Increased expression of DC-SIGN+IL-12+IL-18+ and CD83+IL-12–IL-18– dendritic cell populations in the colonic mucosa of patients with Crohn's disease
Abstract
Dentritic cells (DC) as antigen-presenting cells are most likely responsible for regulation of abnormal T cell activation in Crohn's disease (CD), a chronic inflammatory bowel disease. Wehave analyzed the expression of activation and maturation markers on DC in the colon mucosa from patients with CD compared with normal colon, using immunohistochemical techniques. We found two distinct populations of DC present in CD patients: a DC-specific ICAM-3 grabbing non-integrin (DC-SIGN)+ population that was present scattered throughout the mucosa, and a CD83+ population that was present in aggregated lymphoid nodules and as single cells in the lamina propria. In normal colon the number of DC-SIGN+ DC was lower and CD83+ DC were detected only in very few solitary lymphoid nodules. Co-expression of activation markers and cytokine synthesis was analyzed with three-color confocal laser scanning microscopy analysis. CD80 expression was enhanced on the majority of DC-SIGN+ DC in CD patients, whereas only a proportion of the CD83+ DC co-expressed CD80 in CD as well as in normal tissue. Surprisingly, IL-12 and IL-18were only detected in DC-SIGN+ DC and not in CD83+ DC. A similar pattern of cytokine production was observed in normal colon albeit to a much lesser extent. The characteristics ofthese in-situ-differentiated DC markedly differ from the in-vitro-generated DC that simultaneously express DC-SIGN, CD83 and cytokines.
Abbreviations:
-
- CD:
-
Crohn's disease
-
- CLSM:
-
Confocal laser scanning microscopy
-
- DC-SIGN:
-
DC-specific ICAM-3 grabbing non-integrin
-
- IBD:
-
Inflammatory bowel disease
1 Introduction
Antigen-dependent activation of Th1 lymphocytes is believed to be a major pathogenetic mechanism in Crohn's disease (CD), but the phenotype of the antigen-presenting cell responsible for induction and maintenance of the disease has been poorly defined. The therapeutic approaches today are aimed at reduction of T cell activation, and result in transient relief from disease. After complete removal of pathogenic T cells, relapses recur at the same locations as the primary lesions, indicating a role for local resident cells. A candidate cell type responsible for stimulation and maintenance of local immune responses is the tissue DC.
DC are professional antigen-presenting cells that efficiently pick up antigen in the periphery and present it in the T cell areas of draining lymph nodes to naïve T cells, resulting in T cellactivation and differentiation 1. The initiation of a Th1 response is dictated by a specific subset of DC that have the capacity to produce a large amount of IL-12. On the contrary, Th2 responses are promoted in the absence of IL-12 2. In CD, the expression of IL-12 is up-regulated, resulting in a condition that maximally promotes Th1 immune responses 3. In addition, the expression of IL-18, also involved in Th1 development, is up-regulated in CD as well, but the cells that produce these cytokines are not fully characterized 4, 5.
Most of the studies on human DC are performed with DC populations generated from monocytes in vitro. Little is known about the activation and maturation of mucosal DC in their microenvironment, especially in a situation of chronic inflammation. We used in situ immunohistochemistry analysis to further examine phenotypic and functional characteristics of DC subsets in CD colon tissues.
Previous findings in our laboratory indicate that the expression of CD11c, a marker commonly used to type DC, did not correlate with the expression of other more specific DC markers in a two-color confocal laser scanning microscopy (CLSM) analysis 6. This may be due to a much wider expression spectrum of CD11c, including macrophages and granulocytes that are present in the colon lesions as well. Therefore, in the present study we focused on DC-specific markers, like DC-specific ICAM-3 grabbing non-integrin (DC-SIGN) 7, a 44 kDa type II membrane protein with an external mannose-binding, C-type-lectin domain involved in antigen recognition and presentation 8, and CD83 9, a marker for mature DC that plays a role in antigen presentation 10 and in CD4+ T cell development in the thymus 11. We show that DC-SIGN+ DC form a network of cells in the colonic mucosa, and produce IL-12 and IL-18 in active disease, and that CD83+ DC are negative for IL-12 and IL-18 and are mainly located in lymphoid structures. These findings are compared with the characteristics of in-vitro-generated DC from peripheral blood.
