β-Catenin expression in thymocytes accelerates thymic involution
Abstract
Age-related thymic involution in mammals is accompanied by decreased generation of naïve T cells without significant reduction in the number of peripheral T cells. This leads to inefficient immune responses and inadequate combating of infections and other challenges to the immune system in older mammals. The molecular mechanisms that underlie this phenomenon are not known. In this report we show that expression of β-catenin in thymocytes enhances thymic involution. The effect of β-catenin expression is seen in all the thymic sub-populations, suggesting that an age-related developmental process is accelerated. We also show that, as in normal mice, thymic involution does not lead to a drastic reduction in splenic T cells in β-catenin-transgenic mice. This study identifies β-catenin expression in thymocytes as a molecular target of age-related thymic involution.
Abbreviations:
-
- DN:
-
Double-negative
-
- DP:
-
Double-positive
-
- SP:
-
Single-positive
-
- TCF:
-
T cell factor
1 Introduction
Immuno-senescence is the age-related decline of the immune system that results in inadequate responses to transformed cells and infectious agents 1–3. The decrease in naïve T cell populations is due to a decline in thymic function that has been attributed to thymocytes as well as the thymic environment. Convincing evidence shows that both T cell precursors and the environment must be derived from young mice to obtain a ‘young-immune system’ phenotype in bone marrow chimera experiments 4–7. Indeed, recent studies suggest that essentially all aspects of the immune system change with age. These changes manifest as reduced functionality and altered cellular phenotypes. It has been documented that a decrease in the generation of naïve T cells, but no reduction in peripheral T cell numbers, accompanies age-related immunodeficiency. T cells in the peripheral lymphoid compartments show a shift from naïve CD44lo cells to cells expressing high levels of CD44 8. Thus, an increase in the number of memory-type T cells, probably a homeostatic response to the decrease in naïve T cells, maintains T cell numbers in the peripheral lymphoid organs of aged mice.
Other cells of the immune system also show changes that are relevant to immune function (reviewed in 1), suggesting that aging of the immune system is continuous with development and maturation and is intrinsically complex.
T cells develop in the thymus through a series of stages defined by the expression of the cell surface markers CD4 and CD8. Most of the immature thymocytes express neither CD4 or CD8 and are referred to as double-negative (DN); these cells develop into cells expressing CD4 and CD8, which are called double-positive (DP) thymocytes. These DP thymocytes express the TCR and are subjected to selection such that only self-non-reactive but self-restricted CD4 or CD8 single-positive (SP) thymocytes develop and emerge as mature T cells into peripheral lymphoid organs.
In mice, thymus development starts around day 11–12 of gestation and the thymus acquires all the thymocyte populations described above by birth 9. Recent consensus suggests that normal maturation of the thymus extends to age-related thymic involution as the animal matures. There is even a suggestion that the involution process is an essential part of maturation and maybe beneficial to the organism 1. Regardless, the observation that older individuals suffer because of inefficient responses to neoplastic changes as well as to infectious agents poses a challenge to the immunologists who wish to provide remedies to combat the situation efficiently.
However, our understanding of the involution phenomenon at a molecular level is not yet at a stage to permit such remedies to be effectively developed. For example, circulating levels of growth hormone and insulin growth factor 1 are reduced with age. Treatment with these hormones increased thymic cellularity but did not rejuvenate thymus function or enhance T cell development 10. This corroborated the notion that thymic involution is a complex process with additional components. A molecular understanding of the mechanism of the thymic involution process may afford the means to restore naïve T cell populations and partially relieve age-related immunodeficiency.
