The proinflammatory peptide substance P promotes blood–brain barrier breaching by breast cancer cells through changes in microvascular endothelial cell tight junctions
Abstract
Neuropeptide substance P (SP) has been implicated in inflammation, pain, depression and breast cancer cell (BCC) growth. Here, we examined the role of SP in trafficking of BCCs (human MDA-MB-231 and MDA-MB-231BrM2 cells) across the blood–brain barrier (BBB) and brain microvascular endothelial cells (BMECs) using in vitro and in vivo models. SP was secreted from BCCs and mediated adhesion and transmigration of BCCs across human BMECs (HBMECs) in vitro. SP induced activation of HBMECs, leading to secretion of Tumor Necrosis Factor alpha (TNF-α) and angiopoietin-2 (Ang-2) from HBMECs, resulting in changes in localization and distribution of tight junction (TJ) ZO-1 (tight junction protein zonula occludins-1) and claudin-5 structures as well as increased permeability of HBMECs. Using spontaneous breast cancer metastasis mouse model (syngeneic) of GFP-4T1-BrM5 mammary tumor cells administered into mammary fat pads of Balb/c mice, SP inhibitor spantide III inhibited in vivo changes in permeability of the BBB and BMEC-TJs ZO-1 and claudin-5 structures as well as decreased tumor cell colonization in brain. Thus, SP secreted from BCCs induces transmigration of BCCs across the BBB, leading to activation of BMECs and secretion of TNF-α and Ang-2, resulting in BBB impairment and colonization of tumor cells in brain. Therefore, therapies based on SP inhibition in combination with other therapies may prevent breaching of the BBB by BCCs and their colonization in brain.
Abstract
What's new?
Breast cancer cells migrate across the blood-brain barrier in more than one-fifth of patients with metastatic breast tumors. According to this study, that feat is accomplished in part by substance P-mediated changes in the tight junction proteins ZO-1 and claudin-5 in brain microvascular endothelial cells (BMECs). Substance P activation of BMECs led to secretion of TNF-α and angioprotein-2, which resulted in modifications in ZO-1 and claudin-5 localization and distribution and increased BMEC permeability. The findings suggest that substance P inhibition may be key to the success of therapies designed to prevent tumor cell colonization of the brain.
Abbreviations
-
- Ang-2
-
- angiopoietin-2
-
- BBB
-
- blood–brain barrier
-
- BMEC
-
- brain microvascular endothelial cells
-
- DMEM
-
- Dulbecco's Modified Eagle Medium
-
- FAK
-
- focal adhesion kinase
-
- FBS
-
- Fetal Bovine Serum
-
- HBMECs
-
- human brain microvascular endothelial cells
-
- MMPs
-
- matrix metalloproteinases
-
- NK-1R
-
- receptor for substance P
-
- SP
-
- substance P
-
- Tie-2
-
- TEK tyrosine kinase endothelial receptor
-
- TJs
-
- tight junctions
-
- TEER
-
- transendothelial electrical resistance
-
- ZO-1
-
- tight junction protein zonula occludins-1
About 20–30% of breast tumor patients with metastatic disease develop breast tumor metastasis in the brain years after primary cancer is diagnosed.1-3 The blood–brain barrier (BBB) is “a dynamic interphase between the peripheral circulation and the central nervous system (CNS).”4 Impairment of the BBB is critical in the development of diseases that affect the CNS including tumor metastases.4-9
The BBB is comprised mainly of brain microvascular endothelial cells (BMECs) that exhibit many specialized properties, including highly selective permeability and high transendothelial electrical resistance (TEER).10-12 BBB functions are primarily maintained by the BMEC-tight junction (TJ) protein complexes between adjacent BMECs in which claudin-5 is considered as “sealer” of the BBB and tight junction protein zonula occludins-1 (ZO-1) acts as a scaffolding protein between actin and BMEC-TJ proteins.4
Most forms of brain injury resulting in neurodegenerative diseases as well as tumor transmigration to the brain are associated with BBB disruption.6-8, 13-15 Disruption of the BBB by brain metastasis was observed in tumors of triple-negative and basal type breast tumor cells.6 Several genes were shown to facilitate the development of brain metastases and include the cyclooxygenase-2 (COX-2), the epidermal growth factor receptor (EGFR) ligand HBEGF and the αlpha-2,6-sialyltransferase ST6GALNAC5 as mediators of cancer cell passage through the BBB.16 Recent studies also described the changes in the brain microenvironment during the initial steps of breast tumor metastasis to the brain.17, 18
The vascular basement membrane was shown to serve as the “soil” in brain metastasis.19 The majority of in vivo experimental models of brain metastasis and analysis of human clinical specimens showed that more than 95% of early micrometastasis examined demonstrated vascular cooption with little evidence for isolated neurotropic growth.19 The existing microvasculature is a key niche for malignant progression and therapies targeted to the BBB should hold significant promise for the treatment of tumor metastasis in the brain.