2 Results
2.1 DC-SIGN and CD83 expression
To analyze the expression and localization of the two DC markers DC-SIGN and CD83 in CD colon tissues, we performed immunohistochemical analysis. In Fig. 1A the expression of CD83 in the colon of a CD patient is shown. CD83 expression was demonstrated in the lamina propria in single cells, and further restricted to massive aggregated lymphoid structures, that stretch out into the submucosa. Expression of DC-SIGN in the colon of a CD patient (Fig. 1B) is found scattered throughout the whole mucosa. In CD colon tissue the DC-SIGN+ population showed a similar distribution pattern as in normal tissue, only the number of cells was increased, both in the lamina propria and in the submucosa (see Table 1). The CD83+ population was detected only in very few solitary lymphoid nodules in normal colon (results not shown).
The observed distribution pattern of CD83+ and DC-SIGN+ DC analyzed with immunohistochemical techniques demonstrated that these DC subsets localized at different sites. Whereas CD83+ DC were found associated with dense lymphoid aggregates, we did not observe any staining of DC-SIGN within these areas. As shown in Fig. 2, CLSM analysis of the border of a dense lymphoid aggregate demonstrated that CD83 and DC-SIGN do not co-localize and stain two distinct subsets of DC in colon mucosal tissue.
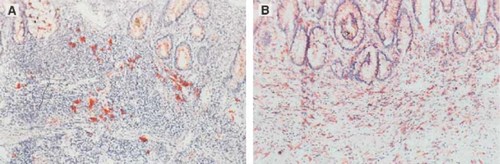
Immunohistochemistry picture of the colonic mucosa of a patient with CD. (A) Staining with CD83; (B) staining with DC-SIGN. Original magnification ×400. The staining within the crypts is also seen in the negative isotype control (see also Fig. 3) and is considered to be nonspecific.
|
Localization |
Donor |
Number of DC-SIGN+ DC per fielda) |
Comparison with normal |
|---|---|---|---|
|
Lamina propria |
Normal |
18.6 ± 1.3 |
– |
|
Lamina propria |
CD |
27.8 ± 2.5 |
p < 0.006 |
|
Submucosa |
Normal |
15.0 ± 0.8 |
– |
|
Submucosa |
CD |
23.5 ± 1.6 |
p < 0.0001 |
- a) Original magnification ×400. At least 20 fields in each tissue were counted for each donor.
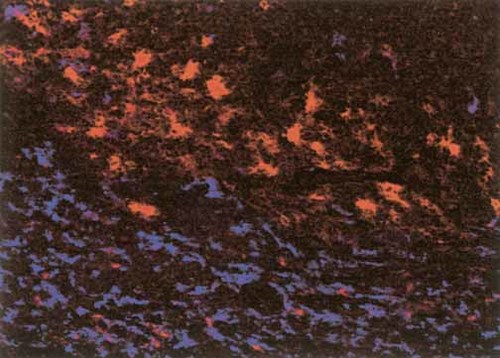
CLSM of DC-SIGN+ DC (in blue) and CD83+ DC (in red). Original magnification ×200.
2.2 Co-expression of activation markers
To investigate the level of activation of the different DC subsets in CD compared with normal colon tissue, we performed three-color CLSM analysis using CD80 as a marker for activation. We counted the number of DC-SIGN+, CD83+ and CD80+ cells in a minimum of 10 microscopic fields (×400 magnification) of samples from different patients. The absolute number of DC-SIGN/CD80 double-positive cells in CD patients was 18.7±3.1 cells per field compared with 4.9±1.3 cells per field in normal tissue (p<0.0001). Taking into account the differences in the absolute numbers of DC-SIGN+ cells in CD compared with normals, the percentage of CD80+ cells within the DC-SIGN population is similar in CD (58.5±5.4%) compared to normals (44.1±8.7 %, p<0.24). In the CD83+ population the percentage of CD80+ cells is also similar in CD compared to normal (36±4.5% and 32.3±1.5%, respectively).
