Association of T cell antigen CD7 with type II phosphatidylinositol-4 kinase, a key component in pathways of inositol phosphate turnover
Abstract
CD7 is a 40-kDa glycoprotein that is expressed on prothymocytes and persists during T cell differentiation. CD7 has been demonstrated to generate, like other costimulatory molecules, intracellular signals that modulate T cell function. However, although it binds to phosphatidylinositol 3-kinase (PI 3-kinase), the signaling events mediated by CD7 are not completely understood. In this context, phosphatidylinositol 4-kinase (PI 4-kinase) is a key enzyme involved in a variety of events, from the modeling of the actin cytoskeleton to the activation of protein kinase C. In this study, we show for the first time that PI 4-kinase of 55 kDa can associate with CD7. The enzyme activity was insensitive to wortmannin, but was inhibited by adenosine, a characteristic for type II PI 4-kinase. Together, our findings demonstrate that type II PI 4-kinases are integral components of the CD7 signaling pathway and may play a role of CD7 in co-stimulation and thymic differentiation.
Abbreviations:
-
- PI 3(4)-kinase:
-
Phosphatidylinositol 3(4)-kinase
-
- PI-4,5-P2:
-
Phosphatidylinositol 4,5-diphosphate
-
- IP3:
-
Inositol-1,4,5-triphosphate
1 Introduction
CD7, a 40-kDa glycoprotein, is one of the earliest T cell-specific surface proteins to appear in T cell ontogeny 1, 2 and persists in expression in mature Tcells 3. Similar to CD28 4, CD7 has been demonstrated to act as a costimulatory molecule 5. Anti-CD7 mAb have been reported to be mitogenic 6, increase calcium fluxes 7 and augment IL-2 production 8. In addition, Chan et al. 9 have shown that CD7 ligation modulates integrin-mediated adhesion. In vitro cross-linking with CD7 mAb leads to association with CD3 and CD45 10. Like CD28, CD7 binds to the phosphatidylinositol 3-kinase (PI 3-kinase) by means of a cytoplasmic tyrosine based YEDM motif 11. The exact signaling mechanisms mediated by CD7-PI 3-kinase interaction are still unknown.
One of the major pathways of signaling in mammalian cells involves the turnover of phosphatidylinositol (PI) and the generation of diacylglycerol (DAG) for protein kinase C (PKC) activation and inositol-1,4,5-triphospate (IP3) for intracellular Ca2+ mobilization 12. PI 4-kinases convert PI into PI-4-phosphate (PI-4-P), a highly relevant intermediate in multiple phosphatidylinositide signaling pathways 13. The enzyme activities have been classified into type II and type III, based on their sensitivity to adenosine and wortmannin 14–16. Type II PI 4-kinases are implicated in early signaling cascades during T cell activation 17–19. In addition to their putative role in mitogenic signal transduction, PI 4-kinases are also implicated in integrin-mediated signaling mechanisms, cytoskeletal reorganization and secretion 20–25. PI-4-P and PI-4,5-P2 themselves can interact with actin-binding proteins to regulate actin polymerization 26–28.
Recently, CD7 has been shown to have a role in regulating integrin-mediated adhesion. Ligation of CD7 on CD4+ human T cells with mAb increased binding to fibronectin, ICAM-1, and VCAM-1 29. Chan et al. 30 reported a CD7-mediated induction of integrin adhesion that can be abolished by pretreatment with the tyrosine kinase inhibitor herbimycinA. The same phenomenon was observed when cells were pretreated with PI 3-kinase inhibitors wortmannin and LY294002, suggesting a role of PI 3-kinase in CD7-induced integrin adhesion 9.
In this study, we demonstrate for the first time that CD7 associates with type II PI 4-kinase. The enzyme was insensitive to wortmannin, but sensitive to adenosine, a property of a type II PI 4-kinase. CD7 binding to PI 4-kinase provides a new connection between this receptor and signaling events in T cells.
