Retroperitoneal Doege–Potter syndrome with intraoperative blood glucose monitoring
Abstract
Background
Doege–Potter syndrome, characterized by solitary fibrous tumors and non-islet cell tumor hypoglycemia, is rare. Here, we report a case of Doege–Potter syndrome in which retroperitoneal tumor resection was performed with continuous intraoperative blood glucose monitoring.
Case presentation
A 37-year-old man presented with hypoglycemia-related symptoms, and a 10 × 12 × 9 cm tumor was found in his right kidney. Following tumor resection, insulin secretory abnormalities improved, and intraoperative blood glucose monitoring showed no hypoglycemic events. High levels of insulin-like growth factor-II confirmed the diagnosis of an insulin-like growth factor-II-producing tumor with non-islet cell tumor hypoglycemia. Postoperative serum insulin-like growth factor-II levels normalized, with no recurrence observed over 3 years.
Conclusions
This case highlights the rarity of primary retroperitoneal Doege–Potter syndrome, emphasizes the safety of intraoperative blood glucose levels during surgery, and suggests rapid recovery of insulin secretion postoperatively.
Abbreviations & Acronyms
-
- BG
-
- blood glucose
-
- DPS
-
- Doege–Potter syndrome
-
- IGFBP
-
- insulin-like growth factor binding protein
-
- IGF-II
-
- insulin-like growth factor-II
-
- isCGM
-
- intermittently scanned continuous glucose monitoring
-
- IV
-
- intravenous
-
- NICTH
-
- non-islet cell tumor hypoglycemia
-
- SFT
-
- solitary fibrous tumor
Keynote message
We report intraoperative blood glucose trends and safety of surgical operations in Doege–Potter syndrome.
Introduction
Although IGF-II primarily stimulates fetal growth and cellular differentiation, it exerts similar effects as insulin, albeit with reduced potency.1 NICTH, a recognized tumor-associated syndrome, is caused by the secretion of high-molecular-weight forms of IGF-II (Big IGF-II). Furthermore, when a tumor presenting with NICTH is pathologically characterized as a SFT, DPS is diagnosed,2, 3 and surgical excision of the tumor is the preferred therapeutic modality.4
We present a DPS with a primary retroperitoneal lesion. Moreover, an isCGM device was used to measure intraoperative BG levels every 5–15 min. This is the first report of intraoperative BG trends in IGF-II-producing tumors.
Case presentation
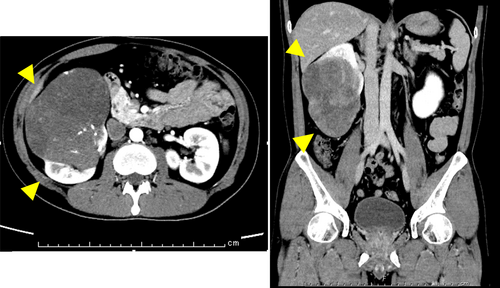
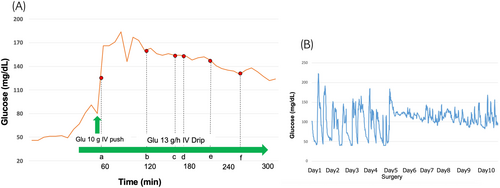
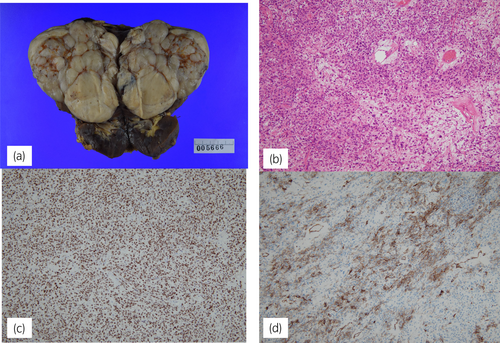
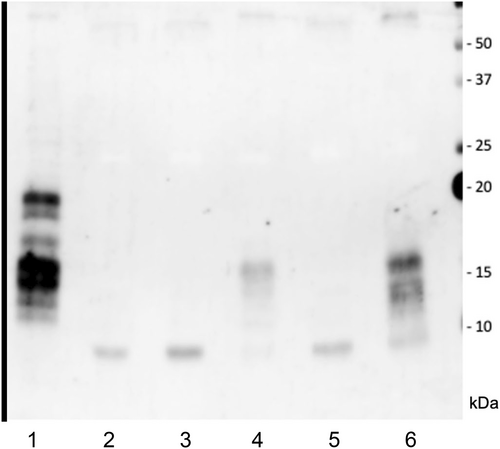
The patient was a healthy 37-year-old man who underwent routine annual physical examinations at his workplace. The patient had no abnormal or irregular blood test results at the time. However, approximately 1 month after his most recent examination, he began to experience general malaise and a decreased ability to think. Over the next 3 months, these symptoms increased in frequency and severity, resulting in incontinence and impaired consciousness, leading to admission to the emergency room. A blood draw showed fasting BG concentration of 26 mg/dL(70–100 mg/dL), insulin antibody concentration of <0.4 U/mL(>0.4 U/mL), and blood insulin concentration of <0.2 μU/mL(2.7–10.4 μU/mL). Fasting blood C-peptide immunoreactivity concentration of 0.1 ng/mL (0.7–2.5 μU/mL) and IGF-I level of 32 ng/mL (37 years old: baseline 97–272 ng/mL) were also observed. A computerized tomography examination revealed a 10 × 12 × 9 cm tumor around the right kidney (Fig. 1), but no other neoplastic lesions were observed. Based on these findings, NICTH caused by a retroperitoneal tumor was suspected. After hospitalization, the patient was able to correct BG with oral glucose until surgery. The patient underwent a 257-min open right retroperitoneal tumor resection, and his BG level was monitored every 5–15 min using isCGM (Fig. 2A). An IV push of 10 mg glucose was administered before surgery to correct the hypoglycemia, but an additional IV push was not required during the procedure. The patient's BG levels remained stable throughout the surgery; only an IV drip of extracellular fluid with glucose was administered, and it did not cause hypoglycemia. Additionally, direct mechanical stimulation of the tumor did not cause