2.3 Cytokine synthesis
Because an important function of DC is the production of IL-12 and IL-18, we analyzed the expression of these cytokines in the colon microenvironment. In Fig. 3, representative pictures of the mucosa of a CD patient are shown. The patients studied for IL-12 and IL-18 expression did not receive corticosteroids, because corticosteroids have an inhibitory effect on DC function and cytokine synthesis 12. In each panel of Fig. 3 a lymphoid aggregate is present in the center. In Fig. 3A, IL-12 staining is present throughout the lamina propria and the submucosa, but not in the lymphoid aggregate. In Fig. 3B, staining for IL-18 demonstrates that apart from positive staining in the lamina propria and the submucosa, some cells are also stained within the lymphoid aggregate. In addition, we used CD68 staining in the mucosa as a positive control (Fig. 3D).
In Fig. 4, a three-color CLSM overlay of a similar spot in the mucosa of a CD patient is shown, clearly demonstrating that DC-SIGN+ DC, but not CD83+ DC, are producing IL-18. To more closely assess the cytokine synthesis in the two DC populations at different locations, an additional three-color CLSM analysis was performed (shown in Fig. 5). It was clearly observed that DC-SIGN+ DC in the submucosa also produce IL-12 and IL-18, whereas CD83+ DC that are localized in aggregates are completely negative for IL-12 and IL-18. As demonstrated earlier and shown in Fig. 5, DC-SIGN and CD83 do not overlap at any site.
In normal colon specimens a baseline expression of IL-12 is found, whereas there is no IL-18 expressed (Table 2). However, production of IL-12 as well as IL-18 is strongly up-regulated during the CD disease progression. Moreover, if CD patients not using corticosteroids were compared with patients on corticosteroids, the latter demonstrated a lower number of IL-12+ and IL-18+ DC and a lower expression pattern: for IL-18 expression analyzed on a scale from 0–4 in n=3 patients not using corticosteroids and n=3 patients using corticosteroids, 2.7±0.9 and 0.3±0.3, respectively, in the lamina propria, and 3.7±0.3 and 2.7±0.3, respectively, in the submucosa. In conclusion, both IL-12 and IL-18 are produced only by DC-SIGN+ DC and not by CD83+ DC. A correlation was observed between IL-12 and IL-18 expression and disease activity.
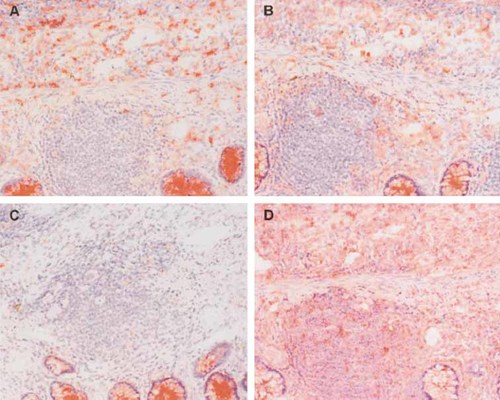
Immunohistochemistry picture of the colonic mucosa of a patient with CD. (A) Staining with IL-12; (B) staining with IL-18; (C) control staining; and (D) staining with CD68. Original magnification ×250. The staining within the crypts is also seen in the negative isotype control and is considered to be nonspecific.

Confocal laser scanning microscopy of DC-SIGN+ DC (in green), CD83+ DC (in red) and IL-18 (in blue) of the colonic mucosa of a patient with CD. DC-SIGN+IL-18+ cells are white/light-blue. The staining within the crypts is also seen in the negative isotype control (not shown) and is considered to be nonspecific. Also the yellow spots, representing granulocytes (arrow) are considered to be nonspecific. Original magnification ×200.
