Association Between Fetal Myocardial Alterations and Congenital Heart Disease Based on Post-Mortem Myocardial MRI
Funding: This work was supported by Zhejiang Provincial Natural Science Foundation of China (Grant/Award number: ZCLTGY24H0401) and Education Department of Zhejiang Province (Grant/Award number: Y202352970).
ABSTRACT
Background
Congenital heart disease (CHD) results from abnormal heart development during fetal development, leading to life-threatening complications. This study aimed to evaluate the feasibility of applying myocardial parametric mapping in post-mortem magnetic resonance imaging and to examine differences in the left ventricular myocardium between fetuses with CHD and controls.
Methods
This prospective case–control study was conducted on 14 deceased fetuses with CHD (CHD group) and 24 fetuses without CHD (control group). Fetuses with CHD were further stratified into the cyanotic (n = 9) and non-cyanotic (n = 5) CHD groups. T1, T2, and proton density relaxation times of the left ventricular myocardium were calculated and compared using multiple-dynamic multiple-echo post-mortem magnetic resonance imaging technology.
Results
The myocardial T2 relaxation time was significantly different between the groups (p = 0.033), with no difference in T1 or proton density relaxation times between the groups. A one-way analysis of variance with Tukey's test showed that the mean cyanotic CHD group showed a longer myocardial T2 relaxation time than the control group (98.000 ± 13.143 vs. 83.542 ± 9.491 ms, p = 0.003). Additionally, the correlation coefficient in the CHD group was significantly different between the myocardial T2 relaxation time and peak systolic velocity of pulmonary artery on a fetal echocardiogram (r2 = 0.681, p = 0.010).
Conclusions
These results suggest that using myocardial alterations in the T2 relaxation time may provide a accurate early warning for myocardial injury and enable noninvasive recognition of cardiac involvement in fetuses with CHD.
Abbreviations
-
- Ao-PSV
-
- peak systolic velocity of the aorta
-
- CHD
-
- congenital heart disease
-
- LV
-
- left ventricular
-
- MAGiC
-
- MAGnetic resonance image Compilation
-
- MRI
-
- magnetic resonance imaging
-
- PA-PSV
-
- peak systolic velocity of the pulmonary artery
-
- pmMRI
-
- post-mortem magnetic resonance imaging
-
- TGA
-
- transposition of the great arteries
-
- TOF
-
- tetralogy of Fallot
1 Introduction
Congenital heart disease (CHD) is the most common type of congenital defect worldwide and one of the main causes of neonatal and child mortality [1]. CHD was described as a large, rapidly emerging, global health burden among children in the 2017 Global Burden of Disease Study [2]. The development of prenatal diagnosis techniques and improvements in pediatric cardiac surgery have led to a positive long-term prognosis in some children with complex CHD [3]. Epidemiological studies have shown that CHD causes a range of high-risk neurodevelopmental alterations and deficient myocardial organization, and may be associated with long-term management of major complications and chronic health problems [3-5]. However, whether short- or long-term outcomes in offspring are directly associated with early changes in myocardial impairment in utero remains unknown.
Prenatal and neonatal multimodality imaging has greatly contributed to the understanding of congenital heart defects and the development and advancement of surgical and interventional treatments [1, 6]. Prenatal Doppler echocardiography remains the cornerstone of cardiac imaging in fetuses with CHD [7]. Fetal or post-mortem cardiovascular magnetic resonance plays an important role in myocardial anatomical and functional assessment [8, 9]. However, fetal post-mortem cardiovascular magnetic resonance is limited by several factors, such as artifacts caused by maternal respiration and gross fetal movements, an elevated heart rate, and immature cardiac gating. Post-mortem magnetic resonance imaging (pmMRI) may be an alternative to conventional autopsy because of its reasonable sensitivity and specificity, particularly for imaging the brain, spinal cord abnormalities, and heart [8, 10]. Tang et al. [11] showed that when using an optimized scanning protocol, cardiac structures of fetuses terminated early in gestation on 9.4-T pmMRI can be clearly displayed. As reported by Ulm et al. [12], pmMRI at 3.0 T from 12 weeks' gestation onward provided high diagnostic accuracy for fetal congenital structural heart defects with a sensitivity of 87.8%, specificity of 97.9%, and concordance with autopsy of 95.3%. Additionally, the minimally invasive method, high-resolution techniques, and advanced computer visualization tools in pmMRI to explain congenital cardiac anomalies to patients and families have slowly been accepted [13]. With the development of these innovative and non-invasive imaging techniques, parents will have more choices of acceptable options to investigate fetal CHD, especially using pmMRI [14].
