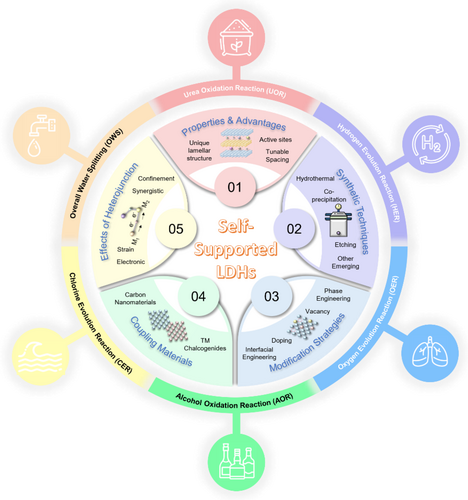
Fueling the future of clean energy with self-supported layered double hydroxides-based electrocatalysts: A step toward sustainability
Abstract
Amid the ongoing transition toward renewable fuels, the self-supported layered double hydroxides (LDHs) are envisioned as propitious electrocatalysts for reinvigorating the electrocatalysis realm, thereby facilitating environmental remediation and bolstering sustainable global energy security. Exploiting appealing attributes such as unique lamellar structure, abundant active sites, tunable intercalation spacing and compositional flexibility, LDHs boast remarkable activity, selectivity and stability across diverse energy-related applications. By virtue of addressing the technological and time prominence of excavating their renaissance, this review first encompasses the facile state-of-the-art synthetic approaches alongside intriguing modification strategies, toward deciphering the authentic structure–performance correlations for advancing more robust and precise catalyst design. Aside from this, heterostructure engineering employing diversified ranges of coupling materials is highlighted, to construct ground-breaking binder-free LDHs-based heterostructures endowing with unprecedented activity and stability. Subsequently, the milestone gained from experimental research and theoretical modeling of this frontier in multifarious electrocatalytic applications, including HER, OER, UOR, AOR, seawater splitting and other fundamental conversion reactions is rigorously unveiled. As a final note, a brief conclusion is presented with an outline of future prospects. Essentially, this review aspires to offer enlightenment and incite wise inspiration for the future evolution of innovative and resilient next-generation catalysts.
1 INTRODUCTION
In the face of addressing the dual crises of escalating environmental conundrum and energy shortcomings, renewable fuels have inspiringly offered a critical and elegant solution to help reduce dependency on conventional fossil fuels while mitigating environmental impact.1-5 Among myriads of renewable fuel sources, hydrogen (H2) fuel emerges as a prime candidate in this arena. In particular, the H2 fuel stands out as an exceptional energy carrier, known for its distinctive features including carbon-free emissions, high energy density (140 MJ kg−1) and renewable sourcing.6-8 Intriguingly, tremendous endeavors have been devoted to the advancement and development of energy storage and conversion devices toward accomplishing clean and sustainable energy storage, environmental remediation and even contributing to the production of a plethora of valuable chemicals/fuels.9-13 Electrocatalysis technology hits the limelight by involving multitudes of redox and conversion reactions in the core of these clean energy devices for greener, cleaner and more efficient production of H2, hence delivering several significant merits over conventional methods such as industrial steam methane reforming and coal gasification.14-17 This would pave an environmentally-benign path to promote a more sustainable energy landscape and address the as-mentioned pressing issues, well-aligning with the United Nations' Sustainable Development Goals (SDGs, e.g., SDG 7, SDG 11, SDG 13) and support the urgent call by the United Nations' International Panel on Climate Change (IPCC) toward accomplishing carbon neutrality in 2050.
Conventionally, the water-splitting cells pair the two primary half-cell reactions namely the hydrogen evolution reaction (HER) at the cathode with the oxygen evolution reaction (OER) at the anode.18, 19 Nevertheless, OER is notoriously sluggish, requiring high voltage and extensive energy consumption for efficient hydrogen generation, hence remains the major hurdle for commercialization. This is ascribed to its complex intrinsic kinetic of four-electron oxidation reaction (4 OH− ↔ O2 + 2 H2O + 4 e−) and elevated thermodynamic oxidation potential (1.23 V vs. RHE).20-22 Furthermore, another underlying bottleneck that aggravates the practical utilization of water electrolyzers arises from the safety concern of overall water splitting (OWS), due to the formation of explosive H2/O2 mixtures during the occurrence of gas crossover in the electrochemical cell. On the contrary, coupling HER with an alternative oxidation reaction (EHCO) to replace OER is a promising blueprint that renders a splash in this arena to tackle the previously mentioned challenges. The oxidation reaction of the more thermodynamically favorable small organic molecules such as alcohols,23, 24 N-containing molecules25, 26 or furan compounds27, 28 is gaining burgeoning research momentum in the catalysis community, as these anodic reactions are much more efficient in assisting hydrogen generation than the OER counterpart. The EHCO stands out ahead of OER, primarily due to its low theoretical thermodynamic potential and the added advantage of generating value-added chemicals with potential applications in the agrochemical, pharmaceutical and food industries.29, 30 Notably, these small molecules derived from biomass are carbon-neutral sources, hence offering sustainable and readily accessible alternatives to fossil fuel-derived fuels or chemicals.31, 32
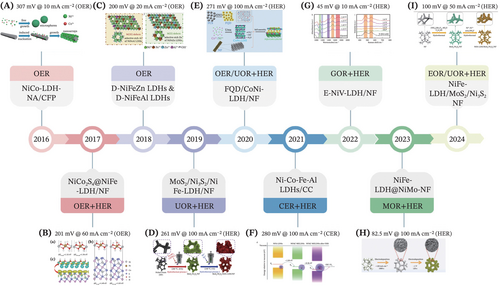
In this era of accelerating change, electrocatalysts play an utmost fundamental role as the core component of multifarious energy conversion devices, by offering adsorption and active sites for reactants while greatly dwindling the activation energies for multitudes of electrochemical redox processes involved.33-43 Notably, layered double hydroxides (LDHs) possess a range of attractive attributes, including abundant active sites, compositional flexibility, a unique lamellar ionic structure with brucite-like layers (comprising positively charged host layers and charge-balancing interlayer anions) and tunable interlayer spacing.44-46 These features have led to the remarkable utilization of LDH-based catalysts, introducing a revolutionary approach to electrocatalysis aimed at developing more robust and advanced catalysts, particularly in the field of electrochemical energy storage and conversion. Regard, the self-supported architecture of LDHs-based electrocatalysts supported by conductive substrates (e.g., carbon cloth, Nickel [Ni] foam, Copper [Cu] foam) offers a more viable and superior alternative to the conventional-coated powdery form. This is indexed to the utilization of additional insulating polymeric binders for assembling the powdery catalysts onto the electrode, rendering drawbacks such as aggregation, enhanced charge-transfer resistance due to poorly conductive nature and catalyst collapse risks from strong bubble formation.47 On the other hand, the self-standing LDHs nanoarrays shed light on accelerated mass and charge transport owing to the intimate interfacial connection between LDH and substrate, and greater accessible electroactive surface area; hence earning a commendable reputation in the realm of electrocatalysis.48 The timeline in Figure 1 vividly outlines their captivating journey of self-supported LDHs-based electrocatalysts, from being an obscure discovery to becoming a thriving research hotspot.
As of the present moment, the prodigious efforts of both theoretical chemists and experimentalists have sparked immense interest in this research area, with intensive studies focusing on the synthesis, characterization, electrocatalytic applications and performance evaluation of LDHs-based electrocatalysts. Nevertheless, although there are surging reviews in the domain of “self-supported electrocatalysts”,49-51 “LDHs”,34, 52, 53 and “EHCO”,54-56 limited attention has been directed to presenting an extensive and up-to-date review of the unprecedented advancements gained by the self-supported LDHs-based electrocatalytic system. There is a lack of recent reviews presenting a comprehensive summary of the potential application of self-supported LDHs-based electrocatalysts toward realizing sustainable H2 production via the coupling of anodic EHCO and cathodic HER, because attention is mostly dedicated to the application of water splitting. Also, the more facile and precise synthetic techniques, modification strategies and precise mechanistic insights into discerning authentic structure–activity correlation are in their infancy. There remains a pronounced dearth of attention toward critically reviewing the modifications performed on LDHs via coupling with different materials to form self-supported LDHs heterostructure for further ameliorating their electrocatalytic performance.
Hence, this review grabs the opportune moment to uncover these conspicuous research gaps while providing a comprehensive review with critical remarks on the recent advances of this frontier in myriads of applications in Figure 2. Herein, this review first highlights the state-of-the-art synthetic techniques to fabricate self-supported LDHs electrocatalysts, followed by an eloquent on the role of different coupling materials in forming self-supported LHDs-based heterostructures. Following this, the latest development of self-supported LHDs-based catalysts for multifarious electrocatalytic applications encompassing OWS (including HER and OER), EHCO and other emerging applications will be, respectively, deciphered. Finally, a summary and future perspectives are outlined to provide noteworthy prospects for the renaissance of this ground-breaking electrocatalyst. This review is anticipated to serve as the cornerstone and scaffold for accelerating and further exploring more novel and robust designs of self-supported LDHs-based electrocatalysts and even other related catalytic systems.
2 SYNTHETIC STRATEGIES OF SELF-SUPPORTED LDHs
Delving into the fundamentality of furthering the advancement of ultra-efficient electrocatalysts, it is crucial to precisely design and synthesize self-supported LDHs to enable the creation of catalysts with distinct structure, morphology and composition. This unlocks new possibilities for grasping valuable insights into their genuine structure-activity correlation, essential for extensive practical or industrial electrocatalytic applications. Herein, multifarious state-of-the-art fabrication techniques for preparing self-supported LDHs are meticulously discussed and presented, encompassing hydrothermal, co-precipitation, etching and other emerging synthetic methods.
2.1 Hydrothermal
Among the manifold synthetic strategies, the hydrothermal method is widely adopted for fabricating LDHs, attributed to several remarkable merits such as having facile operational procedures and simple chemical reactions.66, 67 Intriguingly, this approach has hit the limelight for devising efficient self-supported LDHs, whereby the constructed electrocatalysts successfully exhibit homogeneously ordered structures alongside splendid crystallinity, uniform morphology and immense surface area.68-71
The general procedures for preparing the self-supported LDHs via hydrothermal approach are outlined as follows: (1) Certain ratios of salt solutions (containing bivalent/trivalent metal ions), precipitating agents (urea, CO(NH2)247, 72-76) and in some occasions the structure-directing agents (ammonium fluoride, NH4F75) are well mingled in presence of the aqueous or organic solvent medium (deionized [DI] water73, 74, 77, 78 and methanol76) under an intense stirring. (2) Discretionarily, the substrate is subjected to pre-treatment such as treating carbon fiber paper with O2 plasma to enhance hydrophilicity,76 ultrasonication for substrate cleaning74 and etching Ni foam with HCl for removal of surface oxide layers.47, 78 (3) Subsequently, the uniformly mixed solution and pre-treated substrate are transferred to a Teflon-lined autoclave for undergoing thermal treatment at 100°C–120°C. The common heating duration ranges from 8 to 24 h to create a mild and stable reaction environment for LDHs' growth, before allowing them to be cooled down naturally to room temperature.79, 80 (4) The final step involves separating the constructed self-supported LDHs from the mixture, washing them several times with DI water and ethanol to remove attached materials, and drying in the hot air78 or vacuum oven.73 Of note, the precipitators play a pivotal role in this approach by first offering hydroxyl anions (OH−) for attracting metal ions to form metal hydroxides during the initial nucleation step, while assisting in constructing the layered structures during the succeeding hydrothermal treatment.48, 67
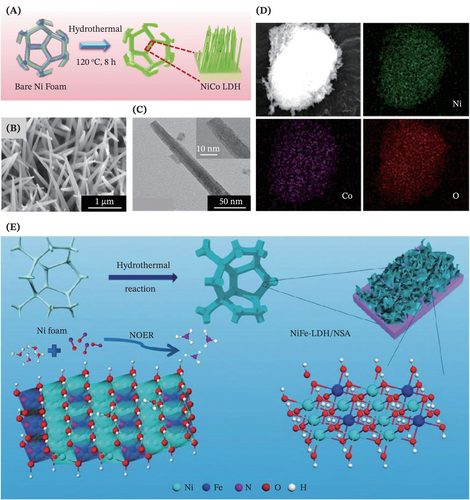
Herein, the binder-free LDHs have earned their rank as fascinating electrocatalysts where their rational design is well accomplished via harnessing the benefits of the hydrothermal route. For instance, Patil et al. developed a simple, one-step hydrothermal synthetic approach for fabricating the bimetallic NiCo-LDH nanowires supported by Ni foam toward the electrooxidation of methanol and water (Figure 3A).78 The field emission scanning electron microscopy (FE-SEM) characterization result revealed the structure and morphology of the as-synthesized electrocatalysts, manifesting uniform densely loaded and vertically aligned NiCo-LDH nanowires fully covered the Ni foam skeleton without disturbing the substrate's three-dimensional (3D) microporous structure (Figure 3B,C). Moving on, the energy dispersive spectrometer (EDS) mapping as depicted in Figure 3D further examined and confirmed that the elemental composition (Ni, Co and O) was uniformly distributed throughout the prepared catalyst sample. Likewise, Meng et al.81 crafted tremendous NiFe-LDH nanosheet arrays on Ni foam substrate surface for reducing nitric oxide (NO) to ammonia (NH3) via a one-step hydrothermal method (Figure 3E). Intriguingly, owing to the unique lamellar structure, exceptional porosity and synergistic effect between Fe and Ni,81-85 the easily formulated FeNi-LDH via hydrothermal approach have elicited burgeoning attention of researchers to deliver decent performance in varied electrochemical reactions such as O2 evolution76, 86 and NO reduction81 reactions.
