Prenatal Ultrasound and Magnetic Resonance Imaging Features and Postnatal Outcomes of Congenital Hepatic Hemangioma: A Retrospective Analysis
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Congenital hepatic hemangioma (CHH) is a rare benign vascular tumor that occurs prenatally. However, only a few cases have been summarized and evaluated for the prenatal and postnatal imaging features of CHH, and no studies have conducted long-term follow-up on it. This study aimed to explore the ultrasound and magnetic resonance features, growth patterns, and clinical outcomes of CHH.
Methods
Thirty-six pregnancies with a prenatal fetal diagnosis and postnatal diagnosis of CHH were studied. CHHs were grouped into those with a diameter ≥ 4 cm and those with a diameter < 4 cm according to the largest diameter. Fisher's exact test was used to compare the imaging characteristics between the groups. The volume of CHHs was measured at each follow-up visit to plot the growth pattern of the tumors, and the volume of CHHs was compared before and after birth using a rank sum test analysis.
Results
Thirty-three cases of CHHs were confirmed by postnatal imaging, and three were confirmed by a biopsy. Mixed echoes were more common in the diameter ≥ 4 cm group than in the diameter < 4 cm group (p = 0.026). Complications were more likely to occur in the large-diameter group. Eighteen (54.5%) cases were classified as rapidly involuting congenital hemangioma, nine (27.3%) as partially involuting congenital hemangioma, and two (6.1%) as noninvoluting congenital hemangioma. A new type of CHH was identified in which four (12.1%) cases continued to proliferate after birth and spontaneously subsided in subsequent months. The CHH volume decreased with age and was significantly decreased at 9 months postnatal compared to birth (p = 0.001).
Conclusion
This study showed the imaging features of CHH were associated with the lesion size. Based on postnatal follow-up, a new type of CHH was identified. If there are no complications at birth in CHH cases, a good prognosis is indicated.
Abbreviations
-
- AFP
-
- alpha-fetoprotein
-
- CHH
-
- congenital hepatic hemangioma
-
- DWI
-
- diffusion-weighted imaging
-
- HASTE
-
- half-Fourier single-shot turbo spin-echo
-
- MCA-PSV
-
- middle cerebral artery peak systolic velocity
-
- NICH
-
- noninvoluting congenital hemangioma
-
- PICH
-
- partially involuting congenital hemangioma
-
- RICH
-
- rapidly involuting congenital hemangioma
-
- T1WI
-
- T1-weighted imaging
-
- T2WI
-
- T2-weighted imaging
-
- TrueFISP
-
- true fast imaging with steady-state precession
1 Introduction
Congenital hepatic hemangiomas (CHHs) are uncommon benign vascular tumors that proliferate in utero and are fully grown by birth [1]. On the basis of the different clinical regression cycles, CHHs are currently categorized into three types: rapidly involuting congenital hemangioma (RICH), noninvoluting congenital hemangioma (NICH), and partially involuting congenital hemangioma (PICH). RICH completely regresses within 12–18 months, NICH grows in proportion to body growth, and PICH initially shows regression similar to RICH but stops growing later [2-4].
At present, the prenatal diagnosis of CHH mainly depends on an imaging examination. According to the clinical treatment guidelines [3], fetal CHH can be diagnosed by the clinical outcome and imaging methods and generally does not require a pathological examination. To better understand the natural history of CHHs, the “Guidance Document for Hepatic Hemangioma (Infantile and Congenital) Evaluation and Monitoring” recommends continued monitoring of a CHH for at least 1 year [3].
In addition, although benign by pathology, CHHs have variable presentations ranging from asymptomatic to life-threatening complications in utero [5]. These complications include high-output cardiac failure with and without hydrops, abdominal compartment syndrome, hemolytic anemia, severe thrombocytopenia with disseminated intravascular coagulopathy (Kasabach–Merritt sequence), and hepatic dysfunction [6-8]. Therefore, a prenatal diagnosis, postnatal follow-up, and prompt detection of complications are essential for identifying and treating CHHs. To date, there have only been a few case studies that evaluated prenatal and postnatal imaging (ultrasound and magnetic resonance imaging [MRI]) features of CHH. To the best of our knowledge, no studies have examined the differences between imaging features and morphological characteristics of different-sized CHHs or performed a long-term follow-up.