Recently, signal transduction by the β-catenin pathway was shown to be important in lymphoid cells (11–13; Mulroy et al., manuscript submitted). β-Catenin protein levels in the cell are regulated by GSK-3β-mediated phosphorylation and ubiquitin-mediated degradation. For example, in the presence of Wnt (a growth factor) the Frizzled (receptor)-mediated signal blocks the β-catenin-phosphorylating enzyme GSK-3β and stabilizes β-catenin. Stabilized β-catenin participates in transcription of target genes in conjunction with T cell factor (TCF)-family transcription factors. TCF-1 was found to be essential for the development of the immune system 14, 15. Recently, β-catenin-binding sites on TCF-1 were found to be essential for the function of TCF-1 in DP thymocyte survival 11. In a separate study, transgenic expression of Axin was found kill immature DP thymocytes 12. In most current models of the Wnt-Frizzled-β-catenin signaling pathway, increased expression of Axin is expected to lower the level of β-catenin expression. Therefore, these data further implicated β-catenin signaling in thymocyte survival.
Expression of stabilized β-catenin would then be expected to enhance survival of thymocytes, leading to hyperplasia and tumors. Instead, expression of very high levels of stabilized β-catenin was found to block thymocyte maturation at early stages and induced moderate levels of apoptosis in thymocytes at all stages of development 13. We have previously shown that expression of moderate levels of a stabilized β-catenin transgene (ΔCat-Tg) in thymocytes did not interfere with early stages of development but enhanced the generation of SP thymocytes, most specifically CD8 SP thymocytes. It did not provide a survival advantage to DP thymocytes when cultured in vitro with or without dexamethasone (which induces apoptosis in DP thymocytes). We also showed that TCR and CD28 signals induce nuclear β-catenin in thymocytes, suggesting enhanced positive selection of thymocytes (Mulroy et al., manuscript submitted).
In this paper, we provide evidence that expression of β-catenin enhances thymic involution. ΔCat-Tg mice were born with normal numbers of thymocytes but the involution process began at an earlier age and proceeded at a faster pace than in control mice. The most remarkable observation was that the thymus of 4–8-month-old ΔCat-Tg mice looked similar to the thymus of 12–18-month-old control mice.
2 Results and discussion
2.1 Expression of stabilized β-catenin in thymocytes accelerates thymic involution
To study the Wnt-β-catenin-TCF signaling pathway in T cell development, we expressed stabilized β-catenin in thymocytes using the proximal Lck promoter cassette. Stabilized β-catenin was expressed in DN, DP and SP thymocytes (data not shown). To study the effect of β-catenin expression on thymic aging, the number of thymocytes was assessed in mice at various ages. Control (non-transgenic littermates) and ΔCat-Tg mice showed comparable numbers of thymocytes at birth, suggesting that similar number of thymocytes were generated in young mice. As the mice aged, we noticed that thymocyte numbers decreased dramatically in ΔCat-Tg mice compared with age-matched littermates (Fig. 1). Thymic involution was evident in ΔCat-Tg mice by 3 months of age, unlike in littermate control mice and was accelerated as the mice aged. The striking conclusion is that 4–8-month-old ΔCat-Tg mice resemble 12–18-month-old control mice (Fig. 1).
Data from large numbers of ΔCat-Tg and littermate control mice at various ages are summarized in Fig. 2. These data suggest that thymic involution is augmented earlier in adulthood in ΔCat-Tg mice and the process continues to be accelerated as the mice age (Fig. 2a).
The phenotype of thymocytes in ΔCat-Tg mice remains comparable as they age (Fig. 2b). In 1–3-month-old ΔCat-Tg mice (right top panel) the proportion of DP thymocytes was decreased compared with littermate controls (left top panel), the proportion of CD4 SP thymocytes was slightly increased compared with controls and the percentage of CD8 SP thymocytes was significantly increased in ΔCat-Tg mice compared with controls. As the mice aged this phenotype remained consistent (Fig. 2b, middle and bottom panels). Therefore, no significant difference in phenotype of thymocytes could be attributed to age in ΔCat-Tg mice.
