Prevention of mucosally induced uveitis with a HSP60-derived peptide linked to cholera toxin B subunit
Abstract
Oral administration of the uveitogenic peptide (aa 336-351) derived from human HSP60 induced clinical and histological manifestations of uveitis in 65.8% (48/73) of Lewis rats. Uveitis was significantly decreased to 16.7% (11/66) in parallel experiments with the peptide linked to recombinant cholera toxin B subunit (rCTB), also given by mouth (χ2=34.2, p<0.0001). The protective efficacy between tolerized and immunized animals was 74.7%. Adoptive transfer of mesenteric lymph node cells from tolerized rats prevented the development of uveitis. A significantly higher proportion of regulatory CD4+CD45RClowRT6+ subset of Th2 memory cells were found in the mesenteric lymph nodes (p<0.005) and spleens (p⩽0.05) of tolerized rats without uveitis, as compared with immunized rats and uveitis. In situ hybridization studies of mesenteric lymph nodes and/or the uveal tract showed significant increases in IL-10 and TGF-β mRNA but decreases in IFN-γ and IL-12 mRNA in tolerized, as compared with immunized animals. Thus, the mechanism of tolerance, preventing the development of uveitis may involve a regulatory subset of memory cells and a shift from Th1 to Th2 and Th3 cytokines. We suggest that mucosally induced uveitis can be prevented by oral administration of the peptide-rCTB conjugate.
Abbreviations:
-
- CTB:
-
Cholera toxin B subunit
-
- BD:
-
Behcet's disease
-
- IRBP:
-
Interphotoreceptor retinal binding protein
-
- MBP:
-
Myelin basic protein
-
- MLN:
-
Mesenteric lymph node
1 Introduction
Experimental uveitis has been induced in Lewis rats by oral (or nasal) administration of peptide 336–351 which is derived from the sequence of human 60-kDa heat shock protein (HSP60) 1, 2. The rationale for exploring HSP was that it is a common microbial agent that might account for a variety of microorganisms, such as Streptococcus sanguis that have been implicated in the etiology of Behcet's disease (BD) 3–6. The peptide was first identified by epitope mapping of mycobacterial HSP65 using the T cell proliferative response in BD 7, 8. A homologous epitope was identified within the human HSP60 7, 8. The specificity of a major T cell epitope (aa 336–351) in BD was confirmed in Japan 9 and Turkey 10. The significance of the BD-specific HSP peptide was greatly enhanced by the experimental evidence that it induced uveitis in Lewis rats when administered subcutaneously with adjuvants 11.
Oral or nasal administration of myelin basic protein (MBP) prevents the development of experimental allergic encephalitis 12, 13. This has also been demonstrated with collagen type II in collagen or adjuvant arthritis 14–16. Retinal S antigen or interphotoreceptor retinal binding protein (IRBP) administered orally also prevented uveitis in rats 17, 18. The surprising immunogenicity of oral or nasal administration of p336–351, instead of the conventional induction of tolerance2, led to difficulties in devising a regime that would suppress the development of uveitis. However, we have taken advantage of the finding that recombinant cholera toxin B subunit (rCTB), linked to an antigen, enhances oral tolerance 19. Thus, MBP conjugated to rCTB prevented or suppressed experimental autoimmune encephalomyelitis (EAE), using lower concentrations of the conjugate than is usually necessary to induce tolerance with protein alone 19, 20. This is in contrast to the adjuvant properties elicited by cholera toxin 21–24.
In this study we demonstrate that p336–351 derived from the human HSP60, covalently linked to rCTB significantly decreased the development of uveitis induced by oral administration of p336–351 alone, from 65.8% (48/ 73) to 11/66 (16.7%) (χ2=34.2, p<0.0001). In situ cytokine expression studies of mesenteric lymph nodes showed a significant increase in IL-10 (p<0.05) and a decrease in IFN-γ mRNA in tolerized, as compared with immunized rats. The uveal tract also showed a significant decrease in mRNA of the Th1 type of cytokines (IL-12 and IFN-γ; p<0.05) but an increase in TGF-β (p<0.001) in tolerized rats without uveitis. Flow cytometric studies of the regulatory CD4+CD45RClowRT6+ T cells in the mesenteric lymph nodes (MLN) and spleen suggest that this subset of Th2 type of memory cells is significantly increased in tolerized rats in which uveitis is prevented (p<0.005 in MLN). Furthermore, adoptive transfer of MLN cells from tolerized rats prevented the development of p336–351-induced uveitis.