Therefore, this study aimed to analyze the prenatal ultrasound and MRI findings of CHHs, to examine the imaging characteristics of CHHs of different sizes, to describe the natural history of CHHs, and to report the clinical outcomes of these patients. Our findings will provide a basis for the clinical diagnosis and evaluation of the prognosis of CHHs.
2 Materials and Methods
In this observational, retrospective study, all ultrasound and magnetic resonance images of fetuses diagnosed with CHH from 1 January 2016 to 1 December 2023 were reviewed. The diagnosis of CHH was made according to the International Society for the Study of Vascular Anomalies classification system, using multimode diagnostic methods combined with clinical manifestations, imaging features, and/or histopathological confirmation [2].
Using the maximum diameter of the lesion, we divided the patients into two groups (diameter < 4 cm group and diameter ≥ 4 cm group). The inclusion criteria included a liver mass diagnosed as CHH by a combination of prenatal ultrasonography and MRI and confirmed by postnatal MRI examinations, serial ultrasound examinations, or pathology results. Cases of CHH without follow-up results, those with termination of the pregnancy, or those proven to be hepatoblastoma were excluded. In fetuses who met the inclusion criteria, pregnancy and delivery outcomes were retrieved and reviewed. In live-born infants, neonatal evaluations with clinical confirmation of CHH were reviewed. In asymptomatic cases, serial images were observed to monitor the regression. This study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University (No. 2019-001). Informed consent was obtained from the parents/guardians of all patients.
2.1 Ultrasound Examinations
All prenatal ultrasound examinations were performed using the GE Voluson E10 (GE HealthCare, Zipf, Austria) and Samsung W10 (Samsung Healthcare, Gangwon-do, Republic of Korea) ultrasound diagnostic devices with an abdominal probe (3.5–5.0 MHz). During routine antenatal ultrasound screening, a comprehensive scan was conducted if a liver mass was detected. The location, size, echo, boundary, and blood flow distribution of the lesion and cardiothoracic ratio were recorded.
2.2 MRI Examinations
Fetal and infant MRI examinations were performed on a 1.5 T MAGNETOM Amira system (Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China) using a 13-channel body coil in combination with a spine coil. The fetal MRI protocols included half-Fourier acquisition single-shot turbo spin-echo, true fast imaging with steady-state precession, diffusion-weighted imaging (DWI), and T1-weighted imaging (T1WI) with and without fat saturation (FS). The infant dynamic enhanced MRI protocol included T1WI, T2-weighted imaging (T2WI), DWI, and FS-T1WI. The details of the acquisition parameters for all sequences are shown in Table 1. The images of the lesion were interpreted by two associate chief physicians or those with higher qualifications. The location, size, signal, and internal condition of the lesion were recorded.
| Parameters | HASTE | TrueFISP | DWI | T1WI | T1WI-FS |
|---|---|---|---|---|---|
| TR (ms) | 1300 | 4.1 | 5000 | 241 | 287 |
| TE (ms) | 100 | 1.76 | 97 | 4.62 | 4.62 |
| Flip angle | 180° | 79° | 90° | 90° | 90° |
| FOV (mm2) | 380.0 × 308.8 | 380.0 × 310.0 | 380.0 × 380.0 | 380.0 × 320.6 | 380.0 × 320.6 |
| Slice thickness (mm) | 3.5 | 4.0 | 5.0 | 3.5 | 4.0 |
| Acquisition matrix | 256 × 198 | 304 × 199 | 176 × 176 | 162 × 256 | 162 × 256 |
| Voxel size (mm3) | 1.5 × 1.5 × 3.5 | 1.3 × 1.3 × 4.0 | 2.2 × 2.2 × 5.0 | 1.5 × 1.5 × 3.5 | 1.5 × 1.5 × 4.0 |
| Bandwidth (Hz/Px) | 698 | 685 | 1136 | 150 | 150 |
| Scanning plane | Axial, sagittal coronal | Axial, sagittal coronal | Axial | Axial | Axial |
| Diffusion direction | — | — | 3 | — | — |
| b (s/mm2) | — | — | 0 and 800 | — | — |
| Acquisition time (s) | 28 | 12 | 87 | 18 | 21 |
- Abbreviations: DWI, diffusion-weighted imaging; FISP, true fast imaging with steady-state precession; FOV, field of view; HASTE, half-Fourier single-shot turbo spin-echo true; TE, echo time; TR, repetition time; T1WI, T1-weighted imaging; T1WI-FS, T1-weighted imaging-fat suppression.