Recently, defect engineering has enlightened the design optimization of LDHs for developing feasible and more novel self-supported electrocatalytic systems.87 This is because by leveraging additional exposed active sites as the “docking” sites for anchoring more intermediate reactive compounds, the adsorption abilities of the intermediate species are further optimized, hence contributing to electrocatalysis revitalization in the realm of energy-related applications and environmental remediation.53, 88-92 In particular, Lyu et al. reported an electronic structure regulation of self-standing NiFe-LDH via the hydrothermal approach, where Zn dopants are introduced through the addition of Zn(NO3)2 ∙ 6H2O into the mixture of solutions before immersing the cleaned Ni foam substrate to undergo the subsequent thermal treatment.75 Notably, both heteroatom doping strategies, which encompass the use of either highly valence metallic atoms (e.g., Zn,75 Ru, Ir74) or non-metallic atoms (e.g., P93 and S47) during the synthesis of binder-free LDHs are ingenious, as they can trigger perturbation in the electronic structure and coordination environment of active sites.
Moving on, benefiting from the advanced synergistic interplay of different elements, abundant active adsorption sites and flexible electronics configurations, the multielement LDHs electrocatalysts have been endowed with greater charge transferability leading to propitious catalytic activity.62, 94 For example, implementing the material design framework as outlined in Figure 4A, Enkhtuvshin et al. reported a pioneering work for constructing high-performance quaternary multimetallic LDHs (NiFeAlCo-LDH) for alkaline and seawater oxidation, by performing electrochemical incorporation of the fourth transition metal (Co) into the ternary NiFeAl-LDH grown via a typical hydrothermal reaction.62 The incorporation of Al into the quasi-semiconductive pristine NiFe-LDH matrix induced a metallic characteristic in the catalyst, thereby facilitating charge transfer between the active catalytic centers and intermediates during the OER. Remarkably, the introduction of cobalt via ZIF-67 further reduced the Fe elements within the catalyst, as evidenced by the lower Fe K-edge spectrum position. The flexible oxidation state of Fe, in conjunction with Co, played a key role in enhancing the OER efficiency of NiFeAlCo-LDH. This multielement electrocatalyst effectively modulated the electronic properties of multiple surface metals, leading to regulated adsorption energies of intermediates and a facile overall water oxidation process. Hence, this work establishes an unprecedented trail for fabricating the promising multielement LDHs electrocatalysts.
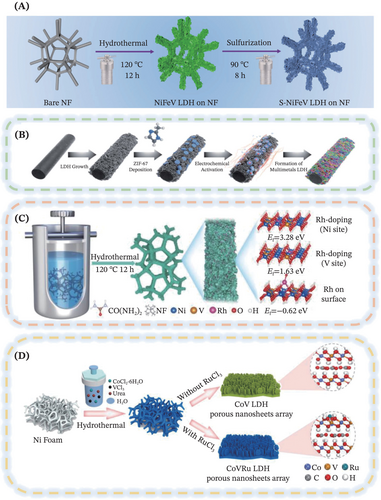
Exploiting and coupling the merits of both defect engineering and multi-element LDHs, Peng et al. recently reported a facile and novel two-step hydrothermal process to prepare the advanced sulfur-doped ternary NiFeV-LDH nanosheets supported on the nickel foam (Figure 4B).47 In detail, there occurred an anion exchange process during the sulfurization step, by undergoing a partial replacement of oxygen with sulfur from Na2S, to ultimately obtain the S-NiFeV-LDH. Inspiringly, the sulfur atoms were chosen as the anion dopants owing to the similar ionic radii and same group origination with the large amount of oxygen content in the NiFe-LDH, thus easing the doping and anion modulation processes to effectively accelerate the electrocatalytic reaction.47, 95 Meanwhile, the incorporation of the high-valence vanadium (V) element was indispensable in maintaining the catalyst surface configuration, by well-stabilizing the surface oxygen species to safeguard the robust stability of the constructed electrocatalyst.
On another note, studies on structural reconfiguration and composition modulation are hotly pursued to gain valuable insights for designing advanced electrocatalysts. For instance, Zhang et al. carried out the sodium dodecyl sulfate (SDS) intercalation modification strategy to construct the CoNi-SHS-LDH vertically grown on Ni foam.77 Remarkably, this intercalation technique via simple anion modulation effectively triggered a structural transformation of the CoNi-LDH electrocatalysts prepared from the typical hydrothermal method, leading to roughening of the nanosheet edges. This inevitably elevated the number of electrochemically active edge sites and addressed the easy agglomeration issue of the conventional NiCo-LDH catalysts, thus being beneficial to bolster the efficiency and performance of their electrochemical applications.77, 96, 97
Next, in light of the numerous merits of single-atom catalysts (SACs) such as unique coordination environment and outstanding atomic utilization, the synergy of SACs with LDHs triggers sapient inspiration of the catalysis community to further thrust the advancement of LDHs electrocatalysts.98, 99 For instance, Sun et al. developed a facile ethylene-glycol-assisted hydrothermal route to synthesize a multifunctional electrocatalyst termed Rh/NiV-LDH, by anchoring the Rh SACs on NiV-LDHs nanosheets72 (Figure 4C). As aforementioned, it was inferred that the incorporation of the V atom into the Ni-based LDHs could help to promote the suppression of surface restructuring and preservation of surface configuration, hence ensuring that the single atoms could be well-anchored on the catalyst surface. Interestingly, this work implied that the monodispersed single atoms were capable of altering the electronic structure of the parent catalyst matrix and circumventing the reaction barrier, thus escalating the electrocatalytic kinetics of the anodic urea oxidation reaction (UOR). Inspired by the previous work, a parallel example was demonstrated in the work of Zeng et al., where there was an incorporation of the atomically dispersed Ru on the binary CoV-LDH porous nanosheet73 (Figure 4D). Attributed to the negatively charged Ru clusters which possess more active electronic states and show prominent adsorption capacity of intermediates, the introduction of the Ru SACs was feasible and promising toward expediting the reaction kinetics and alleviating the HER activity.73, 100-104 In short, the exploration of the synergy of SACs with the LDHs is deemed imperative to further thrust the advancement of LDHs electrocatalysts.
Remarkably, the hydrothermal approach allows effective regulation over the size, crystal growth level and morphology characteristics of the self-supported LDHs by altering the reaction parameters such as the heating temperature, pressure, pH, reaction period and metals ratio.48, 67, 105-108 Briefly, it is foreseeable that the hydrothermal method could incite ingenuity for the fabrication of self-supported LDHs. However, to realize the practical employment of the hydrothermal approach in industrial settings, the demerits of having a long thermal treatment duration required must be taken into account and addressed so that it is conducive to large-scale manufacture.
2.2 Co-precipitation
By making the most of its preponderance such as cost-effectiveness, simplicity and uniform chemical composition, the co-precipitation method is esteemed as one of the most conventional fabrication approaches to synthesize the free-standing LDHs electrocatalysts.69, 79, 106, 109 In essence, the normative procedures to facilely prepare the LDHs consist chiefly of adding the alkaline solution functioning as precipitating agent (e.g., NaOH,110, 111 Na2CO3112 and ammonia113) dropwise to the aqueous solution (containing divalent and trivalent metal ions of the targeted LDHs product) at a proper concentration and molar ratio.105 Continuous stirring is performed for the crystallization process to occur under certain temperatures and pH conditions.79, 114 Of note, pH carries an extraordinary significance in tailoring and influencing the textural, structural and chemical features of the phases, hence diligent control of pH during the entire synthetic process is suggested to yield pure LDHs with optimal chemical homogeneity.67, 69, 115, 116 Various types of substrates including carbon cloth,112, 117, 118 carbon paper111, 119 and Ni foam109, 110, 120 are reported for the construction of binder-free LDHs via the co-precipitation approach for the electrocatalytic applications.
Excitedly, the formulation of self-supported Ni-based LDHs electrocatalysts is prevalently performed via the co-precipitation approach. One of the trailblazing employments of this method was showcased in the work of Wang et al., whereby the synthesis of NiCo-LDH supported on carbon cloth was reported using the one-pot co-precipitation preparation strategy.117 The constructed catalyst possessed an inverse spinal structure of both Co3+ (distributed on octahedral and tetrahedral sites) and Ni2+ (distributed on octahedral sites), where the numerous catalytically active sites allowed rapid reversible Faradaic reaction for the glucose oxidation reaction. The rationales behind selecting carbon cloth to be the conductive substrate for depositing the electrochemically active materials was ascribed to its fascinating conductivity, impressive flexibility and loosely arranged fiber network structure, thus facilitating the glucose diffusion for electrochemical reactions at the electrode interface.117, 121, 122 Likewise, the work of Zhang et al. concurred with the previous work, demonstrating the uniform growth of NiCu-LDH on the carbon cloth via the proposed one-pot co-precipitation method to form a 3D nano-architecture that successfully exhibited superior OER performance.
Following this, in a recent work by You et al., a coprecipitation process of the metal precursors (Ni(NO3)2 ∙ 6H2O and IrCl3 ∙ 3H2O) in the presence of formamide was conducted to obtain the NiIr-LDH supported on the Ni foam (Figure 5A).110 The introduction of the 5d transitional metal, Ir into the Ni-based catalysts was assigned to its prowess of inducing outstanding electronic structure optimization and considerable stability for OER, while harnessing its environmentally benign nature and high recycling efficiency. It was further elucidated that the addition of surfactant—23 vol% formamide solution was indispensable because the carbonyl group of the formamide possessed powerful interactions with the LDHs hydroxyl slabs to form hydrogen bonds, hence profoundly promoting the successful formation of ultrathin monolayer LDHs nanosheets electrocatalysts (thickness ~1.1 nm) (Figure 5B). As a result, the characterization results disclosed the formation of porous nanosheet structure of the electrocatalyst, revealing the crumpling of nanosheets in wavy and curly shapes with rich corner sites, edges and ultrahigh surface area (Figure 5C,D). The x-ray photoelectron spectroscopy (XPS) measurements revealed a notable enrichment of Ni(III) species on the NiIr-LDH surface, with levels exceeding those observed in NiFe-LDH, which is key to forming the active NiOOH phase and prominently boosting OER activity (Figure 5E). As confirmed by density functional theory (DFT) calculations and x-ray absorption fine structure (XAFS) spectroscopy, the integration of Ir into the Ni(OH)2 layer significantly modified the electron density at the Ir and Ni sites. Coupling these insights with in situ Raman spectra (Figure 5F) and electrochemical measurements, it was further verified that the presence of NiO and IrO bonds contributed abundant active sites that drove the superior OER performance of NiIr-LDH. The generation of rate-limiting *O and *OOH intermediates was accelerated at Ni and Ir sites, thereby underpinning the high seawater splitting efficiency. Impressively, Bhowmik et al. excavated the prospective utilization of the ultrathin monolayer graphitic carbon nitride (g-C3N4) as the supporting substrate to steadily host the CoFe-LDH sheets via a one-pot co-precipitation method at ambient temperature, whereby the constructed electrocatalyst (Co0.4Fe0.6-LDH/g-CNx) presented robust durability and exceptional bifunctional catalytic activity for both OER and HER.113 This was contributed by the presence of highly oxidized transition metal (Co3+ as revealed by the XPS study) for facilitating the formation of adsorbed O atoms due to their pronounced electrophilicity, accelerating the reaction between OH and O to produce OOH species, thereby boosting OER activity. Additionally, the presence of pyridinic and tertiary N species in g-CNx induces an asymmetric charge separation, in which this electronegativity difference imparted a highly positive charge density on the neighboring sp2-hybridized carbon atoms, which then favors the reactants' adsorption (H* for HER and OH− for OER).