Substance P (SP) is an important proinflammatory neuropeptide that functions as an immunoneuromodulator in the brain.20-23 Interestingly, it was reported that SP as well as its receptors NK-1 and NK-2 are expressed in breast cancer cells (BCCs).24-26 In breast tumor, the involvement of SP and its receptor in the acquisition of oncogenic properties and in facilitation of bone marrow metastasis has been described.25 Activation of NK-1 by SP resulted in the activation of PI3K, the NF-kB pathway and mitogen-activated kinases (MAPKs).24-26 Further, exposure of breast tumor cells to SP enhanced the aggressiveness of tumor cells by promoting the activity of Her2 and EGFR.27 Targeting of SP by NK-1 antagonist L-733, 060 induced cell death in BCCs and decreased the steady state of EGFR and Her2,27 as well as induced cell death in BCCs that have developed chemoresistance to anti-Her2 therapies.27 In these studies, tumor cells were treated with 500 nM of SP (a very high dose) and with NK-1 antagonist L-733, 060 (at various doses up to 100 μm).27 Further, recent studies by Ghabriel's group28 showed that rat mammary tumor cells walker 256 tumor cells increased SP immunoreactivity locally and modified the properties of the BBB during extravasation and brain invasion.28 However, little is known on the molecular mechanisms for the effects of SP on BMECs and BCC transmigration across the BBB in vitro and in vivo and their metastasis to the brain.
In our study, we analyzed the role of SP secreted from BCCs on their transmigration across the BMEC-TJs and the possible pathway(s) by which SP induces changes in BMECs that lead to BCC colonization in brain. These studies were performed using in vitro and in vivo models with human MDA-MB-231 cells and murine GFP-4T1BrM5 mammary epithelial cells. As we obtained similar results with both cell lines, we present here in vitro data with MDA-MB-231 cells and the in vivo data with GFP-4T1BrM5 murine cells using the syngeneic mice model (Balb/c mice) for breast cancer metastasis in the brain.
Material and Methods
Please see Supporting Information data for methods and reagents used in our study.
Results
SP secretion by BCCs
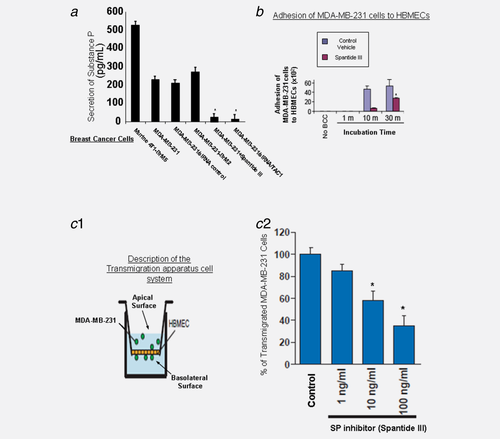
We first analyzed SP secretion in several BCC lines. Using SP ELISA assay, BCCs (1 × 106 cells/ml) were analyzed for secretion of SP within 24 hr of culture growth (Fig. 1a). BCCs secreted high levels of SP and the SP secretion was blocked in the presence of siRNA TAC-1 treatment as described previously24, 26 or in the presence of SP inhibitor, spantide III (100 ng/ml) (Fig. 1a).