2 Results
2.1 Induction of uveitis
The onset of uveitis in the immunized rats was on day 10, with a rapid increase up to day 22 after which there was only a small rise up to day 28 (Fig. 1). At this time 48/73 (65.8%) rats developed uveitis, as assessed by the combined clinical and histological criteria.
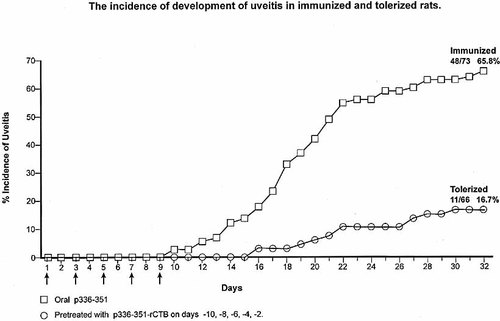
The development of uveitis was assessed by both clinical and histological criteria over a period of 32 days in 73 rats treated orally with 62.5 μg/ml of p336–351 (×5) on alternate days, compared with that in 66 rats pre-treated with 15 μg/ml of p336–351 covalently linked to recombinant CTB (×5) on alternate days before start of the immunization schedule.
2.2 Prevention of uveitis
To prevent the development of uveitis rats were pre-treated by gavage with CTB-linked p336–351, followed by the immunizing regimen of p336–351, in parallel with rats receiving only the immunizing peptide. The development of clinical and/or histological uveitis in the tolerized rats was delayed to day 16 with a slow progressive increase up to day 28 (Fig. 1). Of the 66 tolerized rats, only 11 (16.7%) developed uveitis after treatment with CTB-linked p336–351 and this was a very significant decrease in uveitis (χ2=34.2, p<0.0001). The results of 13 experiments are presented separately for clinical and histological development of uveitis, in order to appreciate the variations between the two modes of assessment and between the experiments (Table 1). The use of a control peptide 136–150 linked to CTB and administered by gavage, followed by the immunizing p336–351, failed to affect the development of uveitis, as 14/20 (70%) of the rats showed the disease (Table 2). Thus, prevention of uveitis was specific to p336–351-CTB. Protective efficacy was 74.7%, calculated as follows: 100×(frequency of uveitis in tolerized/frequency of uveitis in immunized animals).
|
|
Immunizationa) |
Tolerization – Immunizationb) |
||||
|---|---|---|---|---|---|---|
|
Exp. |
Clinical |
Uveitis Histological |
Combined |
Clinical |
Uveitis Histological |
Combined |
|
31 |
8/10 |
6/10 |
9/10 |
2/5 |
1/5 |
2/5 |
|
31 |
4/5 |
3/5 |
4/5 |
– |
– |
– |
|
33 |
4/5 |
5/5 |
5/5 |
2/5 |
0/5 |
2/5 |
|
34 |
3/5 |
3/5 |
3/5 |
1/9 |
3/9 |
3/9 |
|
35 |
2/4 |
1/4 |
2/4 |
0/4 |
1/4 |
1/4 |
|
36 |
2/4 |
2/4 |
2/4 |
0/4 |
0/4 |
0/4 |
|
37 |
3/5 |
3/5 |
3/5 |
0/5 |
1/5 |
1/5 |
|
38 |
2/5 |
1/5 |
3/5 |
0/5 |
0/5 |
0/5 |
|
39 |
3/7 |
1/7 |
4/7 |
1/7 |
0/7 |
1/7 |
|
40 |
3/6 |
– |
3/6 |
0/6 |
– |
1/6 |
|
41 |
6/8 |
5/8 |
7/8 |
1/12 |
1/12 |
2/12 |
|
42 |
2/4 |
4/4 |
4/4 |
1/4 |
0/4 |
1/4 |
|
43 |
3/5 |
2/5 |
4/5 |
0/4 |
0/4 |
0/4 |
|
Total |
45/73 |
36/67 |
53/73 |
8/70 |
7/64 |
14/70 |
|
% |
61.6 |
53.7 |
72.6 |