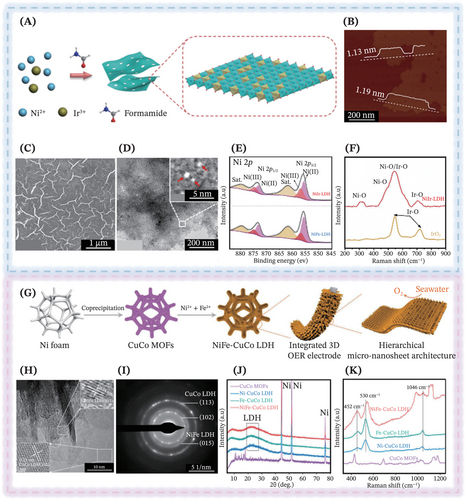
Progressing toward the synergistic catalysis of multimetallic LDHs, Yu et al. reported the construction of CuCo MOFs on Ni foam via the room temperature coprecipitation approach, followed by an ingenious etching-incorporation-sedimentation transformation process to produce the ultrathin dual-metal co-incorporated NiFe-CuCo-LDH micronanosheet arrays with abundant active sites for the expedition of electrolyte diffusion (Figure 5G).120 In detail, the introduction of Ni and Fe synergistically modulated the electronic structure of CuCo-LDH, which in turn augmented the anti-corrosion properties of the electrocatalyst against the aggressive corrosive nature of seawater, while ameliorating the electrical conductivity to vitally induce rapid charge transfer during seawater oxidation reaction. It was noticeable that the final catalysts were composed of both NiFe-LDH and CuCo-LDH, as evinced in the high-resolution TEM (Figure 5H) and well-resolved diffraction rings in the selective area electron diffraction (SAED) pattern (Figure 5I). Both the XRD and Raman spectra substantiated the complete phase transformation of the catalyst, thus affirming the successful conversion of MOFs into multimetallic LDHs via the designed approach (Figure 5J,K).
Later on, several modification strategies have come into researchers' sights and earned prominent acclaim recently, rendering more promising and advanced designs of the self-supported LDHs for myriads of electrocatalytic applications. Availing vacancy construction in the preparation of monolayered NiCo-LDH by using the one-step co-precipitation method, Liu et al. reported that the rich hydroxyl vacancies endowed the catalyst with superior methanol oxidation reaction performance.111 This was because the existence of abundant hydroxyl vacancies helped in boosting the state density to near Fermi level of LDHs, elevating the electrical conductivity during charge transport, as well as promoting the adsorption and further cleavage of methanol. Meanwhile, Cai et al. supplied inspiring insights on designing advanced LDHs electrocatalysts with favorable atomic configuration, by tuning the surface coordination environment to optimize the electrocatalytic reaction.109 Particularly, in this work, the modulation of local structure within NiFe-LDH structure was reported through partial substitution of the Ni2+ with Fe2+, to introduce the double Fe sites to form the FeOFe moieties (Figure 6A). The formation of these moieties functioned as stable high-valent oxygen-bridged metal motifs (OBMM) that were responsible for markedly increasing the OER intrinsic activity.
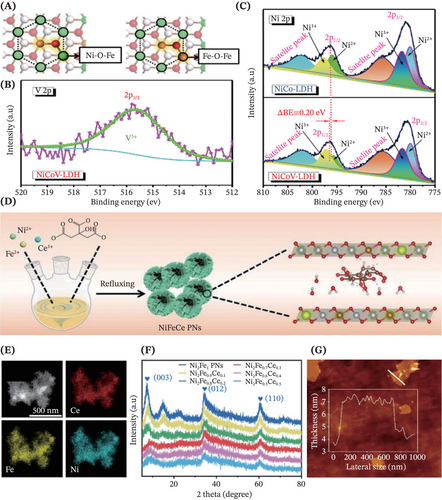
Advancing to address the underlying issues of NiCo-LDH such as the near-to-ground conductivity and stability due to the unescapable aggregation and nanosheet restacking which leads to reduced OER performance, Bera et al. synthesized the binder-free NiCoV-LDH via the co-precipitation method by introducing the foreign vanadium cations in +3 oxidation state (Figure 6B).112 It was revealed that the doping of V3+ into NiCo-LDH synergistically facilitated the electron transfer among metal ions by altering the electronic structure of Ni2+ ions (as shown in the high-resolution XPS spectra in Figure 6C), thus favoring the designed electrocatalysts toward OER studies. To revitalize the electrocatalysis, a promising approach is proposed by Chen et al. by synergistically coupling multiple modification strategy (doping with Ce atoms, intercalation with citrate anions and modulation of anion composition by optimizing Ni:Fe:Ce ratio) for preparing the hierarchical self-supported NiFeCe-LDH electrocatalysts via the co-precipitation method (Figure 6D).119 Particularly, owing to the outstanding electron interaction between 3d and 4f orbitals, the doping of the low-cost Ce element had appreciably contributed to ease morphological change and enhance intrinsic OER activity. The characterization results revealed the successful fabrication of the as-mentioned LDHs with a homogeneous distribution of all elements indicating successful Ce incorporation (Figure 6E), high-phase purity (Figure 6F) and ultrathin nanosheets-like profile showing intimate staking of 4–5 host layers (Figure 6G).
Overall, the co-precipitation strategy sheds light on the rational preparation and design of self-supported LDHs electrocatalysts. Nevertheless, a considerable number of limitations including low reproducibility, less yield, the possibility of contamination and aggregation that may readily hamper the practical utilization of this method must be acknowledged and resolved as a priority to ensure feasible scaling of this technology in industrial settings.
2.3 Etching
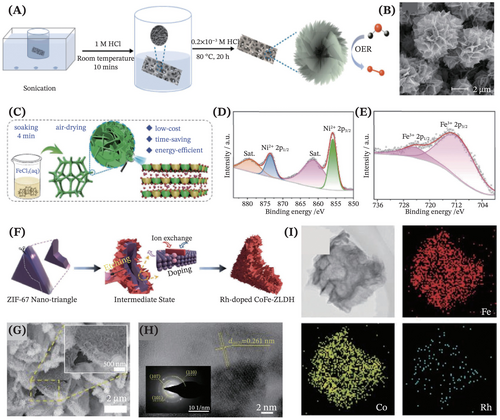
The etching synthesis approach opens a new philosophy in designing and fabricating the self-supported LDHs. This approach sparks the interest of the catalysis community in employing a facile one-pot etching method to directly synthesize binder-free LDHs. For instance, Wang et al. reported an in situ acid wet-etching method to yield the NiFe-LDH supported on the NiFe foam substrate, with Figure 7A portraying the overall synthetic scheme.123 Notably, the acid solution played a crucial role in ionizing the Ni and Fe atoms of the NiFe foam substrate which reacted with each other to obtain the NiFe-LDH. The as-synthesized LDHs exhibited a unique hydrangea flower-like structure (Figure 7B) with abundant active sites and ultrahigh surface area, hence displaying remarkably high OER electrocatalytic performance. Next, Li et al. proposed an ultrafast, exceedingly simple and energy-efficient synthesis of NiFe-LDH nanosheets array vertically anchored on the Ni foam substrate via a one-step solution-phase etching method at ambient temperature and pressure (Figure 7C).124 Briefly, the procedure was as simple as soaking the Ni foam (functioned as both Ni source and substrate) into the FeCl3 (Fe3+ ions served as both Fe source and oxidant) for 4 min, wiping off attached liquid using filter paper and finally air-drying at room temperature to obtain the [email protected] sample. The XPS characterization technique revealed that only NiFe-LDH was formed on the Ni foam with complete coverage on the substrate (Figure 7D,E). Enthrallingly, Zhu et al. reported an efficient simultaneous etching-doping sedimentation equilibrium (EDSE) strategy for designing and preparing the hollow Rh-doped CoFe-LDH supported on Ni foam substrate, by inducing the conversion of zeolite imidazolate framework-67 (ZIF-67) nanotriangles into these template-directed LDHs electrocatalysts (Figure 7F).125 From this work, it was revealed that the Rh doping led to the construction of Fe vacancies and the electron structure modulation of Co and Fe, hence allowing exposure of more active sites and facilitating the mass transfer. Through the EDSE process, it was uncovered that the as-obtained Rh-doped CoFe-LDH inherited the nanotriangle morphology from the ZIF-67 with uniform distribution of Rh throughout the hollow nanotriangles electrocatalysts (Figure 7G–I).
Henceforth, it is captivatingly remarked that the utilization of the etching approach as the subsequent modification step following the first LDHs synthesis step is invoking a great deal of researchers' interest in designing advanced self-supported LDHs with bolstered electrocatalytic performance. For instance, Feng et al. employed a facile two-step method that encompasses hydrothermal treatment and anodic electrochemical etching for constructing NiAl-LDH supporting on the Ni foam (Figure 8A).126 The etching technique synergistically optimized the electronic and geometric structure of the prepared LDHs, via generating coordination-unsaturated active Ni2+ sites and enabling the introduction of oxygen defects. Thus, the resulting electrocatalysts demonstrated preeminent alkaline water splitting performance due to enhanced electrical conductivity and mass transportation. In another case, Xie et al. studied the defect effects created on NiFe-LDH by deliberately introducing the base-soluble Zn(II)/Al(III) sites through a doping strategy, followed by an alkaline etching treatment to selectively create atomic defects.59 This work provides new inroads for carrying out simultaneous defect engineering at the atomic scale to dramatically tune the active sites on the multicomponent binder-free LDHs.
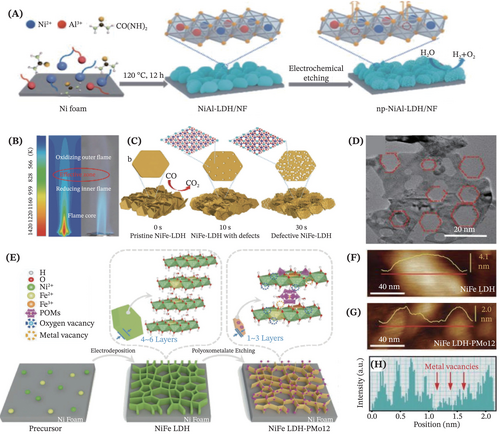
Apart from this, Zhou et al. put forward a fast flame-engraved method for precisely creating abundant oxygen vacancies and etching part of the NiFe-LDH prepared from the hydrothermal method in a safe and open atmosphere without requiring sophisticated equipment (Figure 8B,C).127 As a result, well-defined hexagonal cavities with (110) edges (Figure 8D) were observed in the engraved NiFe-LDH, alongside the existence of abundant oxygen vacancies, more dangling bonds of Ni and Fe metal sites and lower coordination number, thus contributing to substantially enhanced OER activity. Cai et al. ingeniously reported a versatile polyoxometallic acids (POMs) etching approach for reconstructing the NiFe-LDH prepared via electrodeposition strategy (Figure 8E).128 This etching approach represented a new paradigm for reinforcing self-supported LDHs electrocatalysts to accomplish electrocatalytic performance enhancement. A myriad of synergistic effects dominantly contributed to propitious OER performance, including the active species reconfiguration, intercalation of POM polyanionic clusters, 3D morphological nanotailoring such as thinning of NiFe-LDH (Figure 8F,G), as well as the creation of multiple metals (Ni and Fe) and oxygen vacancies (Figure 8H). In brief, the etching approach is a promising strategy that can either be directly employed for synthesizing high-performance self-standing LDHs electrocatalyst, or be employed as a complementary subsequent step for modifying and tailoring the LDHs prepared using other synthesis techniques for producing auspicious electrocatalysts.