Myocardial parametric mapping is a robust cardiac magnetic resonance imaging (MRI) technique that enables pixel-by-pixel myocardium quantification [15]. Parametric mapping has been extensively incorporated into routine clinical practice for cardiomyopathy in adults [16-18] and for cardiology and CHD in pediatrics [19]. These mapping values are measures of non-invasive myocardial tissue characterization, and they correlate with prognostic information [20]. Thayyil et al. [21] reported that fetal and infant post-mortem cerebral parametric mapping could be useful for optimizing pmMRI. However, myocardial parametric mapping in pmMRI has not been investigated in fetuses with CHD, and the related implication of parametric mapping values remains unclear.
Therefore, this study aimed to evaluate the feasibility of applying myocardial parametric mapping in pmMRI and to investigate its potential role in evaluating fetal myocardial alterations associated with CHD during the second and third trimesters.
2 Methods
2.1 Study Population
This prospective case–control study was conducted at the Women's Hospital, School of Medicine, Zhejiang University in Hangzhou, China between 31 August 2023 and 31 July 2024. All procedures were conducted according to the National Health Commission of the People's Republic of China guidelines and regulations in maternal–fetal medicine. The study was approved by the institutional review board of the Women's Hospital School of Medicine Zhejiang University (IRB-20230274-R). Parental written consent was obtained for pmMRI before delivery.
The following inclusion criteria were used in the study: (1) induced abortion, spontaneous abortion, and/or inevitable abortion; (2) stillbirth; (3) fetuses were 17–42 gestational weeks; and (4) a singleton pregnancy. Fetuses with chromosomal or structural anomalies that were identified prenatally or at birth were excluded. CHD was identified using fetal echocardiography (by two technicians each with 12 years of prenatal ultrasonography experience) and confirmed by pmMRI (by two personnel members each with 10 years of fetal MRI experience). The fetuses were divided into the CHD and control groups. We also reviewed transabdominal Doppler echocardiograms and recorded fetal cardiac assessment, such as the measurement of peak systolic velocity of the pulmonary artery (PA-PSV) and the aorta (Ao-PSV).
Cyanosis with CHD occurs in fetuses when persistent venous-arterial mixing occurs secondary to a right-to-left shunt (e.g., hypoplastic left heart syndrome, tetralogy of Fallot [TOF], pulmonary atresia, transposition of the great arteries [TGA], and truncus arteriosus) [22]. There are two CHD subtypes termed cyanotic and non-cyanotic CHD.
2.2 pmMRI Acquisition Protocol
In this study, pmMRI was performed on a GE 3T SIGNA Premier MR system using a 48-channel head coil (GE Healthcare, Milwaukee, WI, USA). The clinical standard, acquisition protocol, and sequence parameters used were as recommended for pmMRI by the European Society of Pediatric Radiology [23]. The following acquisition protocols for cardiac pmMRI were used: (1) Imaging and reporting channel confidentiality was confirmed within the hospital and imaging department. (2) All fetal bodies were stored in the mortuary at 4°C until MRI, which was performed within 24 h. (3) Three-dimensional (3D) isotropic T2-weighted images were acquired in three orthogonal planes with the following parameters: repetition time, 3000 ms; echo time, 90 ms; echo train length, 115; bandwidth, 62.5; acquisition voxel size, 0.6 mm × 0.6 mm × 0.6 mm. (4) Three-dimensional T1-weighted images in the axial plane for body imaging were acquired with the following parameters: repetition time, 550 ms; echo time, minimum; echo train length, 22; bandwidth, 62.5; and acquisition voxel size, 0.6 mm × 0.6 mm × 0.6 mm. (5) Multiple-dynamic multiple-echo imaging technology using a MAGnetic resonance image Compilation (MAGiC) sequence [24] in the short-axis view was used with the following parameters: repetition time, 4000 ms; echo time, auto; echo train length, 12; bandwidth, 22.73; slice thickness, 3 mm; and acquisition voxel size, 0.6 mm × 0.6 mm × 3.0 mm. (6) No exogenous contrast agent was used. The majority of scans were performed out of hours without disrupting the clinical service.