11.4 |
10.9 |
20 |
|
-ND |
|
|
|
|
|
|
- a) p336-351, 62.5 μg (×5), days 11–19
- b) p336-351-CTB, 15 μg (×5) days 1–9; p336-351, 62.5 μg (×5) days 11–19
|
|
Immunizationa) |
Tolerization-Immumizationb) |
||||
|---|---|---|---|---|---|---|
|
Exp. |
Clinical |
Uveitis Histological |
Combined |
Clinical |
Uveitis Histological |
Combined |
|
31 |
4/5 |
2/5 |
4/5 |
2/5 |
4/5 |
4/5 |
|
32 |
4/5 |
3/5 |
4/5 |
2/5 |
3/5 |
3/5 |
|
33 |
4/5 |
5/5 |
5/5 |
3/5 |
3/5 |
3/5 |
|
34 |
3/5 |
3/5 |
3/5 |
3/5 |
3/5 |
4/5 |
|
Total |
|
|
16/20 (80 %) |
10/20 |
13/20 |
14/20 (70 %) |
2.3 Adoptive transfer of lymphocytes from tolerized rats
MLN cells were prepared from tolerized rats and 3×107 cells were administered into the tail vein on day 1 and 8 of starting oral immunization with p336–351. Adoptive transfer of lymphocytes from the tolerized rats showed that 2/12 (16.6%) developed uveitis (Table 3). This compared with the development of uveitis in the control tolerized rats in 1/8 (12.5%) and in 10/11 (91%) of immunized animals carried out in the same experiment.
|
|
Uveitis |
|||
|---|---|---|---|---|
|
Group |
Treatment |
Clinical |
Histological |
Combined |
|
1. |
Immunized with p336 – 351 |
7/11 |
8/11 |
10/11 = 90.9 % |
|
2. |
Tolerized with p336 – 351-CTB followed by (1) |
1/8 |
0/8 |
1/8 = 12.5 % |
|
3. |
Adoptive transfer of cells from tolerized rats, followed by (1) |
1/12 |
1/12 |
2/12 = 16.6 % |
2.4 In situ cytokine expression
In situ hybridization studies of the MLN cells for mRNA expression of Th1 and Th2 cytokines were carried out in immunized and tolerized rats (Fig. 2). A raised mRNA expression of IFN-γ was found in the MLN of immunized (10.1±4.7) compared with the tolerized rats (3.97±1.6), though the 5% level of significance was not reached, because of variation in the immunized rats. However, mRNA expression of IL-10 was significantly increased in MLN cells from tolerized (6.1±2.2), as compared with immunized (2.3±0.9) animals (p<0.05). Examination of the posterior segment of the eye showed a significant reduction in IFN-γ mRNA expressing cells in the tolerized (0.08±0.08), as compared with the immunized (1.1±0.2) rats (p<0.05). Similar results were obtained with IL-12 (0.9±0.1 and 3.3±1.0; p<0.05), but surprisingly, IL-10 showed little or no difference between the two groups of animals (Fig. 2). However, tolerized rats showed a significant increase (p<0.001) in TGF-β expressing cells (2.9±0.35) compared with the immunized rats (0.7±0.1). Thus, tolerized animals which did not develop uveitis express a predominantly Th2 type cytokine pattern in MLN and Th3 in the uveal tract, whereas immunized rats with uveitis express predominantly Th1 type of cytokines in the uveal tract and MLN.