These data indicate that an agent that stabilizes β-catenin in thymocytes may be important in thymic involution under normal circumstances. The effect of this agent is augmented in ΔCat-Tg mice, leading to faster thymic involution. There is evidence of a hormonal basis for thymic involution 16, 17 and, since β-catenin has been shown to participate in signaling from hormone receptors, expression of stabilized β-catenin may mimic hormonal signaling in thymocytes. Alternately, we have recently shown that TCR and CD28 signals stabilize and target β-catenin to the nucleus; therefore, another possibility is that enhanced thymic involution in ΔCat-Tg mice mimics increased chronic signaling by the TCR and CD28 in thymocytes. Finally, the canonical Wnt-β-catenin-TCF signaling pathway may play a role in thymic function and involution in ways not yet defined.
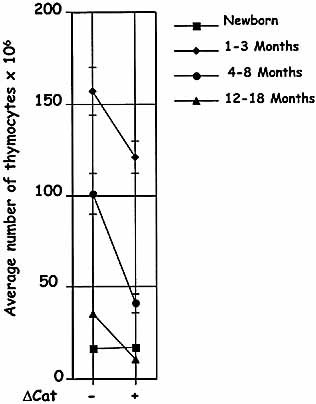
Thymic involution is accelerated in ΔCat-Tg mice. Total numbers of thymocytes in ΔCat-Tg and age-matched littermate control mice in various age groups are presented (these are linked by lines across the page to compare littermate ΔCat-Tg – controls and + mice).
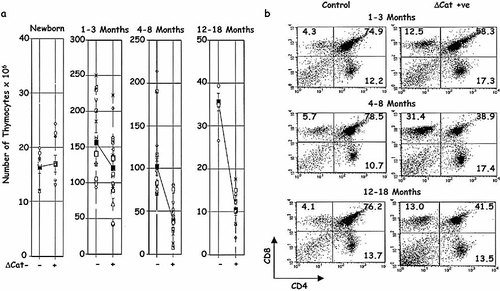
The rate of decline in the number of thymocytes is enhanced with age. (a) A large number of mice in each age group was compared for numbers of thymocytes. In each age group, data for each mouse and average values (these are linked by lines across the page to compare littermate ΔCat-Tg – controls and + mice) are shown. Note the different scales used on for thymocyte numbers. (b) The phenotype of thymocyte subsets is not affected by age; thymocytes from age-matched littermate control (left panels) and ΔCat-Tg+ mice (right panels) were stained with anti-CD4 and anti-CD8. Percentages of thymocytes in each subset are shown in the quadrants. The FACS profiles are representative of more than five experiments.
2.2 Expression of stabilized β-catenin in thymocytes does not lead to a significant change in splenic T and B cells
Age-dependent thymic involution is not accompanied by a drastic reduction in the number of peripheral T cells. Splenic T cells in aged animals express high levels of CD44, indicating a memory phenotype 7. However, we did not address this issue because ΔCat-Tg thymocytes and T cells express intermediate levels of CD44 in the absence of any other memory markers (Mulroy et al., manuscript submitted). It appears that β-catenin expression up-regulates CD44 expression directly.
We characterized splenic T and B cells in young and aged ΔCat-Tg mice (Fig. 3). The number of splenocytes was comparable in age-matched ΔCat-Tg and control mice (Fig. 3a). This showed that, unlike the observations in other tissues such as the breast and colon, expression of stabilized β-catenin did not enhance cellular proliferation in lymphoid tissue. The percentage of T cells was not significantly different in ΔCat-Tg and control mice; neither was the percentages of B cells significantly different in ΔCat-Tg and control mice (Fig. 3b). The number of splenic T cells was also found to be similar; so was the number of B cells (Fig. 3c). Finally, the percentage (Fig. 3d) and number (Fig. 3d and e) of CD4 cells were also comparable in age-matched ΔCat-Tg and control mice; similarly, the percentage and number of CD8 T cells were comparable in control and ΔCat-Tg mice (Fig. 3e). Therefore, aging of the immune system in ΔCat-Tg mice was accelerated but the phenotype of peripheral T cells remained normal. Thus the ΔCat-Tg mouse model system retains an essential feature of normal aging of the immune system.