2.3 Parametric Mapping Analysis
Each case included conventional T1-and T2-weighted, fluid-attenuated inversion recovery, short-tau inversion recovery, phase-sensitive inversion recovery, and proton density imaging from multiple-dynamic multiple-echo imaging. Parametric mapping data were reconstructed using MAGiC software on the Advantage Workstation (GE Healthcare, Milwaukee, WI, USA). The entire left ventricular (LV) myocardium was examined by tracing the contours of the myocardium on a short-tau inversion recovery image. T1, T2, and proton density relaxation times were then calculated (Figure 1e–h). The pmMRI manifestations of typical TOF with a persistent left superior vena cava are shown in Figure 1. These manifestations were an overriding aorta, ventricular septal defect, right ventricular hypertrophy, and LV myocardial T2 hyperintensity.
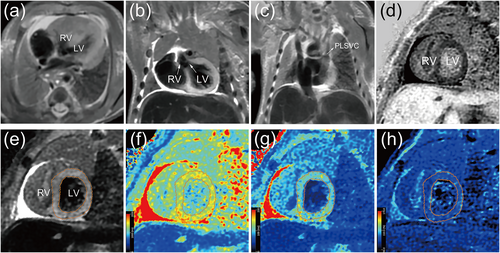
Myocardial parametric mapping analysis and imaging findings in tetralogy of Fallot. (a) Axial T2-weighted image of the heart with right ventricular hypertrophy. (b) Coronal T2-weighted image of an overriding aorta and ventricular septal defect. (c) Coronal T2-weighted image of a PLSVC. (d) Left ventricular myocardium with PSIR showing hypointensity in the short-axis view. (e) Left ventricular myocardium with STIR showing hyperintensity in the short-axis view. (f) T1 mapping (1387 ms). (g) T2 mapping (102 ms). (h) PD mapping (83.1%). LV, left ventricle; PD, proton density; PLSVC, persistent left superior vena cava; PSIR, phase-sensitive inversion recovery; RV, right ventricle; STIR, short-tau inversion recovery.
2.4 Masson's Trichrome Staining
LV heart sections from two fetuses at autopsy, namely one case of TOF and one control, were stained with Masson's trichrome for a histopathological analysis. In brief, the sections on slides were placed in xylene to remove the paraffin wax. The slides were stained with Ponceau-acid fuchsin solution for 10 min and rinsed with weak acid working solution for 30 s. The slides were then rinsed and stained with scarlet-acid fuchsin for 5 min and rinsed again. The slides were then differentiated in phosphomolybdic acid solution for 2 min, stained with aniline blue solution for 2 min, and rinsed with weak acid working solution for 30 s. The slides were dehydrated quickly in 95% ethanol for 3 s, and then dehydrated with absolute ethanol twice for each time 10 s. Finally, the slides were immersed in xylene twice, each time for 2 min, and then sealed with resin to complete the process.
2.5 Statistical Analysis
Data are expressed as the percentage, mean ± standard deviation, or median (interquartile range), as appropriate. The chi-square test was used to compare categorical variables. Continuous data were tested for normality using the Shapiro–Wilk test. Comparison of inter-group continuous variables was performed using the Student t-test or the Mann-Whitney U test. T1, T2, and proton density relaxation times were compared between three groups using a one-way analysis of variance and Tukey's test. A linear regression analysis was conducted to determine the relationship between continuous data. The tests were carried out using SPSS Statistics software (version 21.00; IBM, Armonk, NY, USA). In all comparisons, statistical significance was determined using p < 0.05. R Statistical Software Package (https://www.r-project.org/) was used to generate Figures 2-4.
