference fragments
We successfully cultured PC12 cell line and primary cortical neurons for transfection of SNAP25 interference fragments, and CY3 was used to test transfection rate, and results suggested the transfection rate reached 80%. (Figure 3). After SNAP25-si was successfully transfected into primary cortical neurons. The relative expression of three SNAP25-siRNA fragments (F1, F2 and F3) were detected by qRT-PCR. Compared with NC group, the relative expression of SNAP25-mRNA of F1 fragment was significantly decreased (Figure 3, p < 0.05). The transfection rate of F1 fragment was verified again, compared with the NC group or OGD+NC group, the relative expression of SNAP25-mRNA in the si-SNAP25 (F1) transfection group were decreased obviously (Figure 3, p < 0.05).
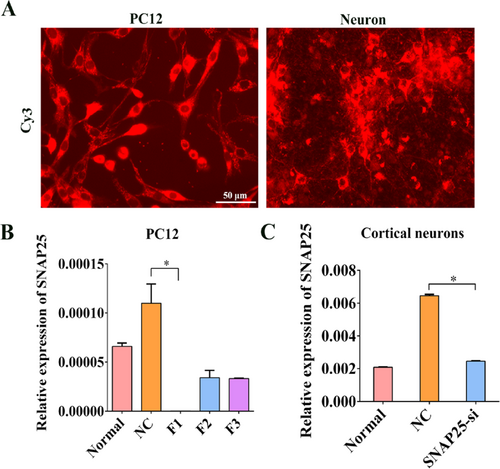
Screening and verification of SNAP25 interference fragments. (A) Transfection rate of SNAP25-si in neurons and PC12 cells were tested with CY3. Scale bar =50 μm. (B) Under normal and OGD conditions, SNAP25-si was successfully transfected into primary cortical neurons. The relative expression of SNAP25-mRNA after three SNAP25-siRNA fragments were transfected. (C) Relative expression of SNAP25-mRNA in neurons after the effective interference fragments was transferred into neurons. *p values ≤ 0.05 vs. NC group (n=6).
Immunofluorescence staining and quantitative detection
Under normal and OGD conditions, SNAP25-si was successfully transfected into primary cortical neurons. Compared with the NC group, SNAP25-si transfection of OGD group lead to worse cell morphology and fewer cells. By using DAPI and Tuj1 immunofluorescence staining (Figure 4) to conduct fluorescence quantitative analysis, we found that compared with NC group, both the cell number and axon length of SNAP25-si group were significantly decreased under normal condition and OGD condition (Figure 4, p < 0.05). Under either normal condition or OGD condition, by using interfering with SNAP25 could cause the reduction of cell number and axonal length, which indicated SNAP25 plays a crucial role in neurons.
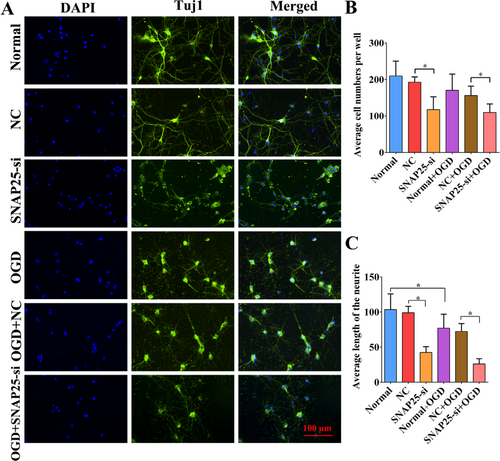
Immunofluorescence staining and quantitative detection. (A) Effect of SNAP25- is in primary cortical neurons. (B) Comparison on average cell number in each group. (C) Comparision on the length of neurites in each group.
The co-expression gene map of SNAP25 by GeneMANIA
The abundance value of SNAP25 (25kDa, ID: 25012) in rats was found in NCBI. It was found that the abundance of SNAP25 in the brain of rat at 2-week-age was 41.482 ± 11.246, and rise to 66.794 ± 7.538 at 6 weeks age. Moreover, the abundance of SNAP25 reach a peak at 70.236 ± 5.587 in the brain at 21 weeks age, and then the abundance of SNAP25 goes down to 65.356 ± 13.531 at 104 weeks age. As a result, SNAP25 act as a critical role in rat brain, which associated with the growth and development of rats (Figure 5). In order to explore SNAP25 adjustment mechanism, we analyzed the genes associated with SNAP25 by using GeneMANIA (http://genemania.org/) software and obtained a gene association map. The results suggested Bcl-2, CREB, nerve growth factor (NGF), stereotaxic needle core biopsy (SNCB), Synaptophysin (SYP), vascular endothelial growth factor (VEGF), JAK1, STAT3, Phosphoinositide 3-kinase (PI3K), AKT and ERK1 are all related to SNAP25 (Figure 5). After SNAP25-si and OGD model was used in primary cortical neurons, the relative mRNA expression of these molecules was detected by qRT-PCR. Compared with the NC group, after the interference of SNAP25 in primary cortical neurons under normal conditions, the relative expression of Bcl-2, NGF and VEGF were up-regulated, while, the relative expression of CREB and SYP were down-regulated (Figure 5, p < 0.05). Moreover, the relative expression of NGF in SNAP25-si group was increased under OGD condition, while, the levels of CREB, SNCB and SYP were decreased (Figure 5, p < 0.05). Taken together, the interference of SNAP25 could lead to the expression changes of the above genes both in normal condition and OGD condition, which indicating that SNAP25 may play a role in neuronal protection by up-regulating these genes.