2.3 Follow-Up
Asymptomatic patients did not have any treatment or a biopsy after birth and were followed up with contrast MRI (age: 0–6 months) and serial liver ultrasound examinations to confirm the prenatal diagnosis. We measured the volume (length × width × height × 0.523) of the CHH at every follow-up time point and continued for at least 12 months until liver ultrasound showed a stable size and vascularity twice consecutively. In patients with symptoms (high-output cardiac status) or patients with imaging evidence of cardiac enlargement (normal cardiothoracic area ratio was not > 1/3 [9]), intrauterine anemia (an increase in the middle cerebral artery peak systolic velocity > 1.5 multiples of the median [10]), or shunt with high flow, the screening interventions included laboratory findings (complete blood counts, liver function tests, thyroid function tests, fibrinogen, and alpha-fetoprotein) and echocardiography. In cases of CHH in which the parents chose to terminate the pregnancy, a fetal autopsy was performed to confirm the diagnosis.
2.4 Statistical Analysis
Categorical data are shown as the number and percentage. Comparisons between groups were performed using Fisher’s exact test. The rank sum test was used to compare the regression trend of tumor volume at different time periods after birth, and growth curves of tumor volume in different time periods were constructed. SPSS 25.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) were used to perform the statistical analysis. p < 0.05 was considered statistically significant.
3 Results
3.1 Clinical Characteristics
Overall, 38 fetal CHHs were diagnosed by prenatal imaging at our institution. After excluding cases lost to follow-up without a biopsy (1 case) and cases with confirmed hepatoblastoma (1 case), 36 pregnant women with fetal CHH were included in this study. Among these cases of CHHs, 33 were confirmed by postnatal imaging, and 3 were confirmed by a biopsy. The age of the mothers in this study was 30.6 ± 4.9 years. The diagnosis of fetal cutaneous hemangioma typically occurred at a mean gestational age of 34 weeks, with a range spanning from 25 to 39 weeks.
3.2 Imaging Features
All 36 cases of CHHs were focal, including 22 cases in the right lobe and 14 cases in the left lobe. The boundary of 34 (94.4%) tumors was clear, and 2 (5.6%) tumors with an unclear boundary were located in the diameter > 4 cm group. In two-dimensional ultrasound images, 75% of the CHH tumors showed mixed echoes, and internal tubular anechoic structures of varying thickness were visible. However, mixed echoes were significantly more common in the diameter ≥ 4 cm group than in the diameter < 4 cm group (p = 0.026). In addition, two fetuses showed abnormal enlargement of the cardiothoracic ratio, and both were in the ≥ 4-cm-diameter group. In color Doppler flow images, 94.4% of the tumors had an abundant blood supply, and the blood supply of the hepatic portal vein was visible. In addition, 95% of the tumors were surrounded by circular or semicircular blood flow signals.
CHH was hyperintense on half-Fourier acquisition single-shot turbo spin-echo and fast imaging with steady-state precession images and relatively hypointense compared with the normal liver parenchyma on T1WI. However, the findings widely varied. In the < 4-cm-diameter group, CHH appeared more homogeneous, and CHHs in the ≥ 4-cm-diameter group had a more complex and heterogeneous appearance, such as necrosis (p = 0.023) and dilated intrahepatic vessels (p = 0.030), than those in the < 4-cm-diameter group (Figure 1). Thin distal abdominal aortas (18.8%) were also observed in large CHH cases. After birth, an early phase enhancement pattern was typically peripheral with a nodular distribution of contrast enhancement, and the delayed phase showed a centripetal fill-in pattern. The details of prenatal imaging features of the CHHs are summarized in Table 2.