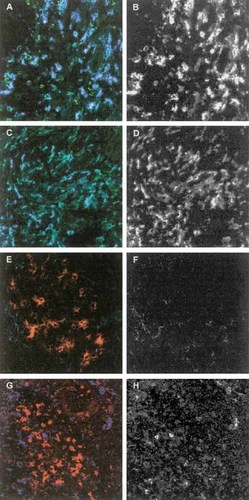
CLSM of colonic mucosa of a patient with CD. DC-SIGN+ DC (in green, A and C) and CD83+ DC (in red, E and G), and IL-12 staining (in blue, A and E) and IL-18 staining (in blue, C and G). Panels B and F (IL-12) and panel D and H (IL-18) demonstrate the single cytokine staining pattern in black and white. Original magnification ×200.
|
Cytokine |
% of total DC-SIGN+ cells |
% of total DC-SIGN+ cells |
Comparison with normal |
|---|---|---|---|
|
|
Normal |
CD |
|
|
IL-12 |
70.1 |
89.5 |
p < 0.0001b) |
|
IL-18 |
8.5 |
45.7 |
p < 0.003 |
- a) The absolute number of DC-SIGN+ DC per field in normal colon mucosa is 11.7+0.9 cells per field and in CD colon mucosa 32.4+2.3 cells per field (p<0.0001). Original magnification ×200.
- b) p values for the comparison of absolute numbers of DC-SIGN/IL-12 and DC-SIGN/IL-18 positive cells per field in normals compared with CD. At least 10 fields in each sample were counted for each donor.
2.4 In-vitro-generated DC
To compare the in situ expression with the expression of in-vitro-generated DC we cultured monocyte-derived DC and determined the expression of DC-SIGN and CD83. As expected these cells express both markers on mature DC at the same time (Fig. 6). These cells were also capable of secretion of IL-12 and IL-18 after additional stimulation (not shown).
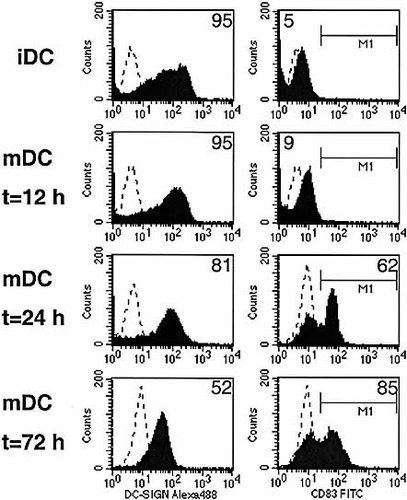
Flow-cytometric picture of in-vitro-matured DC. Immature DC (iDC) and mature DC (mDC) 12, 24 and 72 h after maturation are labeled with DC-SIGN–Alexa488 and CD83–PE. Matched subclass controls and compensation controls were used to set the negative control in the first decade of the log scale and to set the compensation, respectively. For DC-SIGN the mean fluorescence of the total DC-SIGN+ population is depicted in the upper-right corner of the individual histograms. For CD83 the mean fluorescence of the negative gated populations is depicted in the upper-left corners (iDC and mDC at 12 h) and that of the positive gated populations in the upper-right corners (mDC at 24 h and at 72 h), respectively. The percentage of CD83+ DC was 56% at 24 h and 53% at 72 h.
3 Discussion
In this study we describe two distinct populations of DC with unique cytokine profiles that can be distinguished in the colonic mucosa of patients with CD. Much of what is known about the maturation of DC has been learned from in-vitro-generated DC, obtained by differentiation of non-growing human blood monocytes or of proliferating bone-marrow-derived precursors with GM-CSF and IL-4. These in-vitro-differentiated immature DC demonstrate an increased expression of DC-SIGN 13 and lack expression of CD83. Further maturation of in-vitro-obained immature DC with TNF or LPS results in a reduction of DC-SIGN expression and an increase in CD83 and CD80, resulting in a mature population that expresses both DC-SIGN and CD83 (Fig. 6). This is in contrast to the populations we observed in vivo in the colon mucosa tissues, where two completely different populations are present, one expressing DC-SIGN and the other expressing CD83, whereas no intermediate DC forms are present.