2.4 Other emerging preparation techniques
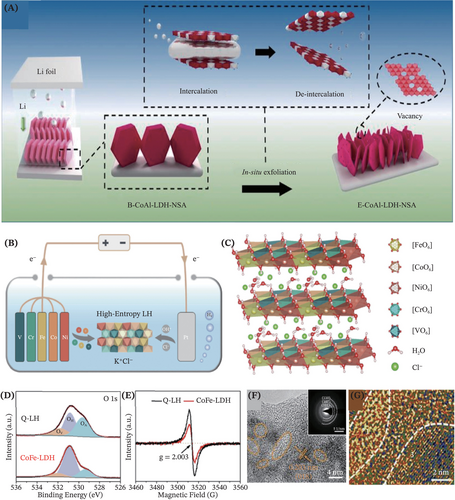
To further propel the advancement of self-supported LDHs, massive efforts have been devoted to designing and proposing varieties of novel synthetic techniques. Specifically, the electrochemical-related approach is offering enticing opportunities in this arena. In a case, an in-situ electrochemical exfoliation method was reported by Song et al.,129 in which an ultrathin LDHs nanoarray was synthesized on the conductive Ni foam, toward achieving a highly efficient oxidation process of 5-hydroxymethylfurfural (HMF) (Figure 9A). This exfoliation strategy comprised intercalation and de-intercalation steps to introduce abundant oxygen vacancies, which simultaneously boosted the active surface area enhancement and electronic structure regulation. Importantly, in the work of Li et al.,130 a green and universal approach based on a self-reliant electrochemical process via controllable anodic oxidation reaction was explored for preparing the high-entropy LDHs (Figure 9B,C). This work is poised to enlighten a more rational design of advanced LDHs, because the electrocatalytic performance of the as-fabricated quinary layered (oxy) hydroxide nanosheets, CoFeNiCrV-LH superseded and outperformed the CoFe-LDH counterpart. The superior performance and stability of the high-entropy LDHs were thankfully contributed by their defect-rich low-crystalline structure, tunable chemical compositions, optimized adsorption energies and abundant exposed active sites (Figure 9D–G). In another case, surface reconstruction was reported by undergoing the electrochemical transformation from Mo-doped Prussian blue CoFe nanocubes into Mo-doped CoFe LDH using Ni foam as the supporting substrate.131 Therein, aside from providing ideas for constructing efficient binder-free LDHs, the electrochemical approach also fascinatingly endows possibilities for site activation, aiming for boosting glycerol oxidation reaction by activating the Ni sites in the constructed NiV-LDH.63
Moving on, the template-oriented method is identified to emerge as a powerful approach for reasonably fabricating the hierarchical LDHs on the conductive substrate. Cao et al. devised a sacrificial template-oriented strategy and subsequent sulfur doping for synthesizing 3D hierarchical S-NiCoFe-LDH porous nanosheets furnished with abundantly exposed active sites and significantly improved electrical conductivity.132 In another study, a simplistic one-pot self-templated pathway was established by Semi et al. for constructing CoFe-LDH on MIL-88 A as a scaffold.133 MIL-88 A was chosen as the fabrication mold owing to several striking properties of this MOF—ultra-high surface area, rich mesopores and abundant coordinatively unsaturated active sites. This ultimately solved the unmastered challenge confining the catalytic performance of most LDHs indexed to low electrical conductivity and slow water dissociation. Nevertheless, although showing great prospects for self-supported LDHs construction, one must take note that the removal of the sacrificial template at a later stage from the coordinated positions may unfortunately lead to a destructive structure of the electrocatalysts.
To solve the technical ravines that are often correlated to complex manipulation and high energy-intensive, multifarious facile and effective methods adopting low energy consumption have been reported for obtaining the desired self-standing LDHs electrocatalysts.134-138 In the work of Yang et al., a Galvanic replacement reaction (GRR) based self-assembly approach with high simplicity and zero energy input was proposed (Figure 10A).134 By means of this approach, the tuning of the Ni-Fe ratio and the embedded anions was easily realized. This facilitated the regulation of multilayer NiFe-LDH nanosheets formation (Figure 10B) for exhibiting remarkable OER catalytic performance. Next, the cost-effective production of NiV LDHs bimetallic electrocatalysts supported on stainless steel via a simple chemical bath deposition route was reported. The electrocatalysts exhibited outstanding performance assigned to synergistic electronic interaction between Ni and V (Figure 10C) and long-term durability.135 Aside from this, a dip-coating approach that was facile, time-saving and green without any extra energy input was employed by Li and coworkers to fabricate partially crystalline NiFe LDHs nanosheet arrays in situ grown on Ni foam.139 Remarkably, corrosion engineering recently also arises as a promising synthesis measure to obtain various self-supported LDHs by simply adjusting the corrosion solution and the substrate. This approach is characterized by its low cost, simple instruments and operation, mild reaction conditions, absence of intentional energy input and high scalability.79, 137 Notably, Zhao et al. conducted an in-depth mechanic investigation into deciphering the correlations between corrosion condition, synthesis and electrochemical properties associated with the NiFe-LDH anchored in the Fe foam (Figure 10D).137
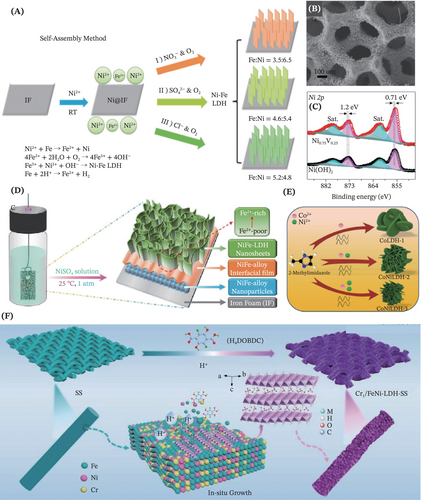
On another note, there is an evolution of growth-related strategy toward becoming a research hotspot for devising ground-breaking binder-free LDHs in electrocatalytic applications. For instance, leveraging an inspiring imidazole-assisted dissolution-regrowth strategy, Shilpa et al. synthesized the CoNi-LDH supported on carbon fiber paper for facilitating the electrocatalytic oxidation of organic molecules (benzyl alcohol, urea, ethanol and glycerol) in the alkaline medium (Figure 10E).140 Moreover, as delineated by Figure 10F, a facile ligand-assisted capping growth approach (LAGGA) was reported for the first time. This approach employed by Xie et al. realized the cost-effective growth of the ultrathin atomic Cr-doped FeNi-LDH nanosheet arrays uniformly supported on the commercially available stainless steel mesh.141 Notably, functioning as both the substrate and metal source, the stainless-steel mesh functioned as a superior semiartificial template that allowed synchronous implementation of in situ doping of the atomically dispersed Cr3+ atoms into the FeNi-LDH for electronic structure modulation. As a result, the constructed electrocatalysts were capable of standing out against other LDH-based catalysts reported to date for OER, assigned to exceptional catalytic performance, low overpotentials and excellent stability. A pioneering work was presented by Jia et al. for employing a novel ultrasonic-assisted oxidation plus vapor-phase-transport growth method for electrode construction.142 Benefited from the successful fabrication of hierarchical assemblies of ultrathin LDHs nanosheets with heterostructure, rich interfaces, phase boundaries and elevated active sites density, the NiCoFe-LDHs sample supported on Ni foam exhibited extraordinary intrinsic activity for OWS.
Overall, the pursuit of synthesizing novel and advanced electrocatalysts is fueled by the development of various emerging techniques. These include electrochemical methods (e.g., electrochemical exfoliation, anodic oxidation reaction and electrochemical transformation), template-oriented approach for fabricating hierarchical LDHs on conductive substrate, multifarious low-energy green methods (e.g., GRR-based self-assembly, chemical bath deposition, dip-coating and corrosion engineering) and growth-related strategy (e.g., imidazole-assisted dissolution regrowth, LAGGA and vapor-phase-transport growth). Although many flourishing works have been reported to guide future researchers in the successful fabrication of self-standing LDH electrocatalysts, a trial-and-error approach remains essential, with the following considerations to shape future catalysis research: (a) careful selection of precursors, substrates and synthetic strategies; (b) optimization of substrate and catalyst architecture through doping, vacancy and phase engineering; (c) refinement of synthesis procedures to enhance efficiency and reproducibility.
3 HETEROSTRUCTURE ENGINEERING OF MODIFIED SELF-SUPPORTED LDHs-BASED ELECTROCATALYSTS
Arriving at the milestone of this illustrious century, the catalysis community witnesses a remarkable journey in advancing the design of more promising self-supported LDHs. Specifically, to harness the benefits of heterostructure engineering, various coupling systems are employed for modifying binder-free LDHs. Great deals of researchers have coupled LDHs with carbon nanomaterials,143-145 transition metal chalcogenides,88, 146, 147 transition metal oxides,148-150 MXENE,151-153 alloy nanoclusters64, 154 and even metal-organic frameworks.155, 156 In this present review, special attention has been diverted toward discussing the coupling of free-standing LDHs with carbon nanomaterials, transition metal chalcogenides (transition metal selenides and transition metal sulfides), transition metal oxides and transition metal phosphides.
3.1 Carbon nanomaterials
Prodigious efforts are made to explore the potential of coupling carbon nanomaterials (CNMs) with the LDHs, due to their remarkable superficial area, exceptional electronic conductivity, as well as superb thermal and structural stability.87, 157, 158 This is to resolve the limitations encountered by most LDHs, where their poor conductivity and relatively low electrochemical surface area (ECSA) assigned to nanosheets agglomeration have typically confined their catalytic performance and inhibited their extensive applications in the electrocatalytic field. The CNMs, encompass the 0D carbon nanodots (CNDs) and carbon quantum dots (CQDs), 1D carbon nanotubes (CNTs), 2D graphene and graphdiyne (GDY) and 3D carbon networks are excavated by both theorists and experimentalists for modifying the self-standing LDHs.
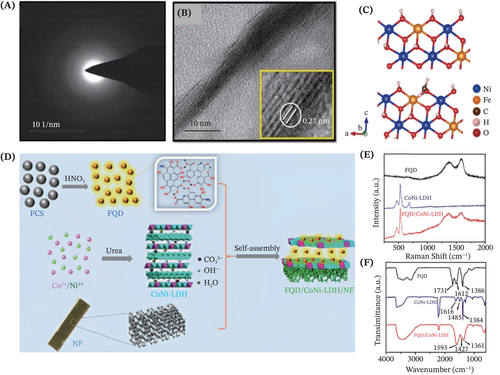
The 0D CNDs and CQDs enable a novel dimension of the material library toward accomplishing a more rational design of advanced binder-free LDHs electrocatalysts. As such, the catalytic properties of the modified self-supported LDHs are meritoriously alleviated due to the versatile features of the CNDs/CQDs such as the supernal specific surface area to create abundant active sites, and excellent electron store and transfer ability to enhance electronic performance. Moreover, thanks to the outstanding compatibility of CNDs/CQDs with myriads surface functional groups, it is conducive to combining these 0D CNMs with the LDHs for favoring the formation of stable carbonaceous composites. In the work of Chandrasekaran et al., a strong intimate integration of Cyamopsis tetragonoloba extract-derived nitrogen-doped carbon dots (NDCDs) with NiFe-LDH to form NiFe-LDH@NDCDs composite stacked sheets was allowed by a simple solvothermal preparation (Figure 11A,B).159 This was contributed by the surface opposite charge between the NDCDs and NiFe-LDH. In addition to this, the presence of functional carboxyl (CO) groups on the NDCDs surface led to the formation of new CONi or COFe bonds, thus assisting an easier formation of the composite. Impressively, the coupling of LDHs with these small-sized NDCDs (≈4.3 mm) brings a larger specific surface area to facilitate the occurrence of electrocatalytic OER reactions. In another work, Guo et al. envisioned a concept of combining the graphene quantum dots (GQDs) and NiFe-LDH by employing the electrostatic adsorption effect, together with the porous conductive Ni foam substrate (Figure 11C).160 Likewise, Feng and coworkers reported novel hybrid electrocatalysts comprising CoNi-LDH nanosheet arrays decorated with the fullerene quantum dots (FQDs) which anchored on the Ni foam for efficient electrocatalytic water splitting and urea electrolysis (Figure 11D–F).61 These two works reveal the exploration of the untapped potential of coupling LDHs with quantum dots leveraging the enhanced ECSA and introduced quantum effects for regulating the electronic structures of the composites.