SP mediates adhesion and transmigration of BCCs through BMECs
We performed an in vitro adhesion assay where MDA-MB-231 cells were cocultured with human BMECs (HBMECs) and assessed for their ability to adhere to HBMECs monolayer based on the kinetic studies as detailed.30, 31 Our results show that these cells adhered to the untreated monolayer by as early as 10 min (Fig. 1b). Pretreatment of HBMECs monolayer with the SP antagonist spantide III (100 ng/ml) blocked adhesion of MDA-MB-231 cells at time points of 10 and 30 min (Fig. 1b).
Various lines of evidence support that SP is expressed in BCCs24, 26 so we examined the role of SP in transmigration of BCCs across BMECs. We employed transmigration assay using in vitro model as described previously,32 where HBMECs were cocultured with breast tumor MDA-MB-231 cells (Fig. 1c-1). MDA-MB-231 transmigration across HBMECs was inhibited by the SP-specific inhibitor, spantide III, in a dose-dependent manner (Fig. 1c-2). Similar results were obtained with BCCs that specifically “home” to the brain, the MDA-BrM2 and CN34-BrM2 (data not shown).
Next, we examined the expression of the SP receptor in HBMECs to confirm their capacity to respond to SP. We observed that HBMECs indeed express NK-1 receptor (NK-1R) (data not shown), in accordance with previous report.24-26
SP inhibitor blocked changes in calcium ion secretion and increased permeability in BMECs
SP induced calcium influx in HBMEC.32 Here, we show that SP significantly increased the calcium ion concentration in cocultures of HBMEC after BCC adhesion and transmigration, which were blocked by SP inhibitor spantide III (at 100 ng/ml) (Supporting Information Fig. 1).
Effects of SP on permeability changes in HBMECs
The permeability changes in HBMECs induced by SP were determined by measuring TEER in 3D cocultures of HBMECs with primary human astrocytes, using fluoresceinated dextran 70. A significant increase in BBB permeability was observed after treatment with SP (10 ng/ml), when compared to control, and these permeability changes were inhibited by spantide III (100 ng/ml) (Fig. 2a).
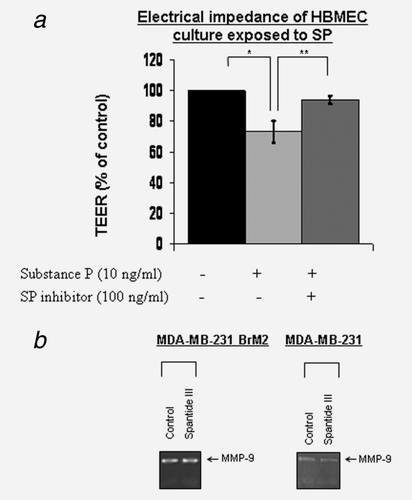
SP does not regulate secretion of MMP-9 in BCCs
Matrix metalloproteinase (MMP)-9 was shown to be involved in breast cancer invasion and metastasis, and circulating BCCs secrete MMP-9.16 Although we observed that BCCs released activated MMP-9 into culture supernatants, this secretion was not inhibited in the presence of SP inhibitor spantide III (100 ng/ml) (Fig. 2b). Thus, induction of MMP-9 secretion from MDA-MB-231/BrM2 tumor cells is not dependent on SP-mediated pathway.
Effects of SP on BMEC-TJ protein expression and cytoskeletal reorganization
SP at 10 ng/ml induced significant changes in ZO-1 (Fig. 3a) and claudin-5 (Fig. 3b) expression in HBMECs. Moreover, SP induced discontinuous lines of TJ complexes in the HBMECs, which were inhibited by SP antagonist spantide III (100 ng/ml) (Figs. 3a and 3b). The changes in TJ expression were quantitated and are shown in Figure 3c.