We found an increased number of DC-SIGN+ and CD83+ DC in the colon tissues of CD patients, compared with normal tissues. In a recent study that analyzed CD11c+ and CD83+ cells on isolated gut DC from patients with inflammatory bowel disease (IBD), no quantitative differences in the DC populations were found between IBD patients and normal controls 14. Since isolated DC were used, the isolation procedure could have been selective or may have affected cell surface marker expression. The enhanced presence of DC in the colon of CD patients that we observed may be the result of stimulation of DC recruitment, local retention or proliferation, or the result of reduced death.
A functional role for the DC-SIGN+ DC population that produces IL-12 and IL-18 in the pathogenesis of IBD seems to be most likely, since these are the two effector cytokines involved in T cell development and activation. Moreover, in experimental models the functional importance of IL-12 and IL-18 for IBD has been shown, because blocking of IL-12 or IL-18 results in reduction of disease in experimental colitis 15–17. Of interest is the observed difference in IL-12 and IL-18 production in our study, suggesting that IL-18, compared with IL-12, is more disease-related. The observation that DC-SIGN+ cells produce IL-12 in the normal colon might indicate that this cytokine is involved in normal gut homeostasis.
From these in situ studies no information about the time-course of the production of these cytokines can be obtained. IL-12 synergizes with IL-18 to induce IFN-γ production by NK cells and Th1 cells 18. In addition, IL-12 allows IL-18 to signal by inducing a functional receptor complex 19. From our data we can conclude that at least someof the cells produce IL-12 and IL-18 simultaneously, although we did not perform double staining of both cytokines. However, it is likely that the combined presence of IL-12 and IL-18 in CD colon tissues may have increased potential to activate T lymphocytes and thus to worsen disease.
Obvious from our study is that in the close vicinity of the DC-SIGN+ DC, concentrated fields of IL-12 and IL-18 are localized that in CD spread throughout the submucosa. Such a local cytokine field 20 can have effect on the local immune polarization. In normal mucosa the strategic localization of DC-SIGN+ DC in the lamina propria and the submucosa enables them to pick up antigens and migrate to the draining lymph nodes. There they activate naïve T cells and determine the fate of the polarizing T cells. According to this hypothesis, the mature DC have a short half-life, and are removed after entering the T cell area of the draining lymph nodes 21. The increased number and expression level of these DC-SIGN+ cellsin the CD lamina propria and mucosa, as observed in our study, could create a situation with a local high IL-12 and IL-18 concentration where effector T lymphocytes could be activated and thereby activate local immune responses.
This is of interest since we recently demonstrated that in contrast to the normal mucosa – where all CD4+CD45RB+ T lymphocytes are of the CD45RO memory phenotype – in CD about 30% of the CD4+CD45RB+ T cells express the CD45RA antigen that is indicative of a naïve phenotype (Ten Hove et al., submitted). Whether or not these T cells have the possibilityto enter a local differentiation process is not clear. An alternative explanation is that expression of CD45RA may indicate effector function of local CD4+ T cells. Further studies are required to evaluate each of these possibilities.
The significance of the presence of CD83+ DC in colon mucosal tissue is less clear. The presence of the CD83+ DC in lymphoid structures could give information about their function. The observation that chronic inflammatory reactions are often associated with the organization of infiltrating lymphocytes lymphoid aggregates is well established in autoimmune-like diseases 22. In an experimental model of diabetes it was demonstrated that DC are responsible for the induction of local lymphoid tissue 23, and in T-cell-rich oral lymphoid follicles in chronic periodontitis and in lymphocytic infiltrates in rheumatoid arthritis mature CD83+ DC are found 24, 25. The development of focallymphoid structures harboring a concentration of mature DC could shift the T cell development in the direction of a Th1 response 26. We demonstrated that the DC present in these structures are mature CD83+ DC that do not produce IL-12 or IL-18. It is not clear what function we can ascribe to these cells, but it is tempting to speculate that these DC are able to attract other leukocytes via production of chemokines and thus play an important role in sustaining the local inflammation. However, the presence of these structures in the normal mucosa of the colon might indicate that these structures could also have a more regulatory function.