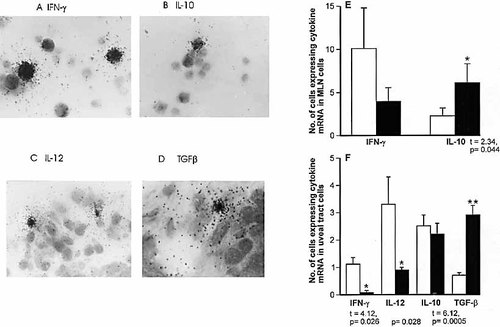
(A–D) Expression of mRNA by in situ hybridization: (A) IFN-γ in MLN, (B) IL-10 in MLN, (C) IL-12 in uveal tract cells, (D) TGF-β in uveal tract cells. (E) The number of cells expressing mRNA of IFN-γ and IL-10 in 105 MLN cells, (F) the number of uveal tract cells expressing mRNA of IFN-γ, IL-12, IL-10 and TGF-β per section.
2.5 CD4+CD45RClowRT6+ regulatory T cells
Preliminary examination of the proportion of CD4+ and CD8+ T cells by flow cytometry in the immunized rats with uveitis and tolerized rats without uveitis showed no significant difference (data not presented). We examined the CD4+CD45RClowRT6+ T cells which are regulatory Th2 type memory cells, by flow cytometry in the spleen, mesenteric lymph nodes and Peyer's patches (Fig. 3). A significant increase in the proportion of CD4+CD45RClowRT6+ splenic cells was found in the tolerized rats without uveitis (61.0±15.8%), compared with the immunized rats with uveitis (43.7±2.1%; t=2.56, p<0.05; Table 4). The most significant increase in the proportion of CD4+CD45RClowRT6+ cells was found in the mesenteric lymph node cells in the tolerized rats without uveitis (74.6±4.3%), compared with the immunized rats with uveitis (50.5%±3.6; t=4.3, p<0.005). Although a similar trend was observed in the cells eluted from Peyer's patches this failed to reach the 5% level of significance because of large variation in the proportion of eluted cells from the intestinal tissues of different animals. Similar flow cytometric analysis confirmed that cells from rats that received MLN cells from tolerized rats showed significantly higher CD4+CD45RClowRT6+ MLN cells (75.4±2.0%) than those of the immunized rats (63.9±2.0%; t=4.0, p=0.01; Table 4]).
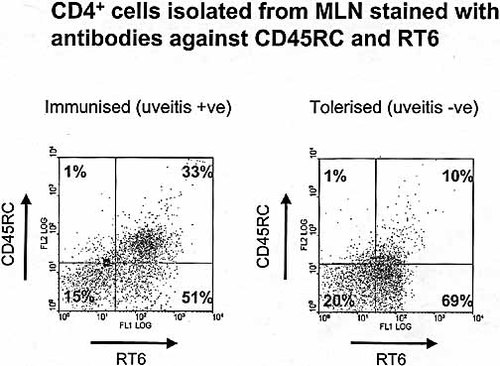
The proportion of the CD4+CD45lowRT6+ T cells was examined by flow cytometry of mesenteric lymph nodes from immunized rats, with uveitis and tolerized rats, without uveitis. PE-conjugated anti-CD45 RC OX-22 and anti-RT6.1 staining was followed by FITC-conjugated anti-rat IgG. The cells were gated on cy-chrome conjugated CD4+ cells. A representative example is shown.
|
T cells eluted from |
Immunized Mean (± SEM) |
Tolerized Mean (± SEM) |
t |
p |
|---|---|---|---|---|
|
(A) |
|
|
|
|
|
Spleen |
43.7 (2.1) |
61.0 (15.8) |
2.56 |
< 0.05 |
|
MLN |
50.5 (3.6) |
74.6 (4.3) |
4.30 |
< 0.005 |
|
PP |
52.6 (13.0) |
67.3 (5.8) |
1.0 |
0.35 |
|
(B) |
|
|
|
|
|
MLN |
63.9 (2.0) |
75.4 (2.0) |
4.0 |
0.01 |
3 Discussion
Although oral or nasal administration of antigens usually leads to tolerance 25, 26, a number of exceptions to this paradigm have been reported 2, 27, 28. Numerous attempts to induce oral or nasal tolerance of systemically induced uveitis by means of HSP60-derived BD peptide (336–351) have failed 2. Indeed, the converse was found, in that oral or nasal administration of peptide 336–351, without an adjuvant, elicited uveitis in Lewis rats. The clinical and histological appearance of the mucosally induced uveitis was indistinguishable from that induced by subcutaneous immunization with the peptide 2.