hypoglycemic attacks. A blood sample taken at the end of the surgery showed BG level of 136 mg/dL, blood C-peptide level of 2.36 ng/mL, and insulin concentration of 9.92 μU/mL, indicating rapid improvement in their own insulin secretion. The patient was discharged from the hospital 8 days after surgery without incident. Fig. 2B presents the BG trends for 5 days before and after surgery. The pathological specimen showed an oval-to-short, spindle-shaped nucleus with increased chromatin at the tumor site. The cytoplasm showed dense proliferation of tumor cells with pale eosinophilic spherocytes. The background contained vitelline blood vessels and connective tissues. Furthermore, immunostaining revealed STAT6(+), CD34(+), and a Ki67 index of 5%–10%, indicative of SFT (Fig. 3). Western blot analysis of the tumor specimen and preoperative blood samples revealed Big IGF-II. DPS was diagnosed based on the SFT of the IGF-II-producing tumor (Fig. 4). One week and 1 month after surgery, Big IGF-II was no longer detected in the serum.
Discussion
For the first time, we report on intraoperative BG trends excisional surgery for DPS of retroperitoneal origin. DPS presents with SFT and hypoglycemia. DPS occurs in <5% of SFT cases.5 To date, approximately 50 cases have been reported,6 most of which occurred intrathoracically; extrathoracic cases are rare.7
The present case was an extrarenal case with a neoplastic lesion around the right kidney, and NICTH was believed to have caused hypoglycemia through the action of IGF-II. The main effect of IGF-II was similar to that of insulin, that is, it promotes fetal growth and cell differentiation. However, its biological activity is low, approximately 14% of insulin's. Tumors produce large amounts of IGF-II, which binds to IGFBP, forming the IGFBP duplication complex (Big IGF-II). These complexes readily enter the interstitial fluid and reach target cells8; they also induce cells to stimulate glucose uptake by skeletal muscles. Continued stimulation of insulin receptors by IGF-II leads to further inhibition of free fatty acid release and gluconeogenesis, glycogenolysis, and ketogenesis inhibition by the liver.9, 10 In IGF-II-producing tumors, patients with metastatic disease, elevated serum lactate dehydrogenase activity, or tumors larger than 12 cm are more likely to produce more IGF-II and become hypoglycemic.11 Our patient had a tumor >12 cm and NICTH.
Complete resection of the tumor is a prerequisite for a cure; however, in many cases, resection is delayed, or the tumor is unresectable. Therefore, there is no clear standard of care for these patients.4 Several case reports have reported that hypoglycemic attacks stopped after surgically resecting the tumor.12, 13 In this case, the tumor was completely resected, and the patient's insulin secretion rapidly improved immediately after surgery. Postoperatively, the patient's BG levels remained stable, without hypoglycemic attacks. Although BG levels were continuously measured during surgery, the patient did not experience hypoglycemic attacks, even when the tumor was mechanically manipulated during surgery.
Conclusion
The results indicate that surgery can be performed without special corrections if the patient is in good general condition preoperatively, if complete resection is possible, or if preoperative hypoglycemia can be treated only with glucose administration. However, it is important to be prepared to respond promptly in each case.
Author contributions
Hirotaka Nagasaka: Writing – original draft. Takahisa Suzuki: Supervision; writing – review and editing. Takuya Kondo: Supervision. Mitsuyuki Koizumi: Supervision. Hideyuki Terao: Supervision. Yuko Murohashi: Supervision. Yoichiro Okubo: Supervision. Tomoyuki Yokose: Supervision. Takeshi Kishida: Supervision.
Conflict of interest
The authors have no conflicts of interest to declare.
Approval of the research protocol by an Institutional Reviewer Board
This study was approved by the Ethics Committee of the Kanagawa Cancer Center Hospital. The approval number was 2022-156.
Informed consent
Written informed consent was obtained from the patient for publication of this case report.
Registry and the Registration No. of the study/trial
Not applicable.