The co-expression gene map of SNAP25 by GeneMANIA and experimental validation. (A) Abundance of SNAP25 in rats from NCBI. (B) The gene map associated with SNAP25 was analyzed by using GENEMANIA software; the purple networks referred to Co-expression, pink networks referred to physical interactions, brown networks referred to shared protein domains and orange networks referred to predicted relation. (C) The relative expression of Bcl-2, CREBNGF, SNCB, SYP, and VEGF molecules after SNAP25 interference in normal condition. (D) In OGD conditions of primary cortical neurons, the relative expression of SNAP25-mRNA in Bcl-2, CREB, NGF, SNCB, SYP and VEGF molecules after SNAP25 interference. *p values ≤ 0.05 vs SNAP25-si group (n=6).
Discussion
In this study, we successfully established a neonatal rat HI model in vivo and a primary neuronal OGD model in vitro, as well as implemented the transfection of SNAP25-siRNA in vitro to explore the role of SNAP25 in HIE induced neurological deficits. Our data revealed that the neuronal number and average length of the neurite were significantly reduced after the transfection of SNAP25-siRNA under OGD condition, which suggesting that interference of SNAP25 increased neuronal damage. In another word, SNAP25 plays an important role in neonatal cerebral ischemia and hypoxia, and its mechanism is associated with CREB signal.
Successful establishment model of HI brain damage model in vivo and in vitro
Here, we established a HI brain damage model in postnatal day 7 (P7) rats based on the classical Rice-Vannucci method, which was most widely used in basic researches (Rice JE, et al., 1981). Moreover, P7 of rats is the age at which the brain grows at its peak in humans at menarche (Bennet L, et al., 2012). Afterwards, the neurological dysfunction and cerebral infarction were evaluated by Zea-longa score and TTC staining, respectively. Meanwhile, OGD is recognized as a model of HI in the world and is widely used in various models of organ ischemia and hypoxia (Tasca CI, et al., 2015). Luo C. and coauthors (2015) established OGD model in vitro to study the neuroprotection of P188 in cultured primary cortical neurons (PCNs), and they found P188 has a protective effect on neuronal injury in vitro (Luo C, et al., 2015). Page found using OGD model for 24 h lead to a decrease in transendothelial electrical resistance (TEER), but failed to show any increase in cell permeability to sodium fluorescein (Page S, et al., 2016). By using OGD model in vitro, HIBD induces IL-10 secretion from astrocytes, and they exert anti-apoptotic effect on injured neurons via the TLR2/NFκB signaling pathway, which may improve learning and memory dysfunction after ischemic injury (He, et al., 2017). SNAP25 plays an important role in the release of neurotransmitters, and we hypothesized that OGD 2 h may be due to post-injury cell self-help. The up-regulation of SNAP25 has a repair effect on damaged neurons and the SNAP25 interference has not been effective in this time point. So, we finally chose OGD for 2 h as the model of neonatal hypoxic-ischemic brain damage in vitro.
The role of SNAP25 on the neurons with OGD
To determine the function of SNAP25 after OGD, SNAP25-siRNA fragment was transfected to the primary cortical neurons after OGD in vitro. The results revealed that after transfecting with SNAP25-siRNA in primary cortical neurons, both the neuronal number and axon length in SNAP25 interference group decreased under normal or OGD conditions, that is to say, the damage of cells were more severe after the decrease of SNAP25 expression level, suggesting SNAP25 could play a neuron protection role in OGD. Evidences have proved that as a cellular calcium modulator, SNAP25 had the function of regulating neural signal transmission (Liu YS, et al., 2017), and it was found that high expression of SNAP25 in adult brain to contribute to neural plasticity (Oyler GA, et al., 1989); furthermore, studies suggested SNAP25 may be involved in axonal growth and synaptic plasticity (Wu CS, et al. 2011; Liu YS, et al. 2017). Previous literatures have reported that SNAP-25 is primarily involved in almost all major neurological disorders, such as Alzheimer’s disease, Down’s syndrome, Schizophrenia, and Attention Deficient Hyperactivity Disorder (Clinton J, et al. 1994; Greber-Platzer S, et al. 2003; Noor A, et al. 2017), and SNAP25 is therefore recognized as an elementary target for the diagnosis and treatment of neurological diseases. In addition, a study based on the HI model in 7-day-old rats found that upregulation of SNAP-25 may be related to the protection effect of erythropoietin and the nonerythropoietic asialoerythropoietin against neonatal HI injury (Greber-Platzer S, et al., 2003). Here, we successfully applied SNAP25-si technology in OGD of primary cortical neurons to explore the mechanism of SNAP25 in neonatal ischemia and hypoxia and found SNAP25 act as a critical role in rat brain, which was associated with the growth and development of rats. Our data demonstrated SNAP25 is one of the important factors to maintain cell growth in normal consition, and may be the effective molecules to protect neurons from OGD injury.