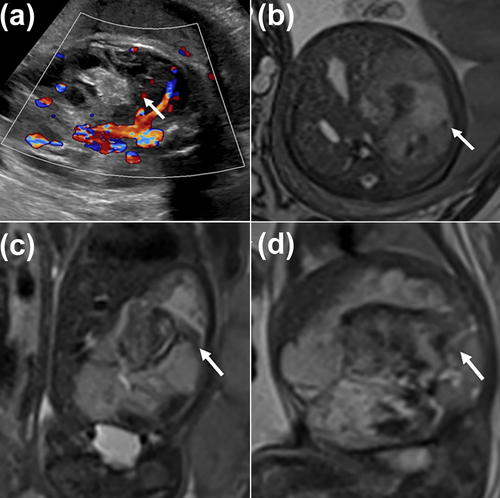
This is a prenatal ultrasound and MR image of a fetus with CHH at 33 weeks of gestation. (a) US shows the CHH is abundant with blood supply. (b–d) The axial, coronal, and sagittal MR images show a giant and complex CHH in the liver's right lobe (white arrowhead). Ultimately, the child developed heart failure and pulmonary insufficiency due to CHH and died shortly after birth. CHH, congenital hepatic hemangioma; US, ultrasound.
| Imaging features | Total (n = 36) | CHH (0–4 cm) (n = 20) | CHH (> 4 cm) (n = 16) | p |
|---|---|---|---|---|
| Sex | 0.999 | |||
| Male | 17 (47.2) | 9 (45.0) | 8 (50.0) | |
| Female | 19 (52.8) | 11 (55.0) | 8 (50.0) | |
| Location | 0.515 | |||
| Right lobe | 22 (61.1) | 11 (55.0) | 11 (68.8) | |
| Left lobe | 14 (38.9) | 9 (45.0) | 5 (31.2) | |
| Boundary | 0.190 | |||
| Clear | 34 (94.4) | 20 (100.0) | 14 (87.5) | |
| Unclear | 2 (5.6) | 0 (0.0) | 2 (12.5) | |
| Echogenicity | 0.026 | |||
| Homogeneity | 9 (25.0) | 8 (40.0) | 1 (6.2) | |
| Heterogeneous | 27 (75.0) | 12 (60.0) | 15 (93.8) | |
| Rich internal blood supply | 34 (94.4) | 18 (90.0) | 16 (100.0) | 0.492 |
| Marginal blood flow | 31 (86.1) | 16 (80.0) | 15 (93.8) | 0.355 |
| Cardiothoracic ratio | 0.190 | |||
| Abnormal | 34 (94.4) | 20 (100.0) | 14 (87.5) | |
| Normal | 2 (5.6) | 0 (0.0) | 2 (12.5) | |
| Intralesional hemorrhage | 7 (19.4) | 2 (10.0) | 5 (31.2) | 0.204 |
| Necrosis | 14 (38.9) | 4 (20.0) | 10 (62.5) | 0.016 |
| Shunting | 2 (5.6) | 0 (0.0) | 2 (12.5) | 0.190 |
| Dilated intrahepatic vessels | 7 (19.4) | 1 (5.0) | 6 (37.5) | 0.030 |
| Thin distal abdominal aorta | 3 (8.3) | 0 (0.0) | 3 (18.8) | 0.103 |
- Note: The bold values indicate that the difference is statistically significant (p < 0.05). Data are presented as n (%).
3.3 Growth Patterns/Natural History of CHHs
Infants who were not treated after birth were followed up regularly and consecutively. The changes in volume of the tumor at each follow-up in each child were also recorded (Figure 2a). A total of 18 (54.5%) cases were classified as RICH (Figure 3), 9 (27.3%) as PICH, and 2 (6.1%) as NICH. Additionally, a new type of CHH was identified in which four (12.1%) cases continued to proliferate after birth and spontaneously subsided in subsequent months (Figure 4). A nonparametric test showed that the average volume of CHHs decreased with an increase in age (Figure 2b). The volume of CHHs did not show significant age-related changes before 6 months of age. However, a significant decrease in CHH volume occurred after 9 months of life compared with that at birth (p = 0.001).