The emergence of 1D CNTs instigates a fresh perspective in rectifying the inherent deficiencies encountered in the unadulterated LDHs electrocatalysis. By hybridizing the CNTs containing 3D network structures with the LDHs comprising 2D nanosheet structures, the unique hierarchical hybrid composites are endowed with the capability of avoiding structural collapse during electrooxidation or electroreduction reactions, thanks to the outstanding chemical stability and mechanical properties of the CNTs.161 Furthermore, the integration of CNTs as the robust conductive supporting skeleton not only offers abundant reactive sites, but is also beneficial for facilitating charge transport. This is evidenced in the work of Yan et al.,162 whereby preeminent catalytic performance and stability were reported by the hybrid composites CoMo-LDH/CNTs in comparison to the undecorated counterparts under identical conditions. It was speculated that equipping LDHs with the CNTs helped to construct an efficient electron transfer channel and facilitate better dispersion of CoMo-LDH on its surface, thus expediting the electron transfer within the modified LDHs electrocatalysts. In another work, a structural engineering strategy was proposed for fabricating efficient trifunctional electrocatalysts, Co@NC-CNTs@NiFe-LDH (Figure 12A).163 Particularly, the interwoven bamboo-like CNTs protruding from the Co-embedded N-doped carbon triangular plates surface carried an extraordinary significance for facilitating electron and ion transport. This was assigned to the prowess of this equipped coupling system (Co@NC-CNTs) in protecting the HER and ORR active sites from being shielded by the NiFe-LDH nanosheets (Figure 12B–D).
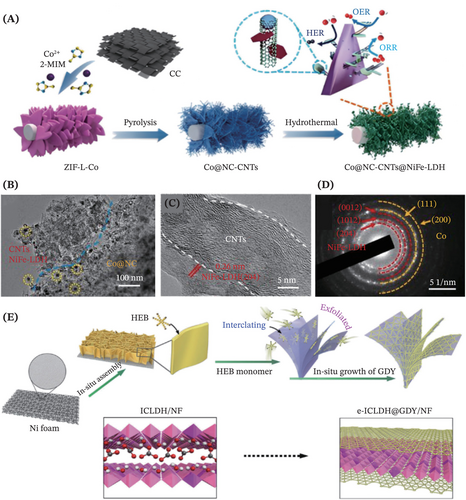
Attempts have also been made to devise and fabricate novel self-standing LDHs composite catalysts by hybridizing LDHs with 2D CNMs such as graphdiyne (GDY)164, 165 and reduced graphene oxide (rGO).144, 166 GDY has drawn substantial interest because it possesses a planar porous structure contributed by the bonding between benzene rings and diacetylenic linkages.165 This porous structure (2.5 Å natural pores) with the presence of high triple bond content favors the exposure of numerous accessible active sites, thus promoting smooth mass transport.167 Furthermore, benefiting from the unique layered structure that features delocalized sp- and sp2-hybridized π-conjugated networks, GDY exhibits superb silicon-like electrical conductivity to further elevate the electrochemical activity.168-172 In 2018, provoked by the intense investigations of GDY in multifarious fields especially as an auspicious cocatalyst in electrocatalysis,173, 174 Shi et al. reported the integration of NiFe-LDH and GDY for the first time. A facile electrodeposition method was employed to construct these promising hybrid electrocatalysts supported on Cu foil toward achieving effective OER.165 In another work by Hui et al., a creative GDY-induced intercalation/exfoliation/decoration strategy was reported to ultimately form the e-ICLDH@GDY/NF product (Figure 12E).164 This strategy allowed modification of the thick bulk iron cobalt LDHs into forming ultrathin exfoliated LDHs nanosheets while simultaneously forming the GDY sandwiched structures via the Glaser-Hay reaction, thus greatly enhancing the system's electrocatalytic performance. Besides, rGO are new 2D CNMs stars for modifying the pristine LDHs to form promising heterostructure electrocatalysts. Chen et al.144 and Guo et al.,166 respectively, reported the in situ growth of the NiFe-LDH (by hydrothermal approach) and CoFe nanosheet arrays (by citric acid-assisted aqueous phase coprecipitation strategy) on the Ni foam uniformly modified by the rGO flakes. To put it briefly, the 3D structure of these composites not only tackles the unsatisfactory stacking structure and poor conductivity of the LDH materials, but also the synergistic effect of the rGO and Ni foam also significantly boosted the ions and electrons transmission capacity ascribed to their superior electrical conductivity, thus drastically improved the electrocatalytic performance.
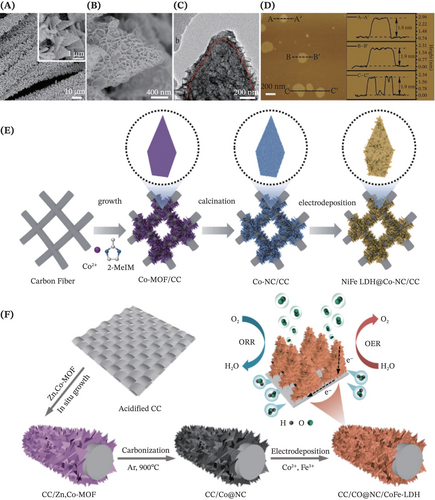
Excitedly, the free-standing electrocatalysis research is recently revolutionized with the introduction of 3D CNMs as the coupling system, complementing the well-established 0D, 1D and 2D materials. Remarkably, the decoration of LDHs with the 3D hybrid carbon nanoflake arrays (Co@NC) plays a crucial role in this significant shift.143, 145, 175, 176 Being embedded with carbon nanofibers and Co metal nanoparticles, the Co@NC nanomaterials offer much more nucleation sites and enable a rougher surface that benefits the uniform growth of ultrathin LDHs nanosheets with rich oxygen vacancies. Li et al.143 and Guo et al.145 unequivocally proved that typical 3D core–shell structures (Figure 13A–C) with good uniformity (Figure 13D) were successfully constructed from the synergistic coupling between the NiFe-LDH and Co-doped carbon nanofibers (Co-NC). It was inferred that there exists cooperation between the interconnected open network and the effective interface electron transfer effect due to the outstanding electrical conductivity of Co-NC. Hence, the constructed heterostructure composite catalysts showed favorable reaction kinetics that led to prominent electrocatalytic performance while exhibiting robust durability. Of note, during the preparation of multifunctional Co-NC, the MOFs are popularly employed as the precursors or sacrificial templates (Figure 13E,F), ascribed to the excellent conductivity, sufficient porosity and uniform heteroatomic doping of the MOF-derived Co-NC.145, 175, 176 Additionally, aside from using Co-NC as the coupling material, Abdelrazek et al. reported the nanocomposites of NiFe-LDH and 3D carbon xerogels (CX) supported on Ni foam substrate for electrochemical methanol oxidation, whereby the presence of CX offered 3D interconnected micro- or mesopores that facilitated both electron and mass transport.177 Furthermore, Deng et al. developed CoFe LDH nanowire arrays on a 3D graphite felt (GF) due to attractive features such as exhibiting outstanding mechanical integrity, high volumetric porosity (ε < 0.98), reasonable cost and even appealing electrical conductivity close to metal (370.37 S m–1).178 In short, the 3D CNMs have fascinatingly paved avenues for the development of attractive catalyst supports.
3.2 Transition metal chalcogenides
3.2.1 Transition metal selenides
In the realm of self-supported electrocatalyst design and development, the transition metal chalcogenides have attained significant considerations for constructing coupled heterostructures with the LDHs nanosheets. This is owing to their multiple morphologies, abundant 3d electron configurations, distinctive electronic conductivity, environmental friendliness and satisfactory stability.179-183 In particular, the transition metal selenides have been a game changer that significantly impacted the design of high-performance LDHs composite catalysts. This is meritoriously contributed by the strong metal-selenium (M-Se) bonds formed by the empty 3d orbitals of selenium with the transition metals, which promisingly facilitates electron transfer during the electrocatalytic reaction.
First, regarding the employment of monometallic selenides, both Ni-based and Co-based selenides are extensively studied. To holistically address the conventional issues faced by the pristine NiFe-LDH especially related to limited active sites, poor intrinsic catalytic activity and low electrical conductivity, the low-cost and abundantly available nickel-based selenides such as NiSe146 and Ni3Se2184 have piqued researchers' interest. Next, Song et al. reported a hydrothermal-selenization-hybridization strategy for hierarchically assembling the CoNi-LDH on the branched CoSe2 nanotubes.88 The strongly coupled ternary hybrid nanostructured arrays (HNA) were successively grown on carbon cloth to form efficient trifunctional electrocatalysts. Inspired by the CoSe2 as the commonly studied polymorph of cobalt selenides, Jin et al. proposed the integrated CoFe-LDH/Co0.85Se architecture to further elevate the OER performance for water electrolysis.185 It was inferred that the nonstoichiometric phase of Co0.85Se exhibited extraordinary conductivity due to its intrinsic half-metallic character, thus rendering the self-supported binary hybrid heterogeneous catalyst constructed with propitious electrocatalytic performance.
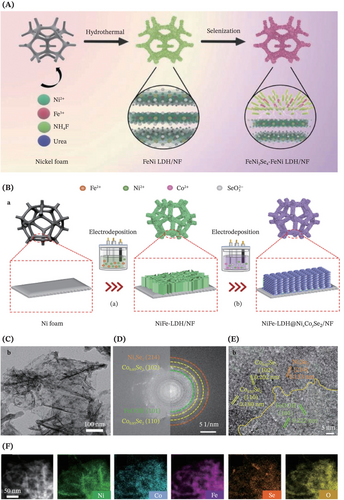
Impressively, in addition to the single-metal selenides, the polymetallic selenides have also captivated a significant amount of interest indexed to higher electrical conductivity and more abundant active sites, thus possessing much better electrocatalytic performance. For instance, bimetallic selenides such as FeNi2Se4 and NixCoySe2 were respectively employed by Yu et al.186 (Figure 14A) and Wang et al.147 (Figure 14B–F) for constructing a rational design of heterostructures involving NiFe-LDH. Particularly, the work of Yu et al. manifested a significant leap that there was electron redistribution owing to the strong electronic coupling between FeNi2Se4 and NiFe-LDH, whereby the optimized electronic structure of the electrocatalyst modulated the building energies of OER and HER reaction intermediates toward accomplishing enhanced OWS.186 As for the work by Wang et al., the introduction of NixCoySe2 nanospheres resulted in the atoms migration from equilibrium positions to the crystal surface, leading to the generation of oxygen vacancies, abundant grain boundaries and large active surface area, hence improving the OER catalytic performance.147 Moving forward, Dai et al. recently synthesized the hierarchical Ni-Co-Fe-Se@NiCo-LDH catalysts involving trimetallic selenides at the heterogeneous interface.187 All these works triumphantly provide new guidance for leveraging the synergistic coupling of transition metal selenides with LDHs for advancing the development of multifunctional self-standing electrocatalysts.
3.2.2 Transition metal sulfides
Transition metal sulfides are another rapidly rising star among the transition metal chalcogenides families on the materials science horizon. These novel coupling materials exert potential electrocatalytic application value, because of their tunable electronic properties, abundant active sites, rich redox chemistry and distinctive structural features.188-194 Furthermore, under a rapidly reversible redox process, there is a creation of the flexible phase structure due to the formation of SS bonds by the sulfur atoms present in the metal sulfide compounds, thus ultimately inducing superior electron transfer and charge capacity.191, 195 When considering and distinguishing by the crystal structure, the transition metal sulfides are predominantly classified into two key categories: layered MS2 (M = transition metals in groups 4–7, e.g., Mo, W, Ta, Nb, etc.) and non-layered MxSy (M = transition metal in group 8–12, e.g., Fe, Co, Ni, etc.) structures.188, 191
Notably, the transition metal sulfides with nonlayered MxSy structure invoke a great deal of researchers' interest to couple with the LDHs for constructing self-supported electrocatalysts. This category of nonlayered metal sulfides primarily shares a marcasite or pyrite structure, where the transition metal atoms located at body-centered (for marcasite structure) or face-centered (for pyrite structure) sites are octahedrally bonded to the neighboring sulfur atoms.188, 191, 196 Interestingly, diverse series of Co-containing nonlayered metal sulfides namely CoSx, Co9S8 and NiCo2S4 have been exploited to form heterostructure catalysts via interface engineering. For instance, Yang et al. reported a newly assembled hierarchical structure integrating the amorphous CoSx and NiFe-LDH, to synergistically favor the composites with propitious HER and OER activity in alkaline conditions.197 In this work, the sluggish HER kinetics issue of the NiFe LDH catalysts due to the large kinetic energy barrier for the Volmer step was holistically addressed, thanks to the disordered structure and isotropic property of the assembled CoSx which exhibited excellent HER catalytic activity as revealed by prior studies.198-201 In another study, the pristine nanoneedle-like Co9S8 arrays on Ni foam surface were reported by Liu et al., where these constructed arrays with 3D architecture played a crucial role as the skeleton for further growth of the NiFe-LDH nanosheets while containing active components beneficial for HER activity and serving as charge transport channel to facilitate charge transfer efficiency.202 The above works exemplify that equipping cobalt sulfides with LDHs for constructing self-standing catalysts is prospective for practical electrocatalytic applications.