2 Results and discussion
2.1 CD7 cytoplasmic tail interacts with PI 3-kinase and PI 4-kinase
The cytoplasmic tail of CD7 contains a consensus sequence motif (YEDM), which has been reported in vitro to bind to the SH2 domains of PI 3-kinase 31. As shown in Fig. 1A (upper panel) and Fig. 1B (lower panel) and by others 11, 31, CD7 ligation could induce a change in receptor-associated PI 3-kinase activity. Anti-CD7 ligation resulted in a marked increase in the level of PI 3-kinase activity precipitated by anti-CD7 mAb. By comparison with unligated cells (lane 2), CD7 cross-linking resulted in a notable increase in activity as early as 1 min, peaking between 5 and 10 min and began to undergo a reduction by 15 min. An anti-p85 precipitate served as a positive control (lane 7), and a rabbit anti-mouse as a negative control (lane 1). These findings demonstrate that CD7 ligation results in a time-dependent recruitment of PI 3-kinase. In addition, anti-CD7 cross-linking showed a notable recruitment of the p85 subunit to the receptor (Fig. 1A, lower panel, lanes 2–6). Maximal binding was observed by 10 min, with a slight decrease starting at 15 min (lane 6).
Jurkat cells, stimulated with anti-CD7 mAb for various periods of time, were lysed, immunoprecipitated with anti-CD7 mAb and assayed for lipid kinase activity in the presence of 1% NP-40 (Fig. 1B). CD7 precipitates showed a constitutive level of lipid kinase activity that was only marginally affected by CD7 cross-linking (lanes 2–6). Importantly, the activity was still present in the presence of 1% NP-40 (lanes 2–6). PI 3-kinase is inactivated by nonionic detergent, whereas PI 4-kinase activity is not altered 32. As a control, PI 3-kinase activity was significantly reduced in the presence of 1% NP-40 when compared to kinase activity without detergent (Fig. 1B, lane 7 vs. 8). The remaining PI 3-kinase activity is most likely due to the incomplete inhibitory effect of the detergent (1:150 of original signal). Immunoprecipitation with rabbit anti-mouse served as a negative control (lane 1). Overall, these experiments strongly suggested that PI 4-kinase might associate constitutively with CD7.
To identify the nature of the precipitated lipid kinase shown in Fig. 1B, the corresponding PI-P spots from the TLC plate were extracted, deacylated, and subjected to HPLC. As shown in Fig. 2, PI-P generated in anti-CD7 precipitates consisted primarily of PI-4-P (upper panel). The position of PI-3-P was defined by analysis of anti-p85 precipitates (lower panel). These findings show conclusively that a form of PI 4-kinase associates with CD7.
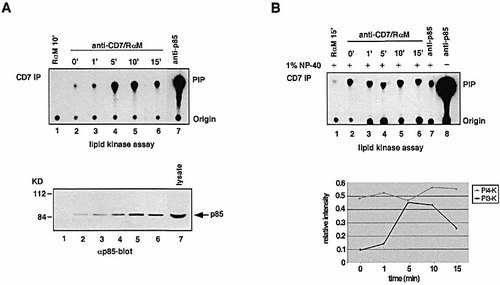
CD7 associates with the lipid kinases PI 4-kinase and PI 3-kinase. (A) CD7 cross-linking induces a time-dependent increase in the recruitment of PI 3-kinase. Upper panel: Jurkat cells were stimulated with anti-CD7 and rabbit anti-mouse Ab for the indicated periods of time (lanes 2–6). Anti-CD7 immunoprecipitations (lanes 2–6) were labeled in a lipid kinase assay. Lower panel: Jurkat cells were stimulated as described above. Cells were lysed, immunoprecipitated with anti-CD7 mAb (lanes 2–6) and immunoblotted with anti-p85 antiserum. (A, B upper, and A lower panels) Rabbit anti-mouse served as a negative control (lane 1), anti-p85 immunoprecipitate/immunoblotting against cell lysates as a positive control (lane 7; A, B, upper, and A, lower panels). (B) CD7 associates constitutively with PI 4-kinase. Upper panel: Jurkat cells were stimulated as described above. Cells were lysed and immunoprecipitated with anti-CD7 mAb (lanes 2–6). Anti-CD7 immunoprecipitations (lanes 2–6) were labeled in a lipid kinase assay in the presence of 1% NP-40. Lower panel: Histogram depiction of the levels of PI 4-kinase and PI 3-kinase activity in CD7 precipitates as detected by densitometric reading.