To prevent p336–351-induced uveitis in rats we utilized rCTB which enhances oral tolerance, if the tolerogen is covalently linked to the rCTB 19. Indeed, p336–351 linked to rCTB, significantly prevented the development of uveitis when administered orally before the tolerizing regime (p<0.0001). The tolerance was specific to p336–351, as an unrelated HSP60-derived peptide (136–150) linked to rCTB failed to prevent uveitis (70%). We also failed to prevent uveitis by tolerization at the same time as immunization or 1, 3, 5 and 7 days after starting the immunizing regime. To confirm that the lymphoid cells were responsible for preventing the development of uveitis we carried out adoptive transfer experiments with MLN cells eluted from rats orally tolerized with p336–351 linked to rCTB. The results clearly indicate that transfer of MLN cells from tolerized rats prevented uveitis (12.5%) to a similar level to that found in tolerized rats (16.6%), whereas 91% of immunized rats developed uveitis in the same experiments.
The mechanism of oral tolerization has been the subject of extensive investigations (reviewed in 25, 26, 29). In this study in situ hybridization of MLN cells showed that the expression of a Th2 cytokine (IL-10) was significantly increased, whereas a Th1 cytokine mRNA (IFN-γ) was decreased in rats which had been tolerized and uveitis was prevented, as compared with the immunized rats which developed uveitis. Furthermore, examination of the uveal tract demonstrated a significant increase in TGF-β (Th3) and a decrease in IFN-γ and in IL-12 (Th1) cytokines in the tolerized, as compared with immunized rats. This suggests that TGF-β may be involved in down-regulation of inflammation of the eyes. This concept is supported by the report that generation of TGF-β secreting T cells is facilitated by IL-4 30, and by our finding that the development of p336–351-driven uveitis is inhibited by treatment with IL-4 from 68% to 30% 2.
The hypothesis was further tested, to ascertain if regulatory memory cells might be involved (CD4+CD45RClowRT6+ cells), as described in inhibition of autoimmune thyroiditis and diabetes 31. A significant increase in this subset of cells in MLN (p<0.005) and spleens (p<0.05) was found in tolerized rats, with no clinical or histological evidence of uveitis, as compared with the immunized rats. Similar results were found with adoptive transfer of MLN cells. The mechanism of protection against autoimmune thyroiditis in rats by CD4+CD45RClowRT6+ cells has been associated with IL-4 and TGF-β 31 and these cells generate a high number of IL-4-producing cells 32. The CD4+CD45RClow cells have recently been found to generate exclusively IL-4, IL-10 and IL-13 cytokines and these cells may control inflammatory and autoimmunediseases 33. These reports are consistent with our previous finding that IL-4 inhibits in vivo p336–351 induced uveitis in rats 2, as well as the present insitu hybridization studies of mRNA expression in the MLN and uveal tract cells which showed increased Th2 and Th3, respectively and decreased Th1 cytokines in the tolerized animals. The mechanism of prevention of uveitis induced by p336–351-linked CTB might be accounted for by a shift from Th1 to Th2 and Th3 in the gut associated lymph nodes and the uveal tract. The strategy of oral tolerization with the uveitogenic peptide covalently linked to rCTB is now undergoing a clinical phase I/II trial of uveitis in patients with Behcet's disease.