Moving forward, in addition to the aforementioned cobalt sulfides, the nonlayered nickel sulfides such as NiS, NiS2 and Ni3S2 have also garnered ever-increasing interest as outstanding electrocatalysts that are easily manufacturable, cost-effective, satisfactorily stable and exhibiting robust electron transfer capacity. Thereinto, among various nickel sulfides within the family, it is intriguing to highlight that there is increasing prominence of employing Ni3S2 as the coupling system of LDHs in synthesizing promising electrocatalysts. This is fascinatingly contributed by the inherent high-conductivity metallic behavior of Ni3S2 due to the presence of NiNi bonds throughout its structure, thus rendering it suitable to be applied as LDHs coupling system for ameliorating the conductivity of the composite electrocatalysts. For instance, Ren et al. fabricated heterostructural NiFe-LDH@Ni3S2 nanosheet arrays supported on Ni foam via a facile two-step hydrothermal approach.179 The synergistic interaction between the NiFe-LDH and Ni3S2 created abundant exposed active sites with enhanced activity interface regions. As a result, the as-prepared catalysts demonstrated excellent electrocatalytic performance alongside admirable electrochemical stability for both HER and OER applications under alkaline electrolytes. Following this, to further elevate the inherent catalytic performance for water-splitting reactions, Sang et al. reported the coupling of 3D Fe doped-Ni3S2 nano-ridges with the amorphous NiFe-LDH nanosheets.203 In this work, cation doping was carried out for incorporating iron atoms into the nickel sulfide catalysts, to activate the charge transfer process while lowering the adsorption energy of the oxygen/hydroxide intermediates, which substantially improved the electrocatalytic water splitting performance of this nickel-based catalyst as confirmed by several works.204-206 In another work, attracted by the high hydrogen chemisorption capacity of the MoS2, He et al. compounded the layered MoS2 with non-layered Ni3S2 before coupling with the LDHs.60 This was to ultimately develop interlaced rosette-like MoS2/Ni3S2/NiFe-LDH/NF which exhibited extraordinary bifunctional catalytic activity toward simultaneously catalyzing anodic UOR and cathodic HER.
3.3 Transition metal oxides
Within the transition metal families, transition metal oxides are emerging as another prominent contender for coupling with free-standing LDHs, aiming to establish highly efficient electrocatalytic systems, particularly for the applications of OER and OWS. Remarkably, the synergy between different single metal oxides (e.g., CeO2,207 CoO,150 Co3O4,208 CuO,148 Cu2O,209 Fe3O4,149 SnO2210 and ZnO211) and LDHs system has been a prominent topic in recent research. For instance, Ouyang et al. constructed the coupling of p-type CuO with n-type FeCoNi-LDH to fabricate a multichannel and hierarchical p–n heterojunction FeCoNi-LDH/CuO nanoarrays with significantly improved stability, mass transfer efficiency and electrical conductivity.148 The d-band centers of the active Fe, Co and Ni atoms shifted upwards, approaching the Fermi level, which strengthened the interaction between the active sites and reactants during the electrocatalytic OER process. As verified by the DFT calculations, the enhanced electrocatalytic performance of this catalyst was also attributed to electron transfer from Fe to Cu, facilitated by the built-in electric field at the interface. In another work, Wan et al. studied the tight heterojunction formed between the n-type SnO2 semiconductor material and NiFe-LDH via the formation of SnOFe (Ni) chemical bond.210 The remarkable OER performance exhibited by this catalyst was assigned to the role of SnO2 as an efficient electron transport layer for fast transfer of electrons generated during the OER process. Impressively, owing to the appealing attributes of bimetallic oxides such as rich valence states, low cost, complicated chemical compositions and excellent intrinsic activity, the coupling of these leading coupling agents (e.g., Co2Mn3O8,212 NiCo2O4213, 214 and NiFe2O4215) with LDHs systems to form self-supported electrocatalysts have also been intensively studied.
3.4 Transition metal phosphides
Transition metal phosphides have also rapidly garnered widespread recognition as exceptional coupling materials with LDHs to form self-supported catalysts, pushing the boundaries of electrocatalysis. Noteworthy, the phosphorus atoms serve as effective proton acceptors benefiting from their strong electrostatic affinity and moderate bonding with hydrogen, while the positively charged transition metals function as hydride acceptor sites, hence this “ensemble effect” synergistically elevates the HER electrocatalytic performance.216-218 For instance, Yang et al.219 and Wang et al.220 constructed Mo-Ni2P@NiFe-LDH/NF and NiCoP@NiMn-LDH/NF respectively for boosting the OWS activity, in view of the moderate interaction between phosphorus atoms and reaction intermediates to efficiently promote the kinetically sluggish hydrogen desorption. In another work, Yang et al. designed NiCoP@FeNi-LDH with the capability of achieving industrial-scale high-current-density water splitting at 1000 mA cm−2, hence delivering crucial insights for scaling up hydrogen production in industrial settings.221 Apart from water splitting application, this coupling material exhibits its superiority in the co-electrocatalysis of methanol/water, especially in the form of ternary transition metal catalysts (e.g., NiCoxP216 and NiFexP222), chiefly due to the reconfiguration of the electronic structure within the catalyst. Simply put, the transition metal phosphides can be highly regarded as exceptional coupling agents with LDHs, driving innovations in electrocatalysis for efficient hydrogen generation.
4 SELF-SUPPORTED LDHs FOR ELECTROCATALYTIC APPLICATIONS
Recognizing the pivotal role of electrocatalysis as the nexus for green energy storage and environmental clean-up, there is a pressing necessity for developing high-performance and affordable catalysts for electrochemical energy conversion processes. As such, this section offers an up-to-date review, featuring notable representative examples of recent self-supported LDHs-based electrocatalysts for electrocatalytic conversion reactions to deepen mechanistic insights. This would help establish a better yardstick for evaluating the potential of these electrocatalysts for large-scale industrial green manufacture while prescribing goalposts for next-generation electrocatalysts to strive to reach.
4.1 The coupling of HER with OER
Proverbially, the anodic OER during electrochemical water splitting presents a challenging, complex and sluggish four-electron transfer process, necessitating a substantial overpotential to surmount the energy barrier for facilitating efficient water splitting. To meet the demands of hydrogen production at scale, a range of functionalized LDH-based electrocatalysts have been devised and developed by the researchers. In this section, a comprehensive analysis summarizing the latest significant advancement in self-supported LDHs-based heterostructure electrocatalyst toward significant enhancement in OER and/or HER efficiency will be presented, with the ultimate goal of accomplishing favorable outcomes in OWS for sustainable H2 production.
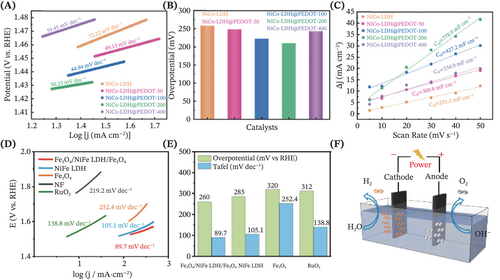
A summary table that demonstrates some representative examples of self-supported LDHs-based electrocatalysts for electrocatalytic HER and OER application is presented in Table 1. It is truly fascinating to witness how heterostructure engineering has ushered in a groundbreaking philosophy toward developing more advanced binder-free electrocatalysts for water-splitting applications. Wang et al. reported a novel self-standing NiCo-LDH composite via the coating of a layer of conductive polymer known as poly(3,4-ethylenedioxythiophene) (PEDOT).223 The optimal hybrid electrocatalysts (NiCo-LDH@PEDOT-200) exhibited stable and stellar performance for both OER (requiring an overpotential of 210 mV at 50 mA cm−2) and HER (requiring an overpotential of 52 mV at 10 mA cm−2) while delivering only 1.57 V for OWS at 10 mA cm−2 (Figure 15A–C). With its sulfur (S)-rich layer, the strong coupling between PEDOT and NiCo-LDH nanosheets had formed a tight contact, wherein the PEDOT thin-film expanded the LDHs' interlayer and established a tunnel for efficient charge transfer. This work has supplied refreshing insights on compounding LDHs with conducting polymers such as PEDOT for fabricating promising electrocatalysts, by virtue of the merits of this polymer including appealing cycling life, unique charge storage mechanism as well as impressive environmental stability.224, 225 Next, the work by Zhang et al. demonstrated the construction of a sandwich-structured Fe3O4/NiFe-LDH/Fe3O4 as a bifunctional electrocatalyst, requiring a low cell voltage (1.648 V to achieve 10 mA cm−2) while endowed with superior stability for highly sustained OWS (Figure 15D–F).149 This work has established a deep insight into the role of confined space within the TM-based electrocatalyst, where there was abundant encapsulation of electrocatalytic active sites inside the Fe3O4 oxide layer, which remarkably stabilized the key charge transfer intermediates, thus promoting the reaction kinetics for OER and HER. Furthermore, some other recently reported coupling materials such as TM oxides (e.g., NiCo2O4214), alloy nanoclusters (e.g., NiCu226), polymetallic selenides (e.g., Ni-Co-Fe-Se187), CNMs (e.g., MOF-derived Co-NC145) and even LDHs227, 228 for the fabrication of self-supported LDH-based heterostructure, have also been evidenced to result in astonishingly ameliorated performance in the realm of OWS applications.