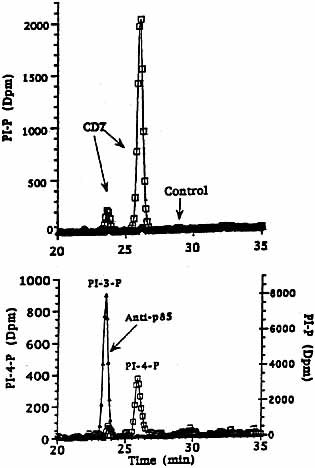
HPLC analysis of lipid products. PI-P spots were extracted from TLC plates, deacylated and subjected to HPLC. PI-P generated in anti-CD7 precipitates consisted primarily of PI-4-P (white quadrangle, upper panel). The position of PI-3-P was defined by analysis of anti-p85 precipitates (black triangle, lower panel). The position of PI-4-P is shown by a PI-4-P standard (white quadrangle, lower panel).
2.2 CD7 associated PI 4-kinase has properties of a type II kinase
To further characterize CD7-associated PI 4-kinase, anti-CD7 immunoprecipitates were separated by 12% SDS-PAGE. The lane containing the sample was cut into 5-mm slices from the top of the gel. After renaturation, each gel slice was assayed for PI 4-kinase activity as described in Sect. 4 (Fig. 3, lanes 2–17). PI 4-kinase activity in cell lysates served as a positive control (lane1). CD7 associated PI 4-kinase activity was only detected in sample 6 (lane 7), which co-migrated within 55 kDa.
Type II PI 4-kinases have been documented to be activated by nonionic detergent and inhibited by adenosine 14–16, while type III PI 4-kinases and PI 3-kinase are inhibited by wortmannin 33, 34. To identify the type of PI 4-kinase associated with CD7, Jurkat cells were immunoprecipitated with anti-CD7 mAb and a lipid kinase assay was carried out in the presence of adenosine. As shown in Fig. 4A, adenosine concentrations up to 60 μM (lanes 1–3) had no inhibitory effect on the enzyme activity when compared without adenosine (lane 6). Adenosine at a concentration of 120 and 300 μM almost completely inhibited PI 4-kinase activity (lanes 4, 5). The slight variation for the IC50 for adenosine in our system is most likely due to the measurement of kinase activity on immune complexes when compared to pure enzyme in a free state (30–50 μM). In contrast to adenosine, wortmannin had no effect on the activity of PI 4-kinase up to 300 nM (Fig. 4B, lanes 1–4). As a control, CD7-associated PI3-kinase showed a significant reduction in activity in the presence of wortmannin when compared to non-treated cells (Fig. 4C, lanes 1–4 vs. lane 5).
In summary, our findings showing for the first time that CD7 can associate with a specific type of PI 4-kinase extends our understanding of the mechanism by which this co-receptor may modulate T cell growth. At a fundamental level, CD7-PI 4-kinase complexes should up-regulate PI-4,5-P2, a key intermediate needed for the production of DAG and IP3. DAG acts to activate PKC, while IP3 regulates the release of Ca2+ from intracellular compartments 12. Alternatively, PI-4,5-P2 can serve as a substrate for PI 3-kinase in the generation of D-3 lipids, which in turn acts to anchor PH domain carrying proteins such as VAV, phosphatidyl 3,4,5-triphosphate-dependent protein kinase-1 (PDK-1 ) and protein kinase B (AKT/PKB) to the plasma membrane of T cells. Therefore, by recruiting PI 4-kinase, CD7 would be expected to have a pleiotropic effect on the signaling environment of T cells. In this context, binding of CD7 to PI 3-kinase would act as a perfect cooperative partner for PI 4-kinase. CD7-associated PI 3-kinase could act upon PI-4,5-P2 produced by CD7-associated PI 4-kinase. The constitutive association of PI 4-kinase with CD7 would make sense in a scenario in which PI 4-kinase induces PI-4,5-P2 prior to the recruitment and activation of PI 