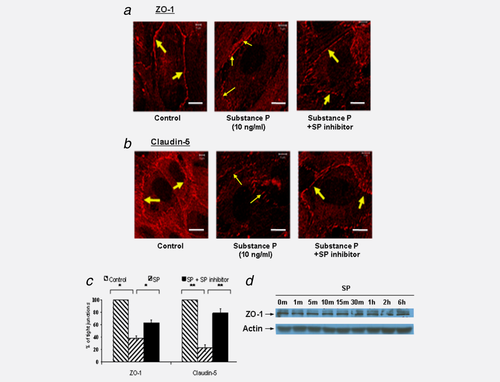
To assess if the expression of TJ proteins is downregulated by SP, a Western blot was done on total HBMEC cell lysates after treatment with SP. No changes in claudin-5 and occludin expression levels were observed (data not shown). Further, ZO-1 expression was not altered by treatment with the SP neuropeptide through 30 min (Fig. 3d) and up to 6 hr (Fig. 3d). Collectively, SP is sufficient to disturb brain endothelial TJs by redistributing ZO-1 away from the cell–cell boundaries and into the intracellular compartment.
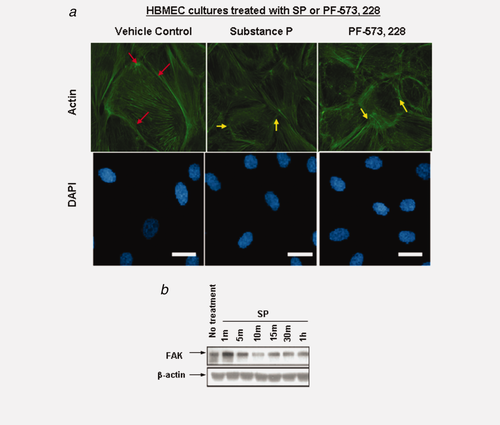
Several lines of evidence have demonstrated that the morphological changes in endothelial cells are associated with proinflammatory cytokines and SP.33 Using focal adhesion kinase (FAK) inhibitor, PF-573,228, we determined the morphological consequence (via actin staining) of downregulating this pathway independent of SP. The selectivity of this inhibitor has been described previously.34 As there are dynamic changes in actin cytoskeleton reorganization, and because ZO-1 protein directly associates with actin in the BMEC-TJs complex,10 we analyzed actin expression. Experiments of untreated HBMECs or HBMECs treated with vehicle control showed that F-actin was denser at these boundaries and was mainly retained within the plane of cell (Fig. 4a, red arrows). However, in cells treated either with SP or PF-573,228, cells exhibited smaller and rounded morphology (Fig. 4a, yellow arrows). Interestingly, in the drug-treated HBMECs, F-actin has also been relocalized away from the cell periphery and into a more stress fiber-like pattern, projecting across cell–cell boundaries and exposing endothelial gaps between the cells (Fig. 4a). Further, we observed changes in FAK expression levels by 10 min (Fig. 4b), although no changes were observed on FAK phosphorylation (data not shown). Taken together, these results imply that FAK may be required downstream of SP.
Effects of SP on TNF-α and Ang-2 secretion by HBMECs
As SP was shown to be involved in inflammatory diseases and is a modulator of inflammation,21-23 we examined secretion of TNF-α from HBMECs. After cocultures of GFP-MDA-MB-231 cells with HBMECs for 24 hr, GFP-MDA-MB-231 cells were removed and TNF-α secretion by HBMECs was detected (Fig. 5a). This secretion was inhibited by SP inhibitor spantide III (Fig. 5a). No secretion of TNF-α by MDA-MB-231 cells was found (data not shown).