| Electrocatalyst | Synthesis technique | Overpotential (mV) @ 10 mA cm−2 | Tafel slope (mV dec−1) | Cell voltage for OWS (V) @ 10 mA cm−2 | Stability (h) | References |
|---|---|---|---|---|---|---|
| Ni-Co-Fe-Se@NiCo-LDH | Two-step hydrothermal + selenization | HER: 113 OER: 286@100 mA cm−2 |
HER: 44.9 OER: 78.9 |
1.55 | 30 | [187] |
| CoCO3@NiFe-LDH | Two-step hydrothermal | HER: 171 OER: 232@20 mA cm−2 |
HER: 168.2 OER: 120.2 |
1.67 | 20 | [232] |
| Ni3Se2@NiFe-LDH/NF | Hydrothermal + electrodeposition | HER: 68 OER: 222 |
HER: 106.2 OER: 61.3 |
1.55 | 24 | [184] |
| NiFe-LDH-Vo@NiCu | Hydrothermal + electrodeposition | HER: 85 OER: 244@50 mA cm−2 |
HER: 92.4 OER: 51.2 |
1.54 | 24 | [226] |
| Co-NC@Ni2Fe-LDH | In-situ growth + calcination + electrodeposition | HER: 87 OER: 233 |
HER: 127.4 OER: 49.1 |
1.55 | 20 | [145] |
| Pt-NiFe-LDH | Electrodeposition | HER: 36 OER: 198 |
HER: 50.0 OER: 21.0 |
1.88 | 3000 | [233] |
| NiCo-LDH@NiCoV-LDH | Hydrothermal + electrodeposition | HER: 80 OER: 260@100 mA cm−2 |
HER: 68.3 OER: 68.8 |
1.55 | 40 | [227] |
| CoFe-LDH/NiCo2O4 | Hydrothermal | HER: 120 OER: 306@50 mA cm−2 |
HER: 53.8 OER: 41.0 |
1.65 | 12 | [214] |
| NiFe-LDH@CoSx/NF | Hydrothermal + electrodeposition | HER: 136 OER: 206 |
HER: 73.0 OER: 62.0 |
1.54 | 24 | [197] |
| FeNi-LDH/V2CTx/NF | Hydrothermal | HER: 151 OER: 222 |
HER: 136.0 OER: 58.7 |
1.74 | 10 | [152] |
| MoO3/NiFe-NF | Hydrothermal + electrodeposition | HER: 85 OER: 212 |
HER: 97.4 OER: 63.6 |
1.43 | 24 | [234] |
| CoFe2O4/CoFe-LDH | Water bath reaction | HER: 337@1000 mA cm−2 OER: 350@1000 mA cm−2 |
HER: 59.0 OER: 55.0 |
1.93@1000 mA cm−2 | 100 | [229] |
| Mo-Ni2P@NiFe-LDH/NF | Two-step hydrothermal | HER: 82 OER: 269@40 mA cm−2 |
HER: 56.0 OER: 44.0 |
1.46 | 18 | [219] |
| NiCoP@NiMn-LDH/NF | Hydrothermal + phosphorization | HER: 116@ 100 mA cm−2 OER: 293@100 mA cm−2 |
HER: | 1.52 | 50 | [220] |
Engrossingly, constructing advanced electrocatalysts capable of delivering exceptional electrochemical performance at high current densities has been a focal point in recent studies. Chen et al. developed CoFe2O4/CoFe-LDH 2D/2D heterojunction that achieved impressive HER performance, requiring a low overpotential of 337 mV at high current density (1000 mA cm−2), outperforming previously reported non-noble metal LDHs and spinels catalysts.229 This was owing to the significant charge redistribution at the heterointerface that enhanced water dissociation kinetics and optimized the adsorption energies of key intermediates. Jiang et al. fabricated Ni3Fe-LDH anode catalyst for the anion exchange membrane water electrolysis application, whereby this stellar catalyst achieved an ultrahigh current density greater than 2000 mA cm−2 at 2.0 V while capable of exhibiting superb long-life durability at 1000 mA cm−2 for 400 hours.230 This impressive performance was associated with the reversible dynamic phase transition of Ni3Fe-LDH into the active Ni(Fe)OOH intermediate during OER, as evidenced by in situ Raman spectra. The phase transformation was driven by the catalyst's optimized local chemical environment, characterized by an ordered atomic structure and enhanced oxygen coordination. Similarly, structure reconstruction of LDHs catalyst was reported by Wu et al., in which Co doping induced structural evolution during OER, forming real surface-active Ni(Co)1−xFexOOH species that catalyzed with low overpotentials at high current densities (500 mA cm−2), maintaining stability over 300 h.231
4.2 The coupling of HER with EHCO
With the blossoming interest in replacing the sluggish OER with EHCO to work in tandem with HER for enhanced hydrogen generation, this review centers on the use of self-supported LDHs-based electrocatalysts in UOR and AOR for EHCO applications. A summary table of the recent representative examples of self-supported LDHs-based electrocatalysts for the coupling of HER and EHCO is presented in Table 2.
| Organic compounds | Electrocatalyst | Synthesis technique | Electrolyte/organics concentration | Potential (V) @ current density (mA cm−2) | Tafel slope (mV dec−1) | Stability (h) | References |
|---|---|---|---|---|---|---|---|
| Urea | S-NiFeV-LDH | Two-step hydrothermal | 1 M KOH/0.33 M | 1.38@100 | 30.1 | 25 | [47] |
| Rh/NiV-LDH | Hydrothermal | 1 M KOH/0.33 M | 1.33@10 | 45.0 | 200 | [72] | |
| FQD/CoNi-LDH/NF | Self-assembly | 1 M KOH/0.50 M | 1.36@10 | 17.0 | 30 | [61] | |
| MoS2/Ni3S2/NiFe-LDH | Two-step hydrothermal | 1 M KOH/0.50 M | 1.40@100 | 36.0 | 16 | [60] | |
| NiFe-LDH/MoS/Ni3S2/NF | Two-step hydrothermal | 1 M KOH/0.50 M | 1.41@50 | — | 50 | [65] | |
| Methanol | NiCoxP@NiCo-LDH | Electrodeposition | 1 M KOH/0.50 M | 1.23@10 | — | 20 | [216] |
| NiFexP@NiCo-LDH/CC | Electrodeposition + phosphating | 1 M KOH/0.50 M | 2.69@50 | 97.0 | 10 | [222] | |
| Glycerol | E-NiV-LDH | Electrochemical regulation | 1 M KOH/0.10 M | 1.23@10 | — | 300 | [63] |
| Benzyl alcohol | CoNiLDH-3 | Imidazole-mediated | 1 M KOH/0.15 M | — | 22.0 | 12 | [140] |
| CoV-LDH/NF | Hydrothermal | 1 M KOH/0.10 M | 1.31@100 | 17.4 | — | [246] | |
| Ethanol | CoNiLDH-3 | Imidazole-mediated | 1 M KOH/0.15 M | — | 24.0 | 12 | [140] |
| NiFe-LDH/MoS/Ni3S2/NF | Two-step hydrothermal | 1 M KOH/0.34 M | 1.43@50 | — | 50 | [65] |
4.2.1 Urea oxidation reaction
In this domain, the electrocatalytic UOR has sparked mounting interest in numerous energy-related applications, namely the direct urea fuel cells (DUFCs), urea-assisted water splitting and urea-assisted rechargeable zinc air batteries. Remarkably, among diversified anodic reactions for promoting energy-saving hydrogen generation, there is an intriguing surge in employing UOR to replace OER. This is by virtue of its intrinsically low thermodynamic potential (0.37 V vs. RHE), the abundance of urea in presenting as raw materials or by-products in industrial wastewater, and low energy consumption for hydrogen production.235-241 Moreover, the standard Gibbs free energy of UOR (36.36 kJ mol−1) is also much lower compared with the OWS counterpart (237.101 kJ mol−1).242 Nevertheless, despite demonstrating a promising future, the state-of-the-art electrocatalytic performance of UOR is still unsatisfactorily, indexed to inadequate adsorption and desorption of the key intermediates such as CO* and NH*. The bestial challenge is also arising from the complexity, diversified intermediate adsorbed species and sluggish kinetics of the six-electron reaction path (CO(NH2)2 + 6OH− → N2 + CO2 + 5H2O + 6e−).241, 243, 244 In light of bolstering the electrochemical performance of overall urea electrolysis, it is of great impetus to explore and devise efficient bifunctional electrocatalysts for promoting both HER and UOR.
Inspiringly, thanks to their sophisticated functionalities, the self-supported LDHs-based catalysts have swiftly earned their rank in the realm of overall urea electrolysis. Heterostructure engineering was hotly pursued for synthesizing promising binder-free nanohybrid electrocatalysts to drive overall urea electrolysis, whereby coupling materials encompass SACs72 and transition metal sulfides60 were reported. Of note, in 2022, Sun et al. reported the integration of NiV-LDH with rhodium (Rh) SACs to form a multifunctional electrocatalyst termed Rh/NiV-LDH, attaining a stable H2 generation and favorable urea elimination rate as high as 93%.72 As revealed both experimentally and theoretically, the as-synthesized catalysts exhibited splendid electrocatalytic activity, demonstrating TOF of 0.125 s−1 at an overpotential of 100 mV and HER mass activity of 0.262 A mg−1. This was assigned to the synergy between the atomically dispersed Rh sites and the NiV-LDH support, which greatly suppressed the reaction barrier in the Heyrovsky and Volmer step in the HER process while facilitating optimized adsorption (urea molecules) and desorption of key intermediates, thereby improving overall urea catalytic efficiency. Another work by He et al. envisioned a concept of integrating the MoS2/Ni3S2 and NiFe-LDH with the conductive Ni foam substrate.60 Ultimately, the interlaced rosette-like bifunctional electrocatalysts demonstrated an efficient performance (only 1.343 V required to attain 50 mA cm−2, which was 216 mV lower than OWS in this case) alongside robust stability (remained at 98 % of the original current density after 16 h operation) for urea-assisted H2 production.
Aside from the previously mentioned coupling materials, it is noteworthily mentioned that the coupling of LDHs with MOFs155 and carbon nanomaterials such as FQD61 have also supplied insightful inspirations for future research works in this field. For instance, Huang et al. successfully synthesized a newfangled electrocatalyst comprising Co-MOFs clusters embedded on CC and interconnected ultrathin CoFe-LDH nanosheets via a facile one-step chemical etching reaction, toward achieving superior bifunctional UOR and HER activity in the alkaline media.155 Feng et al. presented the fabrication of a novel 3D hierarchical hybrid electrocatalysts via a one-step self-assembly approach, by anchoring the FQD-decorated CoNi-LDH nanosheet arrays into the porous Ni foam surface.61 Surprisingly, the experimental results demonstrated that the as-synthesized catalyst (FQD/CoNi-LDH/NF) required a potential as low as 1.45 V for overall urea electrolysis to attain a current density of 10 mA cm−2. In particular, the DFT calculations performed on the model jointly disclosed that there was significant charge transfer from the FQD to the CoNi-LDH, with about 2.71 electrons donated to the LDH moiety, as confirmed by Bader charge analysis, thus elevating Co/Ni 3d orbital's electron states to near Fermi level. Additionally, in comparison to the pristine CoNi-LDH, the as-fabricated catalysts greatly ameliorated the ∆GH* values for hydrogen adsorption on Co sites (from −1.65 to −0.09 eV) and Ni sites (−1.96 to −0.15 eV). These closer-to-zero ∆GH* values triggered superior HER performance, thereby enhancing the catalyst's overall efficiency in urea electrolysis for efficient hydrogen production.
In addition to heterostructure engineering, heteroatom doping is accounted for another promising strategy for modifying the pristine LDHs. This was showcased and exemplified by the work of Peng et al.47 that introduced sulfur to the pristine ternary NiFeV-LDH, hence correspondingly leading to higher electrical conductivity and induced acceleration in the UOR process (ease urea adsorption and reducing the desorption of key intermediated from a ∆G value of 5.72–4.40 eV). In brief, the self-reliant LDHs-based catalysts, especially those functionalized by doping engineering or heterostructure engineering, have endorsed astonishing potential for surmounting the sluggish OER, toward realizing simultaneous urea-rich wastewater purification while generating valuable hydrogen fuel.
4.2.2 Alcohol oxidation reaction
Interestingly, the alcohol oxidation reaction (AOR) has garnered significant attention as a highly sought-after alternative anodic reaction, aiming to supplant the sluggish OER reaction for sustainable hydrogen generation. This encompasses the upgrading of diversified alcohols such as methanol, ethanol, glycerol and benzyl alcohol to be transformed into a wide array of valuable products.
Being the simplest monohydric alcohol, there is a higher preferability for choosing methanol for the anodic oxidation reaction over other alcohols, for coupling with HER to generate H2. This can be ascribed to its low-cost, high-energy density, ease of storage and preparation, as well as wide availability due to massive production primarily derived from the coal chemical industry.78, 245 In this context, the methanol oxidation reaction (MOR) has been fervently recognized as the most prominent AOR utilizing self-supported LDHs as electrocatalysts to facilitate the reaction itself. Herein, it is found that the foremost target products of most of this research work encompass either formate or formic acid, whereby both are essential intermediates for transforming into fine chemicals in the chemical manufacturing industries. Surprisingly, Zhang et al. fabricated binder-free bifunctional electrocatalysts that successfully exhibited preeminent formate's Faradaic efficiency (nearly 100%), by coupling NiCo-LDH with transition metal phosphides such as NiCoxP222 and NiFexP for hydrogen generation accompanied with formate.216 On the other hand, concerning the production of formic acid employing self-supported LDHs-based electrocatalysts, the lack of information on FE was identified as a current gap besetting the anodic MOR. However, although no FE value for formic acid production was reported, Wang et al. reported a decent formic acid yield of 97% with splendid long-term stability while endowing with a 74 mV decrement of required potential compared with the OER counterpart.64 Wan et al. inspiringly paid attention to introducing a conductive SnO2 layer (as an electron transport layer) between the conductive Ni foam and NiFe LDH for the oxidation of methanol to formic acid.