3 Results
3.1 Population
From 31 August 2023 to 31 July 2024, 42 deceased fetuses were first evaluated using pmMRI. Four fetuses were excluded (one case of twins, one case of cri-du-chat syndrome [5p-syndrome], and one case of 22q11.2 deletion syndrome). Thirty-eight fetuses were included in the final pmMRI study. Maternal demographics and perinatal outcomes are shown in Table 1. The baseline data were similar between the groups. The maternal age in the CHD group (n = 14) was 30.714 ± 3.407 years, and the mean gestational age at delivery was 23.857 ± 3.371 weeks. The prenatal diagnosis of 14 CHD cases is shown in Table 2. There were nine cases of cyanotic CHD and five cases of non-cyanotic CHD. Prenatal diagnosis information for the control group is shown in Table 3. An autopsy was performed on one case of intrauterine fetal demise and one case of TOF at the family's request.
| Characteristics | CHD group (n = 14) | Control group (n = 24) | p |
|---|---|---|---|
| Maternal baseline | |||
| Maternal age, y | 30.714 ± 3.407 | 30.542 ± 4.344 | 0.899 |
| GDM, n (%) | 1 (7.143) | 2 (8.333) | 0.991 |
| HDP, n (%) | 0 (0) | 1 (4.167) | 0.741 |
| BMI, kg/m2 | 24.547 ± 3.525 | 24.005 ± 3.825 | 0.667 |
| Nulliparous, n (%) | 10 (71.429) | 16 (66.667) | 0.955 |
| Fertility treatment, n (%) | 4 (28.571) | 1 (4.167) | 0.249 |
| Post-mortem outcomes | |||
| GA at delivery, wk | 23.857 ± 3.371 | 26.083 ± 4.117 | 0.095 |
| Birthweight, g | 715 (550, 859) | 695 (508, 1090) | 0.317 |
| Male to female ratio | 1.800 | 0.500 | 0.180 |
| Fetal cardiac functions on echocardiography | |||
| Ao-PSV, m/s | 0.839 ± 0.218 | NA | NA |
| PA-PSV, m/s | 0.818 ± 0.251 | NA | NA |
- Note: Data are expressed as the percentage, mean ± standard deviation, or median (interquartile range), as appropriate.
- Abbreviations: Ao-PSV, peak systolic velocity of the aorta; BMI, body mass index; GA, gestational age; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy; MRI, magnetic resonance imaging; NA, not applicable; PA-PSV, peak systolic velocity of the pulmonary artery.
| Cases | Prenatal diagnosis |
|---|---|
| Cyanotic CHD | |
| Case 1 | Truncus arteriosus, aortic stenosis, ventricular septal defect |
| Case 2 | TGA, ventricular septal defect |
| Case 3 | TOF, PLSVC |
| Case 4 | TGA, single atrium, ventricular septal defect |
| Case 5 | TOF, PLSVC |
| Case 6 | TGA, PLSVC |
| Case 7 | TGA, ECD |
| Case 8 | TGA, single atrium, pulmonary stenosis |
| Case 9 | TOF, double-outlet right ventricle |
| Non-cyanotic CHD | |
| Case 10 | Single atrium, ventricular septal defect |
| Case 11 | Pulmonary stenosis, ventricular septal defect |
| Case 12 | Pulmonary stenosis, PLSVC |
| Case 13 | RVC, pulmonary stenosis |
| Case 14 | Single atrium, ECD |
- Abbreviations: ECD, endocardial cushion defect; PLSVC, persistent left superior vena cava; RVC, right ventricular cardiomyopathy; TGA, transposition of the great arteries; TOF, tetralogy of Fallot.
| n | Prenatal diagnosis before referral for pmMRI |
|---|---|
| 5 | Intrauterine fetal demise |
| 3 | Threatened abortion |
| 2 | Cleft lip and palate |
| 2 | Fetal growth restriction with reversed end-diastolic velocity of umbilical artery flow |
| 2 | Agenesis of the corpus callosum |
| 2 | Congenital chloride diarrhea |
| 1 | Facio-cervical lymphangioma |
| 1 | Leg hemangiomas |
| 1 | Varus deformity |
| 1 | Holoprosencephaly |
| 1 | Micrognathia, thoracic deformity |
| 1 | Myelomeningocele |
| 1 | Lissencephaly |
| 1 | Unilateral renal agenesis |
3.2 Changes in Myocardial Parametric Mapping
There were no significant correlations between gestational age and any of the myocardial parametric mappings. Figure 2 shows fetal myocardial changes in the CHD group compared with those in the control group in terms of parametric mapping. Fetuses in the CHD group (91.000 [83.750, 101.250] ms) had a significantly higher median (interquartile range) T2 relaxation time than those in the control group (85.000 [78.000, 89.500] ms) (p = 0.033). There was no significant difference in the mean T1 relaxation time or median relative proton density between the CHD and control groups.