3-kinase due to receptor ligation and induced phosphorylation. Given the fact that CD7-associated PI 4-kinase shows a non-inducible, constitutive level of activity, its involvement in signaling may be related to the re-distribution of CD7-PI 4-kinase complexes following ligation. Redistributed complexes may target PI 4-kinase to different sites in the plasma membrane. This would obviate the need for a change in the activity of this lipid kinase. On the other hand, CD7-associated PI 4-kinase activity could also play a role in intracellular compartments of cells. Similar to CD7, PI 4-kinase has previously been shown to associate with a variety of receptors, such as the epidermal growth factor receptor 35, 36, CD4 and CD8 17, 37, 38. In this context, transgenic mice expressing CD8α defective in p56lck binding can nevertheless rescue thymic selection, suggesting a possible role for PI 4-kinase 39. This observation may be of particular relevance to the role of CD7 in thymic differentiation, where increased numbers of thymocytes appear in CD7-deficient mice 40. Coincidentally, PI 4-kinase has been reported to play a central role in thymocyte differentiation with defects in negative selection 41. Our new observation showing that CD7 binds to PI 4-kinase may provide an important link in connecting these two functions. The functional importance of various homologous forms of PI 4-kinase in yeast (PIK1, STT4) 42 has also been illustrated, where null mutants are lethal, and conditional mutants have defects in cell cycle and division 43. Clearly, further studies will be needed to reveal CD7-PI 4-kinase-mediated signaling mechanisms.
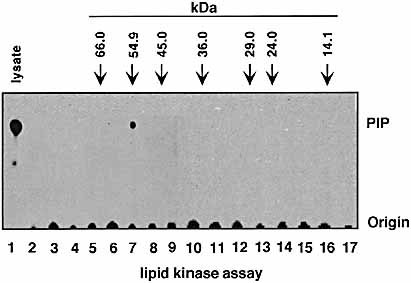
CD7 associates with PI 4-kinase of 55 kDa. Anti-CD7 immunoprecipitates were separated by 12% SDS-PAGE. The lane containing the sample was cut into 5-mm slices from the top of the gel. Each gel slice was assayed for PI 4-kinase activity (lanes 2–17). PI 4-kinase activity in cell lysates served as a positive control (lane 1). CD7-associated PI 4-kinase activity was only detected in sample 6 (lane 7) which co-migrated within 55 kDa. The position of molecular weight markers are shown with arrows.
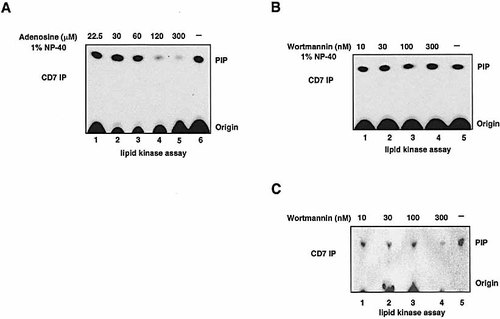
CD7 associates with PI 4-kinase type II. (A) Dose-dependent inhibition of PI 4-kinase by adenosine. Jurkat cells were lysed, immunoprecipitated with anti-CD7 mAb and a lipid kinase assay for PI 4-kinase activity was carried out in the presence of increasing concentrations of adenosine (lanes 1–5). PI 4-kinase activity immunoprecipitated by anti-CD7 mAb without adenosine served as a positive control (lane 6). (B) Wortmannin insensitivity of PI 4-kinase associated with CD7. Jurkat cells were lysed, immunoprecipitated with anti-CD7 mAb and a lipid kinase assay for PI 4-kinase activity was carried out in the presence of increasing concentrations of wortmannin (lanes 1–4). PI 4-kinase activity immunoprecipitated by anti-CD7 mAb without wortmannin served as a positive control (lane 5). (C) Wortmannin sensitivity of PI 3-kinase associated with CD7. Jurkat cells were lysed, immunoprecipitated with anti-CD7 mAb and a lipid kinase assay for PI 3-kinase activity was carried out in the presence of increasing concentrations of wortmannin (lanes 1–4). PI 3-kinase activity immunoprecipitated by anti-CD7 mAb without wortmannin served as a positive control (lane 5).