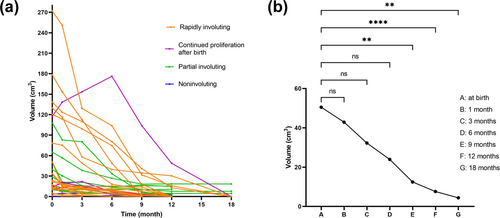
Growth curve and regression trend of congenital hepatic hemangioma. (a) Growth curve of CHH. The curves depict the volume change of each spontaneously regressed tumor over time. Different colors represent different types. Each curve represents the regression of the tumor over time in one child. (b) Changes in mean volume of CHH after birth. Mean volume of CHH tended to decrease with age. There was no significant age-related change in CHH volume before 6 months of age (p > 0.05). There was a statistically significant difference in the mean volume of CHH at 9 months of age. Mean CHH volume at 9 months of age was statistically significant compared to that at birth (p = 0.001). ns indicates no statistically significant difference. ** indicates p < 0.01. **** indicates p < 0.0001.
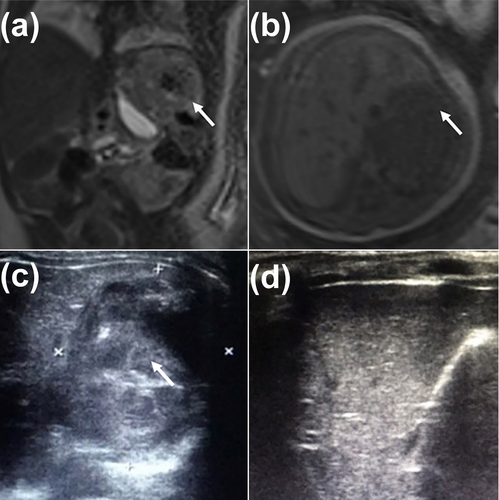
Prenatal and postnatal images of a child with rapidly involuting congenital hemangioma. (a, b) Coronal T2WI and axial T1WI show the fetal CHH in the left lateral lobe of the liver at 38 weeks' gestation (white arrowhead). (c) The US shows no significant change in the lesion's volume at 3 months old (white arrowhead) compared with the fetal stage (white arrowhead), but the blood flow signal is significantly decreased, and an irregularly strong echo is observed in the hepatic parenchyma. (d) The US shows that the mass in the left outer lobe of the original liver disappeared 8 months after birth. T2WI, T2-weighted imaging; T1WI, T1-weighted imaging; US, ultrasound.
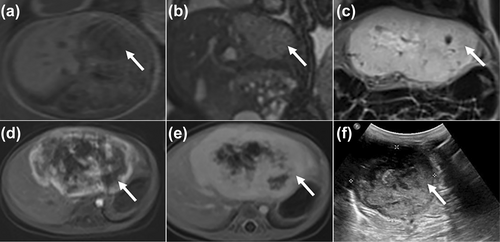
An atypical CHH subtype identified at 25 weeks' gestation (after birth, the CHH initially proliferated and was followed by spontaneous regression). (a, b) Axial T1WI and coronal T2WI show the CHH in the left lobe of the liver at 25 weeks' gestation (white arrowhead). (c) The coronal T2WI shows the lesion is significantly larger (white arrowhead) at 1 month after birth than that in the fetus. (d, e) The dynamic enhanced MRI confirms the prenatal diagnosis of CHH (white arrowhead). (f) The US shows the CHH becoming smaller (white arrowhead) at 3 months after birth. The CHH disappeared at the age of 15 months in the end. CHH, congenital hepatic hemangioma; MRI, magnetic resonance imaging; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; US, ultrasound.
3.4 Clinical Outcome of CHH
After consultations between the parents and healthcare providers, termination of pregnancy was decided in two (5.6%) cases, both involving fetal tumors exceeding 4 cm in diameter. One case of prenatal imaging showed symptoms of intrauterine anemia (middle cerebral artery peak systolic velocity: 1.52 multiples of the median). In another case, pregnancy was terminated because of an enlarged cardiothoracic ratio (0.66).
The majority (94.4%, n = 34) of CHH cases resulted in live births, with delivery occurring between 36 and 41 weeks of gestation (mean: 38 weeks and 2 days). Neonatal birth weight measurements varied from 2.4 to 4.2 kg, averaging 3.3 kg across the cohort. Only one (2.9%) case of CHH did not survive after a preterm birth because of high-output cardiac failure (Figure 1). This neonate had a large CHH with a volume of 376.38 cm3. The remaining 33 (97.1%) cases were asymptomatic and had normal liver function tests, thyroid function tests, and fibrinogen levels. No complications were observed in any of the patients, and all patients showed favorable clinical outcomes and positive prognoses.