4 Materials and methods
4.1 Peptides, CTB, and antibodies
Peptide aa 336–351 (QPHDLGKVGEVIVTKD) was derived from the sequence of the human mitochondrial 60-kDa HSP and was prepared at the Hansen's Disease Laboratory (Centre for Disease Control, Atlanta, GA) as described 7. Recombinant cholera toxin B subunit (rCTB) was produced in a mutant strain of Vibrio cholerae in which the cholera toxin genes were deleted and this was transfected with a multicopy high expression plasmid encoding CTB 34. The rCTB was purified to apparent homogeneity from the culture filtrate by precipitation with hexametaphosphate followed by adsorption chromatography on hydroxyapatite and gel filtration chromatography through Sephacryl S-100 (Pharmacia, Uppsala, Sweden). Peptide 336–351 was covalently conjugated to CTB using N-succinimidyl (3-[2-pyridyl])-dithio) propionate (SPDP) bifunctional coupling reagent 35, according to the manufacturer's instructions (Pharmacia). Briefly, p336–351 and CTB were separately derivatized with SPDP by incubation (23°C, 30 min) at molar ratios of 1:5. The SPDP-derivatized CTB was reduced with dithiothreitol and the resulting preparation was freed ofexcess DTT and pyridine-2-thione by Sephadex G-25 chromatography. SPDP-derivatized p336–351 was mixed in a 2.5-fold molar excess with the derivatized and reduced CTB and incubated for 16 h at 23°C.The resulting peptide-CTB conjugate was isolated by gel filtration through a Sephadex G-25 column (Pharmacia) in PBS. Control peptide 136–150 (aa NPVEIRRGVMLAVDA) was similarly linked to CTB.
Anti-rat mAb used in this work were as follows: RPE-Cy5-conjugated OX-8 (anti-CD8, Serotec, Kidlington, Oxford, GB), PE conjugated OX-22 (anti-CD45RC; Serotec), cy-chrome-conjugated OX-35 (anti-CD4; PharMingen, Heidelberg, Germany) PE-conjugated OX-39 (anti-CD25; Serotec), FITC-conjugated anti-CD28 (Serotec), FITC-conjugated anti-rat IgG (PharMingen), and anti-rat RT6.1 (Serotec).
4.2 Immunization and tolerization
Male Lewis rats weighing 200–220 g were housed in groups of five and given food and water ad libitum. For the induction of uveitis by the oral mucosal route animals were given five consecutive doses on alternate days of 65 μg/ml of p336–351, dissolved in 1 ml of PBS containing type II soy bean trypsin inhibitor (10 μg/ml, Sigma, Poole, GB) by oral gavage (days 0, 2, 4, 6, 8). The optimum concentration of the peptide was determined previously 2.
To induce tolerance animals were treated with five consecutive doses on alternate days of 15 μg/ml of p336–351 conjugated to recombinant CTB and dissolved in 1 ml of 0.35 M sodium bicarbonate buffer prior to immunization with the peptide alone (days –10, –8, –6, –4, –2). Control groups were dosed with 15 μg/ml of CTB followed by either sodium bicarbonate buffer or 65 μg/ml ofp336–351. The optimum tolerizing concentration of the CTB linked p336–351 was determined by prior experiments with different doses of the CTB-peptide. Altogether 13 tolerizing experiments were carried out with the CTB linked p336–351 in parallel with immunization in groups of 4–10 rats. Tolerization was also carried out with CTB linked to a control peptide (136–150) in 4 experiments with 20 rats. Attempts were then made to prevent uveitis by tolerization at the same time as immunization or 1, 3, 5 and 7 days after starting the immunizing regime.
4.3 Clinical and histological examination
All animals were observed daily from day 8 for signs of uveitis by means of slit-lamp microscopy, without any prior knowledge of their treatment. The clinical signs of uveitis were enlarged and tortuous vessels in the iris, constricted pupils and inflammatory cells found in the anterior chamber of the eye as has been reported previously 1. The histological features of anterior uveitis were a mononuclear cell infiltration in and around the ciliary body and iris, with an occasional nodular mononuclear cell infiltrate of the iris 1. Both clinical and histological features were scored using the system described 36. Posterior synechiae between the iris and the capsule of the lens were indicative of uveitis that had cleared. Focal loss of photoreceptors, in the absence of mononuclear cells was also recorded. Animals were killed by cervical dislocation either during clinical disease or 28 days after the first immunization and their eyes were removed for histological examination or frozen in liquid nitrogen prior to sectioning for in situ hybridization. Spleens, mesenteric lymph nodes and Peyer's patches were used to examine phenotypic expression of cell surface molecules by flow cytometry.