In patients with CD the precise cause of the sustained inflammatory reaction is not known, but a dysregulated immune response towards the normal luminal bacterial flora is considered to be a good candidate. This normal flora can have adjuvant activity via DC pattern-recognition receptors; this activity could be different in CD patients compared with normals and, depending on antigen availability, could result in the abundant local production of IL-12 and IL-18 by the DC-SIGN+ DC that we observe. IL-12 production and priming for Th1 responses is induced by products from pathogens, such as lipopolysaccharide, bacterial CpG DNA and double-stranded viral RNA, as well as T cell signals such as CD40 ligand and IFN-γ within a narrow time-window (8–16 h) after induction of maturation 27, and by cytokines 28. IL-18 synthesis is also found after bacterial 29 or viral infection 30. IL-18 is necessary for a recall cell-mediated immune response against Listeria monocytogenes 31. This effect was IL-12-and IFN-γ-independent, but dependent on TNF-α. In addition, blocking of IL-18 with IL-18-binding protein in experimental colitis reduces TNF-α independently of IFN-γ 16.
The presence of DC-SIGN+ DC in mucosal tissues has demonstrated an important function of DC-SIGN in antigen capture. As a C-type lectin, DC-SIGN recognizes carbohydrate motifs presenton pathogens and as an antigen-capture receptor it allows internalization of pathogens into DC to induce antigen presentation 8. It has recently been reported that some pathogenssuch as HIV bind DC-SIGN but circumvent the antigen-processing route of DC-SIGN on DC, illustrating an important function of DC-SIGN if it is expressed on DC at the port d'entrée.
In conclusion, our in situ study of DC populations, which show phenotypically and functionally distinct characteristics, may allow more insight into processes involved in the pathogenesis of CD. These findings may have major implications for the development of therapeutic interventions that focus on the resolution of uncontrolled DC activation.
4 Materials and methods
4.1 Patients and tissue samples
Colon tissues were obtained from surgical resection specimens from patients with CD who underwent partial bowel resection. The mean age of the group was 41.4±3.5 (n=16). Control samples were obtained from 10 patients with a similar mean age of 56.7±6.7 (p>0.05) undergoing a resection for non-IBD-related disorders (diverticulitis, polyposis coli or a tumor). Prior to the resection procedure, 4 of the 16 patients were on corticosteroids.
Specimens were snap-frozen in liquid nitrogen and stored at –80°C until analysis. Histological analysis was performed with cryosections stained with hematoxylin and eosin.
4.2 Antibodies
The following antibodies were used: FITC-conjugated anti-DC-SIGN (AZN D1-FITC), Alexa488-conjugated anti-DC-SIGN and PE-conjugated anti-CD83 were obtained from Coulter/Immunotech (Luminy, France); unconjugated CD80 (B7–24) was provided by Dr. M. de Boer (Tanox, Amsterdam, Netherlands); and unconjugated anti-IL-12 p70 (clone MAB611) and anti-IL-18 (clone 25–2G) were purchased from R&D systems, Inc. (Minneapolis, MN).
4.3 Immunohistochemical staining
Frozen sections were stained using a standard alkaline phosphatase protocol as described previously, with slight modifications 32. Briefly, cryostat fragments of colon tissues obtained from CD patients were cut into 4-μm sections, air-dried overnight, and fixed in acetone for 10 min at room temperature. Sections were pre-incubated in 5% (v/v) normal goat serum [Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (CLB), Amsterdam, The Netherlands]. Then sections were stained with either anti-CD83 as primary mAb (Beckman Coulter, Mijdrecht, The Netherlands), or with FITC-labeled anti-DC-SIGN (obtained from Dr. Y. van Kooyk, Vrije Universitet, Amsterdam, The Netherlands). Upon incubation with primary mAb, sections were incubated with biotinylated goat-anti-mouse-IgG (DAKO A/S, Glostrup, Denmark) (antibody dilution 1/200). Upon incubation with FITC-labeled mAb, sections were incubated with rabbit-anti-FITC antibody (DAKO) (antibody dilution 1/40,000) and biotinylated goat-anti-rabbit antibody (DAKO) (antibody dilution 1/400). Subsequently sections were incubated with streptavidin/biotin-conjugated alkaline phosphatase complex (ABC-protocol) (DAKO). Color was developed using as substrate naphtol AS-MX phosphate (0.3 mg/ml) plus New Fuchsin (0.1 mg/ml) in 0.2 M Tris-HCl buffer, pH 8.0 (ABC-protocol) and sections were counterstained with hematoxylin. Between incubation steps, the sections were extensively rinsed in PBS. Within each test, isotype matched control antibodies were included and found to be negative in the lamina propria and the submucosa but positive within the crypts. This was considered to be nonspecific.