3 Concluding remarks
Our data show for the first time that CD7 associates with a specific isoform of the lipid kinase PI 4-kinase. The molecular mass of 55 kDa, inhibition of the enzyme activity by adenosine and resistance to wortmannin revealed it as a type II PI 4-kinase. Given the central roles of the canonical inositol phosphate pathway in T cells, binding of PI 4-kinase to CD7 provides a mechanism to account for the role of both co-receptor and kinase in thymic differentiation.
4 Materials and methods
4.1 Cells, reagents and antibodies
The human transformed Jurkat T cell line was cultured in RPMI 1640 medium, supplemented with 5% (v/v) fetal bovine serum (FBS; Intergen, Purchase, NY), 100 U/ml penicillin, 100 mg/ml streptomycin (Life Technologies, Grand Island, NY), 2 mM L-glutamine (Life Technologies) and 10 mM Hepes (Whittaker, Walkersville MD). CD7 mAb (3A1) was kindly provided by Dr. Barton Haynes (Duke University, Durham, North Carolina) and anti-p85 of PI 3-kinase by Dr. M. White (Joslin Diabetes Center, Boston, MA).
4.2 Immunoprecipitation and immunoblotting
For immunoprecipitations, cells were lysed in ice-cold lysis buffer containing 1% Triton X-100 in 20 mM Tris-HCl pH 8.3, 150 mM NaCl. The lysis buffer contained protease and phosphatase inhibitors. Postnuclear lysates were incubated for 1 h at 4°C with the indicated antibodies. Protein A-Sepharose beads (30 μl, Pharmacia), were added and incubated for 1 h at 4°C. The beads were washed three times in cold lysis buffer and proteins were eluted by boiling for 5 min in SDS sample buffer, separated by SDS-PAGE and transferred to nitrocellulose for immunoblotting. The membranes were blocked with 5% milk in TBS (10 mM Tris-HCl pH 7.6, 150 mM NaCl) and incubated with the indicated antibody. Bound antibody was revealed with the appropriate secondary antibody, and protein was visualized by enhanced chemiluminescence (ECL, Amersham).
4.3 Lipid kinase assay
4.3.1 PI 3-kinase assay
Immunoprecipitates prepared as described above were washed three times with the lysis buffer, three times with 100 mM Tris pH 7.5, 0.5 M LiCl, and twice with TNE (10 mM Tris pH 7.5, 150 mM NaCl, 1 mM EGTA). The lipid kinase reaction was carried out on the beads using soybean PI liposomes and 10 μCi [γ-32P]ATP. Lipids were then separated by thin-layer chromatography (TLC)as described 44.
4.3.2 PI 4-kinase assay
The PI 4-kinase activity was assayed as described earlier 14, 15, 18 with slight modifications. Briefly, the reaction was carried out in final volume of 0.05 ml containing 50 mM Tris pH 7.6, 10 mM MgCl2, 0.25 mM EGTA, 0.1 mM vanadate, 20 μg/ml PI, 100 μM [γ-32P]ATP (200–300 cpm/pmol) and 1% NP-40. The non-ionic detergent in the reaction mixture is inhibitory to PI 3-kinase, and only PI 4-kinase activity was assayed under these experimental conditions. The reaction was initiated by addition of labeledATP and incubated at room temperature (∼25°C) for 6 min. The reaction was terminated by adding 50 μl of 12 N HCl. The labeled phospholipids were extracted, separated by TLC and visualized by autoradiography.
4.4 Renaturation assay
Anti-CD7 immunoprecipitates were separated by 12% SDS-PAGE. For renaturation, the gel was washed three times in 10 mM Tris pH 7.4. The lanes containing the sample were then cut into 5-mm slices, from the top of the gel. Each gel slice was assayed for PI 4-kinase activity as described above, except for incubation period and volume. The reaction volume was increased to 100 μl, and the incubation was continued overnight.
Acknowledgements
C. E. Rudd is a principal research fellow of the Wellcome Trust, London, GB.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