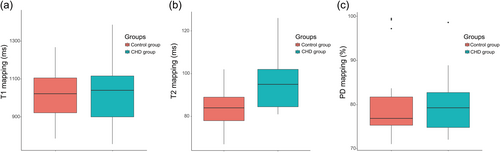
Box plots of parametric mapping of the left ventricular myocardium in the CHD group and the control group. Box plots represent the median, interquartile range (box) and 1.5 interquartile range (whiskers). (a) T1 relaxation time; (b) T2 relaxation time; and (c) PD relaxation time. CHD, congenital heart disease; PD, proton density.
The fetuses were stratified according to the fetal CHD types into the cyanotic CHD, non-cyanotic CHD, and control groups. Figure 3 shows pairwise comparisons of myocardial changes between the groups. Only the T2 relaxation time was significantly different between the groups (one-way analysis of variance, p = 0.004). Interestingly, the mean distribution of the myocardial T2 relaxation time was longest in the cyanotic CHD group (98.000 ± 13.143 ms), followed by the non-cyanotic CHD group (85.600 ± 6.841 ms) and control group (83.542 ± 9.491 ms). The cyanotic CHD group had a significantly longer T2 relaxation time than the control group (Tukey's test, p = 0.003). The myocardial T2 relaxation time was not different between the non-cyanotic CHD group and the control (p = 0.912) or cyanotic CHD (p = 0.089) group. These data indicated that the elevated myocardial T2 relaxation time in the cyanotic CHD group was the most pronounced.
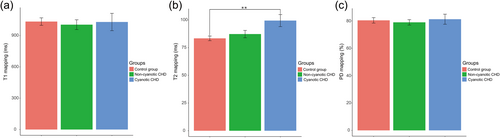
Characterization of myocardial alterations between the cyanotic CHD group, non-cyanotic CHD group, and control group. (a) T1 relaxation time; (b) T2 relaxation time; and (c) PD relaxation time. **p < 0.01 by Tukey-corrected one-way analysis of variance. CHD, congenital heart disease; PD, proton density.
3.3 Correlations Between Myocardial T2 Mapping and Fetal Cardiac Function on Echocardiography
Figure 4 shows scatter plots of the myocardial T2 relaxation time based on pmMRI and PA-PSV and Ao-PSV on a fetal echocardiogram in the CHD group. The correlation coefficient was significantly different between the myocardial T2 relaxation time and PA-PSV (r2 = 0.681, p = 0.010) but not Ao-PSV (r2 = 0.117, p = 0.703).
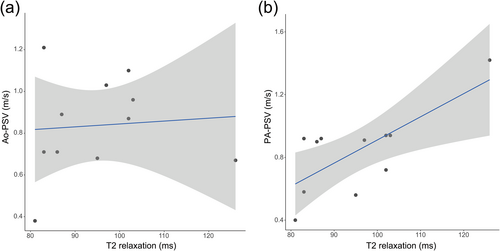
Correlations between T2 mapping and fetal cardiac function on an echocardiogram. (a) Correlation between myocardial T2 relaxation and Ao-PSV. (b) Correlation between myocardial T2 relaxation and PA-PSV. Ao-PSV, peak systolic velocity of the aorta on echocardiography in utero; PA-PSV, peak systolic velocity of the pulmonary artery on echocardiography in utero.
3.4 Masson's Trichrome Staining
Masson's trichrome staining was used to differentiate collagen fibers (blue cytoplasm) from muscle fibers (red cytoplasm) and show the severity of interstitial myocardial expansion (Figure 5). Muscle fibers from the control case showed positive staining. Interestingly, tissues in the TOF case showed substantial blue staining in left ventricular wall, which indicated diffuse fibrosis and diffusely interstitial myocardial expansion of the LV.