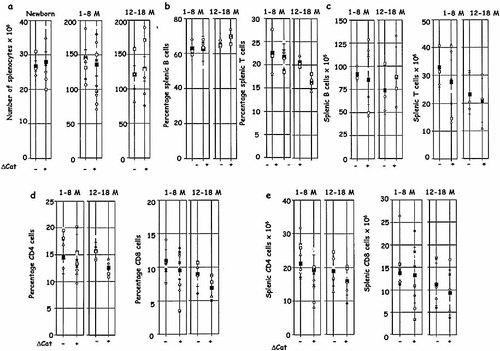
The number of splenocytes is not affected by expression of the β-catenin transgene. (a) Red blood cell-depleted splenocytes from several ΔCat-Tg+ and age-matched littermate control (–) mice were counted and are represented by age groups (M, months; averages are shown by black squares). (b) Percentages of B cells (left panels) and T cells (right panels) in the spleen of control and ΔCat-Tg mice are shown. (c) The numbers of B cells (left panels) and T cells (right panels) in the spleen of control and ΔCat-Tg mice are shown. (d) The percentages of CD4 T cells (left panels) and CD8 T cells (right panels) in the spleen of control and ΔCat-Tg mice are shown. (e) The numbers of CD4 T cells (left panels) and CD8 T cells (right panels) in the spleen of control and ΔCat-Tg mice are shown.
2.3 The mechanism of thymic involution due to expression of stabilized β-catenin
One hypothesis is that age-dependent changes in both the thymic microenvironment and thymocytes affect thymic involution. This suggests that age-related changes in both compartments are responsible for thymic involution. Stabilized β-catenin was expressed only in thymocytes in ΔCat-Tg mice; therefore, the primary effect of this transgene has to be on thymocytes.
We further examined thymocyte sub-populations in young and aged control and ΔCat-Tg mice (Fig. 4). In 1–3-month-old ΔCat-Tg and control mice the percentage of DN populations was comparable but by 4–8 months the proportion of DN thymocytes was higher in the ΔCat-Tg mice (Fig. 4a). The absolute number of DN cells at all ages was lower in ΔCat-Tg mice compared with control littermates. This reflects the decrease in total number of thymocytes starting at 3 months of age. The increase in proportion of DN thymocytes is reflective of the effective decrease in the proportion of DP thymocytes described below.
The proportion of DP population was significantly decreased in ΔCat-Tg mice, starting at 1–3 months of age and it continued to decline sharply throughout the lifetime of ΔCat-Tg mice (Fig. 4b, left panels). The absolute number of DP thymocytes was also significantly reduced in ΔCat-Tg mice compared with control littermates (Fig. 4b, right panels). However, we have ruled out the possibility that the decrease in DP thymocytes was due to enhanced apoptosis of ΔCat-Tg thymocytes. We have previously shown that expression of ΔCat-Tg did not enhance apoptosis in DP thymocytes. It also did not protect DP thymocytes from spontaneous death in in vitro culture or from dexamethasone-mediated death (Mulroy et al., manuscript submitted). Indeed, in light of the observation that β-catenin was important for DP thymocyte survival 11, 12 it was unexpected that expression of stabilized β-catenin did not provide extra protection from cell death in vitro. One explanation is that, although β-catenin may be required for the survival of DP thymocytes, more is not better.
The proportion of CD4 SP thymocytes was comparable in ΔCat-Tg and control mice at 1–3 months of age but increased thereafter (Fig. 4c). The percentage of CD8 SP thymocytes was high in mice at a young age in ΔCat-Tg mice, as has been described previously, and remained high at all ages (Fig. 4c, right panels). The absolute numbers of CD4 and CD8 SP thymocytes declined with age, commensurate with the decrease in the number of thymocytes in aged mice (Fig. 4d).