Potential molecular mechanisms of SNAP25
We obtained a gene-related map of SNAP25 by using GeneMANIA, and found SNAP25-siRNA transfecting into neurons both under normal and OGD conditions could lead to the change of Bcl-2, CREB, NGF, SNCB, SYP and VEGF mRNA levels. Our data revealed that after interference of SNAP25, mRNA levels of Bcl-2, NGF, SNCB and VEGF were increased in normal conditions, but the expression of CREB and SYP were decreased. Whereas, in the OGD condition, all the expression of Bcl-2, CREB, SNCB and SYP mRNA were decreased after the interference of SNAP25, and the expression of NGF remained elevated. Together, gene predication and PCR validation pointed undoubtedly that CREB is all decreased both under normal and OGD conditions after SNAP25-is, while NGR was upregulated. We analyzed their possible role and discussed their fuctional implication.
In a study of HIE, the author found by regulating Bcl-12/Bax ratio, carbamylated erythropoietin pretreatment could reduce neuronal apoptosis and play a protective role on intrauterine HIE (Sung YY, et al., 2018); but we only find the Bcl-2 was changed in normal cultured neurons but not OGD neurons. SYP exhibited a converse change under both normal and OGD conditions, and the implication is waiting to be explained later. Li Sun et al. found acupuncture treatment could improve the motor function of the neonatal rats subjected to HIE by regulating the expressions of SYP and GAP-43 in hmin tissue (Li S, et al., 2015). Moreover, the level of VEGF was reported to increase in cord blood of neonates after neonatal asphyxia, especially in infants with advanced encephalopathy, which can be used as a target gene in the treatment of HIE (Aly H, et al., 2009), but in our study, VEGF is only upregulated in normal group but not OGD group, which is different from previous findings. Moreover, Kataoka M administrated a specific antibody for SNAP-25 phosphorylated at Ser187 in PC12 cells, then revealedprolonged treatment with NGF lead to a preferential localization of SNAP-25 in the plasma membrane by quantitative microfluorometry, which suggested a close relationship between the up-regulation of SNAP-25 phosphorylation and NGF-induced differentiation of PC12 cells (Kataoka M, et al., 2010).
Importantly, we determined the change of CREB both normal and OGD neurous, in which, CREB was consistantly downregulated under two conditions. Therefore, we thought CREB can be regulated by SNAP25 under normal and injury condition. This evidence indicated that the role of SNAP25 may associate with CREB. As a vital molecule, the role of CREB involved in neuronal survival and neurite outgrowth have been reported (Kadkova A, et al., 2019). Therefore, the SNAP25 exerts its influnce on neuron, which may dependent on CREB. This is the first study talking about the mechanism of SNAP25 via CREB.
Conclusion
SNAP25 act as a critical role in rat brain, which associated with the growth and development of rats. In our study, the models of neonatal cerebral HI were successfully constructed, and SNAP25-si was successfully transfected into primary cortical neurons. Our results suggested SNAP25 has protective effect under OGD condition, which is responsible for regulating the expression levels of CREB and SYP on injured neurons. The study provides a new perspective on application of SNAP25 in neonatal cerebral HI.
Ethical statement
The animal study protocol was legally approved by the Animal Care & Welfare Committee of Kunming Medical University (Approval No: Kmmu 2019013). All experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Acknowledgements
Not applicable.
Conflict of interest
There is no conflict of interest in this study.
Funding
This study was supported by grant from the National Natural Science Foundation of China (Grant Nos. 82001604, 82060243 and 81960214) and Joint Fund of Zunyi Science and Technology Bureau-Affiliated Hospital of Zunyi Medical University (No. HZ2020250).
Transparency statement
All the authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Authors' contribution
Yuan Jin and Jie Chen drafted this article; Yuan Jin, Chao Zhang and Ruo-Lan Du analyzed most of the data; Qiao Hu, Xu Fang and Chang-Le Fang assisted to concudt; Ting-Hua Wang and Liang Dong revised this article; Ting-Hua Wang and Zhao-Qiong Zhu contributed to refining the ideas, carrying out additional analyses and finalizing this paper.