4.4 Analysis of cell surface phenotypic expression
Cells isolated from the spleen, mesenteric lymph nodes and Peyer's patches were incubated with the appropriate FITC-, PE- or cy-chrome conjugated mAb, as described in Sect. 4.1. Flow cytometric analysis was performed on a FACScan flow cytometer (Coulter).
4.5 Detection of cytokine mRNA in mesenteric lymph nodes
Expression of IL-10 and IFN-γ mRNA was examined by in situ hybridization as described 37. Briefly, 200-μl aliquots containing 4×105 MLN mononuclear cells were applied to the wells of round-bottom 96-well microtiter plates (Nunc), in triplicate. Aliquots of 10 μg/ml of p336–351 were added to the cell cultures and after 24 h the cells were washed, counted, and 1×105 cells were dried onto electrically charged glass ProbeOn slides (Fisher Scientific, Pittsburgh, PA). Synthetic oligonucleotide probes (Scandinavian Gene Synthesis AB, Koping, Sweden) were labeled using 35S deoxyadenosine-5′-α-(thio)-triphosphate (New England Nuclear, USA) with terminal deoxynucleotidyl transferase (Amersham, Little Chalfont, GB). A mixture of two different oligonucleotide probes was employed for the cytokine. The oligonucleotide sequences were obtained from GenBank using MacVector software. Cells were hybridized with 106 cpm labeled probe/100 μl of hybridization mixture. After emulsion autoradiography, development and fixation, the coded slides were examined by dark field microscopy for positive cells defined as containing >30 grains/cell in a star-like pattern. The intracellular grains were counted by light microscopy. A negative control probe was used in parallel with the specific cytokine probes, with cells from each specimen and these did not show any staining of cells. The results were expressed as the number of labeled cells/105 cells counted.
4.6 Detection of cytokine mRNA in the uveal tract
Cryostat sections (14 μm) of the whole eye were prepared and mounted onto ProbeOn slides. Mixtures of four different labeled synthetic oligonucleotide probes were employed for each of the tested cytokines and the oligonucleotide sequences were obtained from GenBank and probes were designed using MaxVector software. These were as follows: IFN γ (J00219) with complementary bases 507–556, 4682–4729, 4660–4707, 4641–4688 38; IL-12 (M65272, M38444, M65271, M38443) with bases 157–204, 544–591, 191–238, 279–326 39; IL-10 (M57627) with bases 3178, 97–144, 340–387, 376–423 40; and TGF-β (X02812) with bases 1363–1410, 1457–1504, 1766–1813, 1953–2000) 41. In situ hybridization was performedas described above to detect cytokine mRNA expression in cells of the uveal tract. The results are expressed as the number of labeled cells per tissue section using image analysis to measure the tissue section.
4.7 Adoptive transfer of regulatory cells
Cells were isolated from the mesenteric lymph nodes of rats on day 12 of starting the tolerizing regimen of CTB-linked p336–351, administered five times on alternate days. They were resuspended in saline at a concentration of 6×107 cells/ml and 0.5 ml of viable cells were injected intravenously into the tail veins of rats. The cells were injected on the first day of immunization with p336–351 and on day 8. The rats were observed from day 8 daily for signs of uveitis by means of slit lamp microscopy and were killed either during clinical disease or on day 30.
4.8 Statistical method
The χ2 test, Student's t-test or Mann Whitney test was used, as appropriate to analyze the results.
Acknowledgements
This work was supported by a grant from the Iris Fund for the Prevention of Blindness, the Swedish Science Council (Medicine), the Institute of Medical Science, Kawasaki,Japan, the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. We thank Mr. E. G. McGowan for his assistance.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