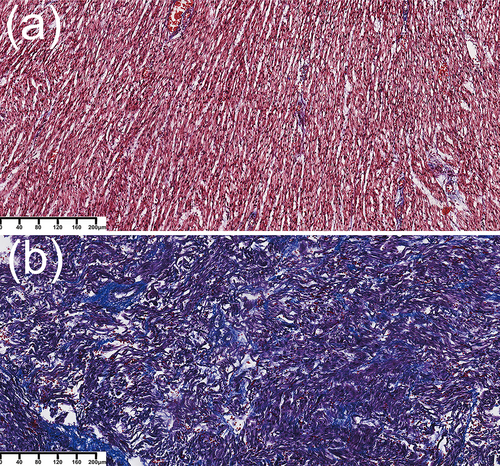
Masson's trichrome staining of myocardial tissue. (a) Case of intrauterine fetal demise. After Masson's staining, the muscle fibers show positive (red) staining. (b) Case of tetralogy of Fallot. Masson's trichrome staining shows substantial left ventricular wall fibrosis and diffusely interstitial myocardial expansion of the left ventricle (blue), as well as extensive structural damage to the myocardium along with irregularly arranged fibers of the left ventricle. Magnification, ×100.
4 Discussion
To the best of our knowledge, this is the first prospective pmMRI study on myocardial parametric mapping technology in the fetal population. We found that elevated T2 relaxation of the LV in fetuses with CHD was more pronounced than that in controls, especially in fetuses with cyanotic heart defects. The increased T2 relaxation time shown by pmMRI had a significant positive association with PA-PSV on a fetal echocardiogram in utero.
Myocardial parametric mapping depends on physiological parameters, such as age, sex, heart rate, and wall thickness. Parametric mapping in infants and fetuses in utero shows an elevated heart rate and a thin myocardium, while the lack of cardiac gating poses significant challenges [25, 26]. In a meta-analysis, the average myocardial T1 relaxation time examined at a magnetic field strength of 3.0 T was 1159 ms (95% confidence interval: [1143–1175 ms]) in healthy adult participants [27]. The pooled mean myocardial T2 relaxation time across the reviewed studies in this meta-analysis was 46 ms at 3.0 T (95% confidence interval: [44–48 ms]) [28]. In the present study, the myocardial T1 relaxation time in fetuses in both groups was slightly lower than that in healthy adult participants, while the myocardial T2 relaxation time in fetuses in both groups was notably higher. According to post-mortem cerebral parametric mapping studies by Thayyil et al. [21] and Arnold et al. [29], an increased T2 relaxation time of white matter may be a normal post-mortem change. These preliminary observations suggest that the application of myocardial parametric mapping based on pmMRI is feasible for non-invasive tissue characterization of the fetal myocardium.
The pathophysiology of myocardial injury in fetuses with CHD is not well understood. Myocardial fibrosis is a common pathological alteration in multiple types of insults to the myocardium in children and adults with CHD and is associated with chronic pressure load [30]. T1 mapping techniques are essential for the assessment of myocardial fibrosis [17]. The assessment of the myocardial T2 relaxation time is contingent upon the presence of free water content within the tissue, and is particularly useful in identifying acute myocardial inflammation or edema [26]. Fetuses with CHD had a higher myocardial T2 relaxation time, especially those with cyanotic heart disease, but not an elevated T1 relaxation time. The myocardial T2 relaxation time is used to index the extent of myocardial edema and is highly sensitive to acute myocarditis, regardless of the symptoms [31]. Studies have shown that T2 mapping technology is useful for determining the extent of cardiac decompensation in severe aortic stenosis [26]. Srisupundit et al. [32] suggested that ventricular overload in fetuses with CHD causes a progressive increase in the number and size of cardiomyocytes, resulting in cardiac dysfunction and hydrops. In our study, Masson's trichrome staining of the TOF case showed that the LV had substantial fibrosis and diffuse interstitial myocardial expansion. Consequently, an elevated T2 relaxation time may be an important myocardial characterization of cyanotic CHD, potentially suggesting myocardial edema and fibrosis. An elevated T2 relaxation time may be the first early sign of myocardial injury on MRI in fetuses with CHD and be different from previously characterized changes in children and adults.