4 Discussion
This study showed that the imaging features of CHHs were diverse. Smaller CHHs were usually homogeneous, whereas larger CHHs tended to be more commonly heterogeneous because of hemorrhage and necrosis. CHH had a natural regression process, and the tumor was significantly regressed after 9 months. We also identified a new type of CHH with postnatal proliferation followed by spontaneous regression. The patients with CHH showed a good prognosis if the lesion did not cause complications at birth, but some complications of CHHs can be fatal. These findings are important because they contribute to clinical outcome predictions for patients with CHHs. There is a lack of large-scale data on the natural history of CHHs. Therefore, this study provides useful data on the interpretation and understanding of the evolution of CHHs.
In our study, most CHHs were detected during the third trimester, and the initial diagnosis was typically established at a mean gestational period of 34 weeks. The time frame for detection spanned from the 25th to the 39th weeks of pregnancy. These findings are consistent with previous case series studies [8, 11]. In this study, all 33 cases of CHHs of fetuses were single lesions, and 61.1% of the tumors were located in the right hepatic lobe. These findings are consistent with a report by Isaacs [12].
Our study also showed that the imaging features of CHH were significantly associated with the size of the lesion. These findings are consistent with a previous study, which showed that smaller hemangiomas were homogeneous, whereas larger hemangiomas included calcifications, cystic spaces, hemorrhages, necrosis, and fibrosis [3]. Severe complications are more likely to occur in CHHs with a tumor diameter ≥ 4 cm than in those with a tumor diameter < 4 cm. Therefore, in the clinical setting, more attention should be paid to large CHHs and timely treatment if complications occur.
The growth patterns of CHHs in our study were different from those in previous studies [13]. In our study, 93.9% of cases were completely or partially resolved, and 6.1% of cases were NICH. However, four cases showed a proliferative trend during the first 6 months of life followed by spontaneous regression in the following months. In previous studies, this pattern was thought to be characteristic of infantile hemangioma. This pattern is different from the definition of CHH, which states that CHHs proliferate in utero and are fully formed at birth. Our study findings complement the current knowledge that CHHs can also show a tendency of initial proliferation followed by spontaneous regression over several months. Nevertheless, we found that the fastest decline in CHH volume occurred 9 months after birth. This finding could help clinical outcome predictions for patients with CHHs.
Regarding long-term clinical outcomes, all cases in our study were asymptomatic, except for one case with a large CHH with a volume of 376.38 cm3. The patient did not survive because of severe complications from congestive heart failure at birth. This finding indicates that a large CHH can be an adverse prognostic factor [14].
This study has some limitations. First, the results might have been affected by the relatively small sample size. Therefore, we will continue to collect further relevant data from new eligible participants to increase the sample size and power of this study. However, because of the rarity of CHHs in fetuses, this study is the largest case–control study on the subject to date. Second, histological or genetic markers were not identified to differentiate between RICH and NICH in our study. Future studies should include more follow-up data to investigate the underlying mechanisms of CHH, examine histological and genetic findings, and determine differences between different types of CHHs.
5 Conclusions
We report the prenatal ultrasound and MRI features of CHHs and found differences in acoustic shadows with different diameters. The trend of tumor regression was examined, and a new type of CHH was identified. The combination of MRI and ultrasound can improve the diagnostic level of CHH, which is beneficial for the reasonable management of pregnancy and the prediction of clinical outcomes of patients with CHHs.
Author Contributions
Luyao Yang: data curation (lead), investigation (lead), validation (lead), visualization (lead), writing – original draft (lead). Jianbo Teng: conceptualization (equal), methodology (equal), supervision (equal), writing – review and editing (equal). Xinhong Wei: conceptualization (lead), methodology (lead), project administration (lead), resources (lead), supervision (lead), writing – review and editing (lead).
Acknowledgments
The authors have nothing to report.
Ethics Statement
This study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University (No. 2019-001).
Consent
Informed consent was obtained from the parents/guardians of all patients.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