These data point to the DP population as being the target of β-catenin mediated involution signals. We have previously shown that the DP thymocytes in ΔCat-Tg mice were not more susceptible to apoptosis than control thymocytes when assayed freshly ex vivo, cultured in vitro or treated with dexamethasone (see above). Thus the decrease in the number of DP thymocytes does not reflect a direct effect of stabilized β-catenin on the viability of DP thymocytes. One interpretation is that the effect of an unknown agent that drives thymic involution in normal mice is enhanced in ΔCat-Tg mice leading to age-related decline in this population by an unknown mechanism.
In conclusion, ΔCat-Tg transgenic mice expressing stabilized β-catenin in thymocytes undergo accelerated thymic involution. Thymocyte maturation, survival and proliferation are controlled by several factors, many of which may be produced by the thymic microenvironment in response to interactions between thymocytes and thymic stromal cells. This paper is one of the first to describe that a protein expressed exclusively in the thymocyte can modulate cellular fate in aging. It shows that thymic involution can be uncoupled from the bone marrow derived precursor input, which would be normal in ΔCat-Tg mice. In normal mice, agents driving β-catenin-mediated involution of the thymus could be TCR and CD28 signaling, hormonal effects or Wnt proteins produced by the thymocytes or by thymic stromal cells. In ΔCat-Tg mice, β-catenin expression in thymocytes could lead to a change in the dynamics of the interaction between thymocytes and stromal cells and/or to enhanced production of factors that accelerate thymic involution.
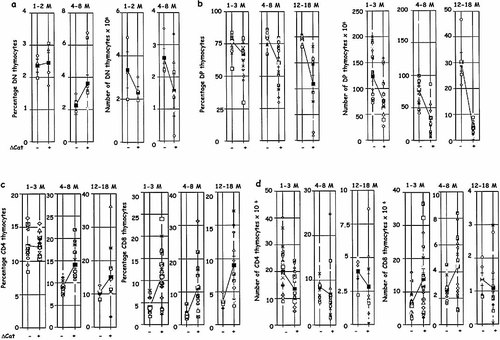
Thymocytes show accelerated decline with age in ΔCat-Tg mice. (a) The percentages (left panels) and numbers (right panels) of DN thymocytes are represented, in different age-groups (M, months). Average values are also shown (these are linked by lines across the page to compare ΔCat-Tg+ mice and littermate ΔCat-Tg– controls). (b) The percentages (left panels) and numbers (right panels) of DP thymocytes are shown, in different age groups. (c) The percentages of CD4 (left panels) and CD8 (right panels) thymocytes are shown, in different age-groups. (d) The numbers of CD4 (left panels) and CD8 (right panels) thymocytes are shown, in different age-groups.
3 Materials and methods
3.1 Generation and analysis of transgenic mice
The ΔCat construct was generated by cloning the BamH I fragment containing the mutant ΔN87βCat gene 18 into the BamH I site in p1017 19. Not-Icut DNA containing the ΔN87βCat gene was injected into FVB recipient mice. Transgenic mice were identified by Southern-blot analysis of DNA extracted from tail cuts using standard protocols. The probe consisted of the BamH I fragment containing the ΔN87βCat gene. Genotyping of transgenic mice will been described elsewhere (Mulroy et al., manuscript submitted).
3.2 FACS analysis
For three-color analyses, thymocytes were immunostained with FITC-, Cy-chrome- or PE-labeled anti-CD4, anti-CD8, anti-TCR, anti-CD5, anti-CD69, anti-HSA, anti-CD62L or anti-Qa-2 antibodies in PBS containing 2% FCS and 0.2% sodium azide in the presence of anti-CD16/CD32 antibodies. All antibodies were purchased from PharMingen (San Diego, CA). Immunostained cells were analyzed using a FACScan flow cytometer and CellQuest software (Becton Dickinson, Mountain View, CA).
Acknowledgements
This work was supported by grants from The Arthritis Foundation, The Barr Foundation and National Institutes of Health and National Cancer Institute awardedto J. S.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