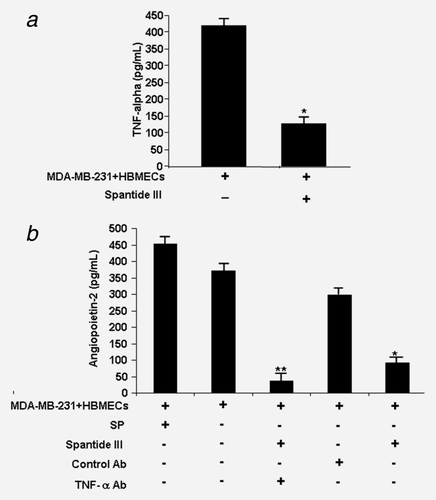
Angiopoietin-2 is highly produced by activated endothelial cells, such as tumor-associated blood vessels,35-38 and may induce blood vessel regression, leading to hypoxia, increased VEGF and Ang-2 expression and vessel sprouting.39, 40 Therefore, we focused on whether SP affects Ang-2 expression in HBMECs. Ang-2 ELISA assay showed that HBMECs exposed to SP or cocultured with MDA-BrM2 induced the secretion of Ang-2 from HBMEC up to 450 pg/ml (Fig. 5b), whereas MDA-MB-231 cells did not secrete Ang-2 (data not shown). Further, the secretion of Ang-2 from HBMECs exposed to MDA-MB-231 cells was inhibited in the presence of spantide III (Fig. 5b). These results suggest a role for SP in inducing the expression and secretion of Ang-2, which may further facilitate the transmigration of tumor cells via increased permeability of HBMECs.
As TNF-α was shown to upregulate Ang-2 in human umbilical vein endothelial cells,36 we examined if induction of Ang-2 is dependent on TNF-α in HBMECs. When cocultures of HBMECs pretreated with TNF-α antibody or control antibody (for 1 hr) were incubated with MDA-MB-BrM2, lower levels of Ang-2 secretion were detected in the supernatants of HBMECs (Fig. 5b), indicating that Ang-2 is induced by TNF-α in HBMECs, and is in agreement with previous studies.35, 36
In vivo effects of spantide III on BBB permeability and 4T1-BrM5 mammary tumor cell colonization in brain
As ZO-1 is the main scaffolding protein and claudin-5 is an important BMEC-TJ “sealer” protein of the BBB,4 we next focused on in vivo analysis of changes in the expression of BMEC-TJ protein of ZO-1 and claudin-5 as a result of transmigration of BCCs across the BBB. Using the spontaneous breast cancer metastasis mouse model (syngeneic) of mammary tumor cells GFP-4T1-BrM5 cells, which “home” to brain16 and form breast metastasis in brain in Balb/c mice, we examined the effects of spantide III on BBB integrity, BMEC-TJs and tumor colonization in brain. GFP-4T1-BrM5 mammary tumor cells were injected into the mammary fat pads of Balb/c mice. After 3 or 4 weeks, tumor cell colonization in the brain, as well as changes in TJ expression in the BBB, was analyzed in these mice. The brain tumor cells immunostained with GFP antibodies to detect tumor cells (red color) or with pan-cytokeratin antibodies (data not shown). Changes in the BBB integrity by analyzing BMEC-TJs were observed when compared to WT BBB brain. Within 3 weeks after the administration of these BCCs, the BBB-BMEC-TJ protein expressions were altered and showed significant reduction in ZO-1 and claudin-5 in BMEC-TJs, which appeared disrupted (Fig. 6b, Panels 1 and 2). Further, significant tumor cell colonization was observed in the brain of these mice (Fig. 6b, Panel 3). To assess the direct role of SP in mediating colonization of BCCs in brain, we have administered i.v. the SP inhibitor spantide III or vehicle control to test its potential in inhibiting BMEC-TJ changes and tumor cell colonization in brain. Reduced disruption of ZO-1 and Claudin-5 was observed in the presence of spantide III (Figs. 6c and 6d) after 3 and 4 weeks, when compared to WT mice. In addition, significantly reduced tumor cell colonization was observed in brain (Fig. 6d, Panel 3 and Fig. 6g). Quantitative analysis of in vivo experiments also showed that Claudin-5 (Fig. 6e-1) and ZO-1 (Fig. 6e-2) at the BBB-BMEC-TJs, as analyzed by confocal microscopy, were markedly altered after 4T1-BrM5 transmigration, whereas spantide III inhibited these changes (Fig. 6e). These changes in TJs were correlated with BBB permeability changes (Fig. 6f).