To quantify the data, the numbers of positive staining cells were counted in microscope fields. In one experiment the positive staining was quantified on an arbitrary scale ranging from 0–4 inwhich 0 indicated no staining and 4 indicated very intense staining.
4.4 Immunofluorescence staining
Triple staining was performed as described previously, with some modifications 33. Briefly, cryostat fragments of colon tissue were cut into 4–6 μm sections, air-dried overnight, and fixed in acetone for 10 min at room temperature. The slides were first incubated with 5% (v/v) normal goat serum (CLB), then with optimal dilutions of primary mouse mAb in PBS containing 1% (w/v) BSA (PBS/BSA) for 30 min at room temperature, followed by incubation with biotinylated goat-anti-mouse-IgG (DAKO) and Cy5-conjugated streptavidin (Jackson Immunoresearch Laboratories, Inc., Palo Alto, CA). Upon blocking with normal mouse serum, sections were incubated with a second PE-labeled mouse mAb, rabbit-anti-PE (Biogenesis) and Cy3-conjugated goat-anti-rabbit (Jackson Immunoresearch Laboratories). Finally, after blocking with normal mouse serum, sections were incubated with a FITC-labeled third mouse mAb. With some FITC-labeled antibodies, a signal amplification step was included using additional incubation in normal rabbit serum and Alexa Fluor 488-conjugated rabbit anti-FITC (Molecular Probes Europe, Leiden, The Netherlands). For each fluorochrome label, negative control antibodies were included.
4.5 CLSM analysis
Confocal fluorescence images were obtained on a Leica TCS SP (Leica Microsystems, Heidelberg, Germany) confocal system, equipped with an Ar/Kr/HeNe laser combination. Images were taken using a×40 1.25 NA objective. Possible cross-talk between FITC, Cy3, and Cy5, which could give rise to false-positive co-localization of different signals, was avoided by careful selection of the imaging conditions.
4.6 Generation, culture and analysis of human DC
All cultures were performed in Iscove's modified Dulbecco's medium with 1% FCS (HyClone, Lagan, UT). Peripheral blood of healthy volunteers was used to generate immature DC. Monocytes were cultured in the presence of recombinant human (rhu)GM-CSF (500 U/ml; a gift from Schering-Plough, Uden, The Netherlands) and rhuIL-4 (250 U/ml; Pharma Biotechnologie). Final maturation was achieved at day 6 by adding rhuIL-1β (5 ng/ml; Boehringer Mannheim, Germany), rhuTNF-α (25 ng/ml; PBH, Hannover, Germany) and LPS (100 ng/ml; Sigma). On day 6 (before addition of the cytokinesand LPS) and 12, 24 and 72 h later, DC were harvested, washed twice, stained with DC-SIGN–Alexa 488 and CD83–PE, and subsequently analyzed with the FACS Calibur and CellQuest Pro software (Becton Dickinson, San Jose, CA).
4.7 Statistical analysis
Differences between the groups were analyzed with the Mann–Whitney test using SPSS 10.1 for Windows.
Acknowledgements
We would like to thank Dr. E. C. de Jong and Dr. M. L. Kapsenberg for their advice, and H. Sibum for his photographic assistance.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