Significantly, in addition to the well-established usage of self-supported LDHs-based electrocatalysts for MOR, the glycerol oxidation reaction (GOR) has also been generating considerable research interest as a viable substitute for the slow and more energy-intensive OER reaction. For instance, the work by Dong et al. showcased the fabrication of Ni site-activated NiV-LDH, which enabled the concurrent production of high-purity hydrogen with value-added formate via glycerol electrolysis. This study reported a high FE of 94% and an impressive GOR performance, achieving 1.23 V at 10 mA cm−2. This enhancement was attributed to the elevated valence state of the Ni site in the electrochemically regulated NiV-LDH, with in situ Raman spectroscopy clearly detecting the presence of highly active Ni(III) species as active sites during the GOR process.63 Noteworthily, Shilpa et al. suggested that a hybrid electrolyzer that coupled HER with AOR including benzyl alcohol oxidation reaction (BAOR), GOR and ethanol oxidation reaction (EOR) possessed better energy conversion efficiency when compared with the conventional water electrolyzer.140 This was evidenced by a shift of onset potential to a lower value, while the FE of H2 related to OWS was demonstrated to be 93%, while the higher values of 87% (BAOR), 92% (EOR) and 94% (GOR) were obtained, respectively, in those hybrid electrolyzers.
In essence, although both experimental evidence and theoretical modeling analysis are still scarce up to the present time, the unprecedented performance of the alcohol-assisted hydrogen production shown in the aforementioned works encourages more devotion to the research effort, toward designing more advanced self-supported LDHs in this arena. Although most of the electrocatalytic cell shown in the research work is still powered by electricity, Liu et al. presented a demonstration of a photovoltaic electrolysis (PV-EC) system for realizing the solar energy-driven MOR-assisted hydrogen generation in an energy-saving manner, by employing monolayered NiCo-LDH as anode and NiCo alloy as the cathode.111 Hence, a similar electrocatalytic system using renewable energy sources for driving hydrogen production is highly recommended.
4.3 Other emerging electrocatalytic applications
In addition to the as-discussed electrocatalytic applications, the self-supported LDHs-based electrocatalysts have recently been found to be vibrant research for several other emerging applications. First, seawater electrolysis offers a promising route for scalable H2 production while allowing seawater desalination compared with conventional water splitting using high-purity freshwater, considering the accessibility of the seawater resources available on the Earth. However, the limitations of sweater electrolysis such as having high corrosivity and insoluble precipitates, that may result in poisoning or corrosion of catalysts have impeded their practical utilization, thus the seeking of stable and active anode materials is urgently needed. In this case, the self-supported LDHs-based electrocatalysts come into sight as promising electrocatalysts for resolving the previously described concerns. For instance, Enkhtuvshin et al. successfully prepared quaternary multi-metallic N-Fe-Al-Co LDHs that demonstrated excellent performance for seawater electrolysis.62 In another study, Wu et al. fabricated a self-assembled CoFe-LDH coupled with Pt clusters, wherein their synergistic effects had jointly contributed to enhanced chemical stability and superb catalytic activity toward seawater splitting, demanding only 1.651 and 1.858 V to attain 100 and 500 mA cm−2 in the seawater electrolyte.247 Yu et al. demonstrated the co-incorporation of Ni and Fe into the CuCo-LDH to form an active and durable OER electrode for achieving high-performance seawater electrolysis, exhibiting propitious OER selectivity (FE of 97.4% for O2 at 500 mA cm−2) over the undesirable chlorine evolution reaction (CER).120
Excitedly, the self-supported LDHs-based electrocatalysts have also found their applications in nitrogen reduction reaction (NRR) and nitrogen oxide reduction reaction (NORR), hence lying an important basis for realizing mass production of ammonia, a pivotal chemical fertilizer to sustain our lives. In the work of Liu et al., the phosphorus doping strategy was reported to effectively regulate the charge distribution in the FeNi-LDH arrays synthesized on carbon nanofibers, hence greatly bolstering the NRR performance to deliver a decent FE of 23% and ammonia yield of 1.72 × 10−10 mol s−1 cm−2.93 In another work, Meng et al. indicated that the NiFe-LDH exhibited exceptional NORR activity for delivering an ammonia yield rate of 112 μmol h−1 cm−2 and maximal FE as high as 82%, while being capable of demonstrating excellent stability for over 30 h.81 To sum up, despite having limited experimental research and studies, the self-supported LDHs-based electrocatalysts have paved new horizons for developing novel electrocatalysts in favor of electrocatalytic applications such as seawater electrolysis, NRR and NORR. Hence, it is speculated that there is prospective employment of this catalyst system for extending to other flourishing electrocatalytic application systems.
5 SUMMARY AND FUTURE PERSPECTIVES
Leveraging appealing features and superiorities such as unique lamellar structure, abundant active sites, tunable composition and enhanced cyclic stability alongside efficient charge/mass transfer, the self-supported LDHs-based electrocatalysts are accredited with superb performance in myriads of electrocatalytic applications. This review highlights different state-of-the-art preparation techniques for synthesizing self-supported LDHs-based electrocatalysts, such as hydrothermal, co-precipitation, etching, electrochemical, template-oriented and growth-related strategies. Tandem adoption of various modification strategies such as heteroatom doping, vacancy construction and phase engineering during the synthesis of the as-mentioned catalyst systems are also exemplified and discussed. Following this, various coupling systems for constructing self-supported LDHs-based heterostructures such as CNMs, transition metal chalcogenides, transition metal oxides and transition metal phosphides are introduced. Finally, this review presents the latest advancement of the catalyst system in interest for a variety of energy-related applications such as HER, OER, OWS, UOR, AOR, seawater oxidation, NRR and NORR. Crucially, this present review encompasses the recent advancements in the design, synthesis and mechanistic insights on self-supported LDHs-based electrocatalysts, and is poised to shed light on and stimulate sagacious inspiration for the creation of more sophisticated and robust next-generation catalysts.
To effectuate the cornucopia of accomplishing large-scale commercialization, several current research gaps accompanied by future recommendations ought to be taken into consideration. First, despite shedding light on the rational and robust design of next-generation electrocatalysts, there is a need to explore more reliable and ingenious preparation techniques for synthesizing self-supported LDHs-based electrocatalysts. This is to allow their fabrication in a precisely tuned, low-cost, mass production, high uniformity with minimal aggregation and environmentally friendly manner. Next, the seeking for more effective substrates other than the conventionally employed Ni foam, Cu foam, carbon cloth and carbon paper deserves more attention, to potentially ameliorate their electrocatalytic performance with long-term stability. Furthermore, in synergy with the conduct of more intensive lab-scale experimental research, theoretical studies leveraging various advanced computational aids such as DFT,248 microkinetic modeling,249 and machine learning (ML)250, 251 are highly encouraged to offer comprehensive guidance for experimental realization. Moreover, further studies should explore self-supported LDHs-based electrocatalysts with novel coupling materials (e.g., MXENE, dual-atom catalysts [DACs] and polymers), while extending application scope to other energy- (or environmental) related fields (e.g., carbon dioxide reduction reaction [CO2RR], oxygen reduction reaction [ORR], nitrate reduction reaction [NO3RR] and oxidation of furfural molecules) with the establishment of descriptors related to the electrocatalysts' performance.252, 253 To facilitate reliable comparisons and fostering advancements in the field of electrocatalysis, it is vital to establish robust and standardized performance indicators (e.g., overpotential required to achieve industrial-grade current density) for the electrocatalysts under consistent conditions. Looking ahead, future research should incorporate isotope labeling, especially for reactions involving molecules such as CO2, N2, NO3, to rule out contamination effects while procuring more comprehensive insights.
To propel progress, investigating the electrocatalysts in laboratory settings through the deployment of primary electrolyzer types such as H-cells, flow cells and membrane electrode assembly (MEA) cells is gaining prominence for realizing the prospective commercialization of self-supported LDHs-based electrocatalysts in energy conversion and storage applications.254-257 Also, there is a pressing need to implement more sophisticated and advanced in situ characterization techniques (e.g., in situ Raman, in situ x-ray absorption spectroscopy [XAS] and in situ TEM), as they are indispensable for real-time monitoring the catalysts' compositional and structural changes under operating conditions.258, 259 This could contribute to resolving the challenges of revealing the nature of LDHs-based electrocatalysts, particularly concerning catalyst reconstruction, actual active sites and the reaction mechanisms at play during complex organic oxidation reactions. To mitigate the typical challenges faced by self-supported LDH electrocatalysts, which tend to maintain high efficiency for organic oxidation only at lower voltages due to competition between OER and EHCO, several approaches can be considered. These include modifying the electrolyte nature by increasing organic substrate concentration and lowering OH− concentration, as well as designing non-OER active materials through rational catalyst design to prevent the coupling of oxygen atoms on the catalyst surface.
Additionally, exploring the feasibility of eliminating the electricity supply to the electrochemical cell, by integrating renewable energy supply such as solar cells to power the electrocatalytic processes is becoming increasingly critical to support sustainable energy practices. Smartly incorporating industrial revolution (IR) 4.0 elements (e.g., neural network [NN],260, 261 artificial intelligence [AI] system,262, 263 cloud computing and big data analysis264) are also instrumental in boosting their commercial viability of the electrocatalysts systems. While the full commercial potential of self-supported LDHs-based electrocatalysts is still on the horizon, it is believed that the explosive research growth and dynamic pace of discoveries are propelling this field forward, reaching new heights and setting the stage for successful commercialization of efficient catalysts for energy conversion and storage devices.
AUTHOR CONTRIBUTIONS
Man-Kei Wong conceived the idea, wrote and revised the manuscript, with input from Jian Yiing Loh and Feng Ming Yap commented the manuscript. Wee-Jun Ong conceived the research topic, supervised the entire project and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge the financial support provided by the Ministry of Higher Education (MOHE) Malaysia under the Fundamental Research Grant Scheme (FRGS) (Ref no: FRGS/1/2024/TK08/XMU/02/1). This work was supported by the PETRONAS-Academia Collaboration Dialogue (PACD 2023) grant, provided by PETRONAS Research Sdn. Bhd. (PRSB). The authors would like to thank the Ministry of Science, Technology and Innovation (MOSTI) Malaysia under the Strategic Research Fund (SRF-APP NanoMalaysia BICEP Project 4, S.22015). The authors gratefully acknowledge Agilent Technologies Malaysia Sdn. Bhd. for their contribution through chromatography. The authors would also like to acknowledge the financial support provided by the National Natural Science Foundation of China (Ref no: 22202168) and Guangdong Basic and Applied Basic Research Foundation (Ref no: 2021A1515111019). This work is supported by the Embassy of the People's Republic of China in Malaysia (EENG/0045). We would also like to acknowledge the financial support from the State Key Laboratory of Physical Chemistry of Solid Surfaces, Xiamen University (Ref no.: 2023X11). This work is also funded by Xiamen University Malaysia Investigatorship Grant (Grant no: IENG/0038), Xiamen University Malaysia Research Fund (ICOE/0001 and XMUMRF/2021-C8/IENG/0041).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Biographies

Man-Kei Wong received her Bachelor of Chemical Engineering with Honors from Xiamen University, Malaysia, in 2024, under the supervision of Prof. Dr. Wee-Jun Ong. She is presently a postgraduate student pursuing a Master's in New Energy Science and Engineering, and is a graduate research assistant (GRA) at the School of Energy and Chemical Engineering at Xiamen University, Malaysia. Her research topic of interest primarily focuses on the tunable nanostructured electrocatalysts for water splitting, urea oxidation, CO2 reduction, NO3 reduction and other emerging energy applications.

Wee-Jun Ong received his B.Eng. and Ph.D. in chemical engineering from Monash University. He is a Professor and Assistant Dean in School of Energy and Chemical Engineering at Xiamen University Malaysia. Starting from 2021, he serves as the Director of Center of Excellence for NaNo Energy & Catalysis Technology (CONNECT). Previously, he was a scientist at Agency for Science, Technology and Research (A*STAR), Singapore. In 2019, he was a visiting scientist at Technische Universität Dresden and a visiting professor at Lawrence Berkeley National Laboratory. His research interests include nanomaterials for photo(electro)catalytic and electrochemical H2O splitting, CO2 reduction, alcohol oxidation, H2O2 production, plastic reforming, nitrate reduction and N2 fixation. For more details, refer to https://sites.google.com/site/wjongresearch/.