In the past decade, there has been an increased acknowledgment of the intricate nature of CHD, which cannot be easily classified into a single category [33]. Cyanotic CHD is a congenital heart defect that results in hemodynamic abnormalities. Fetuses with CHD have altered hemodynamics because of underlying anatomical abnormalities and developmental progression [34]. Interestingly, in this study, the alteration of myocardial T2 relaxation in pmMRI was closely related to PA-PSV on a fetal echocardiogram in utero. CHD involves structural cardiac abnormalities that increase blood flow to the lungs and elevate systemic pressure, resulting in adverse pulmonary vascular remodeling and pulmonary hypertension [35]. A study on central blood flow distribution in fetuses with simple TGA by Blanc et al. [36] showed that fetuses with TGA had a greater fraction of their main pulmonary artery flow perfusing the lungs than normal fetuses, leading to a dominant LV. An experiment using a mouse model investigated the effect of chronic perinatal hypoxia on cardiac development [37]. This previous study showed considerable changes in gene expression, electrophysiological function, and contractile performance, with persistent effects that may contribute to worse myocardial injury and dysfunction observed in cyanotic CHD [7]. Hypoxia–reoxygenation cycles in cyanotic CHD promote oxidative damage and inflammatory cytokine release, perpetuating edema and fibrosis [38, 39]. This dual pathology may explain why T2 elevation is more pronounced in cyanotic CHD than in non-cyanotic CHD. Further studies on fetal CHD need to determine the causal association between hemodynamic changes and myocardial alterations in the T2 relaxation time, particularly in complex cyanotic CHD.
The experience of pregnancy loss can have a profound effect on families, leading parents to opt out of traditional autopsies because of their intrusive nature [14]. The primary limitation to this study was the absence of more cases pertaining to cardiac autopsies and histopathological evidence. Additionally, our study had a limited sample size of fetuses and a heterogeneous study cohort in terms of CHD subtypes. Furthermore, the control group showed local variations in the prenatal diagnosis, contributing to an overall lack of uniformity. The disparities in intrauterine and post-mortem environments further restricted the establishment of reference values for fetal myocardial parametric mapping. Finally, there was difficulty in interpreting and extending the advanced cardiovascular magnetic resonance techniques in complex perinatal settings, thereby limiting their clinical application. Further research and clinical evidence are required to assess the association between fetal myocardial and hemodynamic parameters and early survival and developmental outcomes in patients with cyanotic CHD [40].
5 Conclusion
This study shows that myocardial parametric mapping in pmMRI is promising for identifying LV histological damage in CHD. These insights highlight the potential clinical utility of this imaging approach in improving the management and outcomes of CHD cases.
Author Contributions
Weizeng Zheng: conceptualization (lead), data curation (lead), funding acquisition (lead), investigation (lead), visualization (lead), writing – original draft preparation (lead), writing – review and editing (equal). Xia Ying: investigation (equal), resources (equal). Yuan Chen: data curation (equal), formal analysis (equal), methodology (equal). Le Wang: data curation (equal), investigation (equal). Peiyue Jiang: conceptualization (supporting), data curation (supporting). Ying Jiang: supervision (equal), data curation (equal). Guohui Yan: resources (equal), software (equal). Hong Wang: project administration (equal), resources (equal). Yimin Zhou: validation (supporting). Yun Liang: validation (equal). Yu Zou: supervision (supporting). Liqun Sun: writing – original draft preparation (equal), writing – review and editing (equal). Qiong Luo: conceptualization (lead), funding acquisition (equal), project administration (lead), resources (lead), writing – review and editing (lead).
Acknowledgments
We are grateful to the staff of the Delivery Unit, Women's Hospital School of Medicine Zhejiang University, for their help in obtaining the deceased fetuses.
Ethics Statement
The study was approved by the institutional review board of the Women's Hospital School of Medicine Zhejiang University (Approval Number: IRB-20230274-R).
Consent
All participants provided informed consent.
Conflicts of Interest
This article belongs to a special issue (SI)-Fetal imaging, maternal and children imaging. As the SI's Guest Editor, Professor Liqun Sun is excluded from all the editorial decision related to the publication of this article. The remaining authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data generated and analyzed during this study are included in the published article.




