Prenatal Diagnosis and Management of Congenital Tracheal Stenosis
Funding: This work was supported by the Natural Science Foundation of Zhejiang Province (Grant number: LGF22H040003).
Abbreviations
-
- CTS
-
- congenital tracheal stenosis
-
- FIESTA
-
- fast imaging employing steady-state acquisition
-
- MRI
-
- magnetic resonance imaging
-
- SSFSE
-
- single-shot fast spin-echo
-
- VACTERL
-
- vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities
A 28-year-old pregnant woman with no prior obstetric complications had a normal prenatal workup before 24 weeks' gestation. At 24 weeks, ultrasound revealed gastrointestinal malformations, a persistent left superior vena cava, and polyhydramnios. At 29 weeks, prenatal magnetic resonance imaging (MRI) showed tracheal atresia or stenosis, a tracheoesophageal fistula, distal duodenal atresia, polyhydramnios, and polydactyly (Figure 1a–d). The patient delivered a male infant via cesarean on 6 August 2020. Computed tomography confirmed the prenatal findings (Figure 1e–h), but the infant died 3 h after birth. Autopsy revealed polydactyly, severe tracheal stenosis, esophageal atresia, a tracheoesophageal fistula, and distal duodenal atresia.
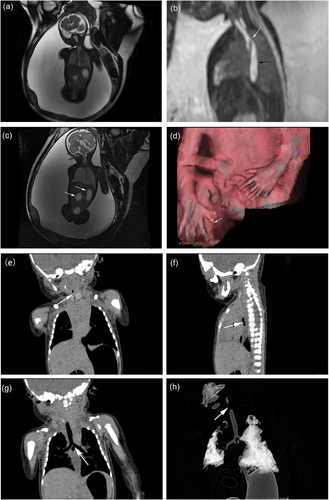
Fetal MRI at 29 weeks' gestation and routine computed tomography scan after birth. (a) Coronal FIESTA image shows an interruption in the upper trachea (white arrow), indicating tracheal atresia or stenosis. (b) Sagittal FIESTA image shows the upper edge of the tracheal carina communicating with the esophagus (white arrow), with expansion of the middle and lower esophagus (black arrow). (c) Coronal FIESTA image shows a distended stomach and duodenum, demonstrating the characteristic “double bubble sign” (white arrow). (d) Three-dimensional reconstruction image reveals polydactyly (white arrow). (e) Coronal reconstruction image shows an interruption in the initial trachea (white arrow). (f, g) Sagittal and coronal reconstruction images reveal a tracheoesophageal fistula at the tracheal carina (white arrow). (h) Volume rendering reconstruction of the trachea shows a discontinuity in the main trachea (white arrow), confirming tracheal atresia or severe stenosis.
Congenital tracheal stenosis (CTS) is a rare condition, occurring in approximately 1 in 64,500 live births and often associated with complete tracheal rings or syndromic conditions, such as VACTERL Isolated CTS accounts for only 10%–30% of cases, with most involving cardiopulmonary anomalies and gastrointestinal malformations, leading to a mortality rate exceeding 70%. Prenatal MRI, particularly FIESTA and SSFSE sequences, effectively delineates tracheal, bronchial, and surrounding structures aiding diagnosis. CTS primarily affects the upper trachea, with T2-weighted imaging revealing partial stenosis or absence, often with a tracheoesophageal fistula, gastrointestinal obstruction, and cardiopulmonary anomalies. T1- and T2-weighted imaging may also show gastrointestinal dilatation (e.g., the “double bubble sign”). These imaging techniques are critical for prenatal diagnosis and clinical decision-making. CTS often presents as a life-threatening emergency, with management complicated by its diverse manifestations and associated anomalies. Optimal outcomes occur in specialized centers with multidisciplinary expertise, with slide tracheoplasty as the preferred treatment.
Prenatal MRI is pivotal in diagnosing fetal CTS, aiding obstetricians in prenatal and perinatal management.
Author Contributions
Guohui Yan: conceptualization (lead), funding acquisition (lead), investigation (lead), methodology (equal), resources (equal), supervision (equal), writing – original draft (lead). Weizeng Zheng: conceptualization (equal), data curation (equal), resources (equal). Yongqing Zhang: data curation (equal), investigation (equal), resources (equal). Yu Zou: data curation (lead), formal analysis (lead), investigation (lead), resources (lead), writing – review and editing (lead).
Acknowledgments
The authors have nothing to report.
Ethics Statement
The present study was approved by the Institutional Review Board of Women's Hospital, Zhejiang University School of Medicine (Approval number: IRB-20210026-R).
Consent
Informed consent was waived for this retrospective study due to the inherent challenges in recontacting participants, in accordance with ethical guidelines governing research involving de-identified medical records.
Conflicts of Interest
The authors declare no conflicts of interest.
Citing Literature
Open Research
Data Availability Statement
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.