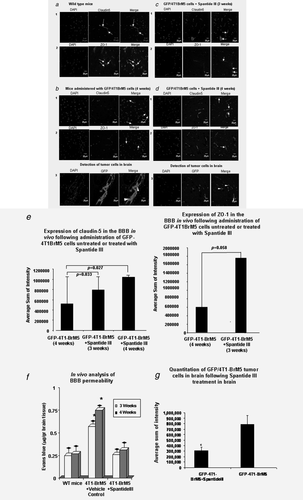
Therefore, in an in vivo mouse model, spantide III may be effective in blocking early stages of tumor cell entry to the brain via inhibition of TJs disruption and BBB permeability changes.
Discussion
Our study investigated a proposed hypothesis by which SP released from breast tumor cells induced changes in BBB-BMEC-TJ distribution and/or expression of ZO-1 and claudin-5. The results presented here determined the changes of TJ proteins ZO-1 and claudin-5 that are affected by SP during the transmigration of BCCs across HBMECs. We showed the secretion of SP by BCCs (Fig. 1a) and the effects of SP in mediating adhesion (Fig. 1b) and transmigration of BCCs across the HBMECs (Fig. 1c-1), which is independent of MMP-9-mediated pathways (Fig. 1c-2). SP also induced activation of HBMECs as indicated by increased calcium ion concentration (Fig. 2a) and permeability changes (Fig. 2b). The permeability changes in HBMECs by SP were through induction of changes in ZO-1 and claudin-5 distribution in HBMECs. SP induced HBMEC activation by inducing TNF-α and led to Ang-2 secretion from BMECs (Fig. 5). These changes allow BCC entry to the brain by breaching the BBB in vivo (Fig. 6b) and inducing changes in the distribution of BMEC-TJ proteins, ZO-1 and claudin-5 (Fig. 6b). Furthermore, administration of SP inhibitor, spantide III, showed in vivo inhibition of changes in BMEC-TJ intensity (Figs. 6c and 6d) and of BCC colonization in brain (Fig. 6d, panel 3 and Fig. 6g). Taken together, the results shown here suggest that SP functions as an important modulator in breast tumor metastasis to brain at the level of BBB BMEC-TJs. These studies are in agreement with a recent study showing that breast tumor cells increased SP immunoreactivity locally and modified the properties of the BBB during extravasation and brain invasion.28 Wistar rats injected with walker 256 tumor cells via the internal carotid artery showed tumor cell extravasation across the BBB at day 3 postinoculation, which corresponded with significantly increased albumin and SP immunoreactivity as well as significantly reduced endothelial barrier antigen labeling of microvessels.28
Previous work in our laboratory demonstrated that the neuropeptide SP involved in HIV-1 Gp120 mediated permeability of BMECs in the brain by downregulating BMEC-TJ protein expression.32 Here, we showed that SP is secreted by BCCs (Fig. 1a) and examined whether BCC transmigration across HBMECs also involved SP. To this end, we used the previously described in vitro coculture model of HBMECs with BCCs30, 32 in the presence of SP inhibitor, spantide III. We have found that spantide III significantly reduced transmigration of MDA-MB-231 cells through a HBMEC monolayer (Fig. 1c). In vitro, SP induced prominent changes in the BMEC-TJ proteins ZO-1 and claudin-5 distribution (Fig. 3) and changes in the distribution of the actin cytoskeleton from cortical arrangement into stress fibers (Fig. 4). In support of the above findings, the decrease in the transmembrane electrical resistance (TEER) in this condition also indicates a decrease in BMEC-TJ function (Fig. 2a). Overall, these studies demonstrate that SP secretion by BCCs mediates their transmigration through the HBMECs by regulating HBMEC-TJs integrity and cytoskeletal rearrangement. It is reasonable to assume that the efficiency of BCC transmigration across the BBB-BMEC-TJ complexes is dependent on several factors that include the resilience of the BBB to minor insult, the aggressiveness of the tumor cells such as TBNC cells, the secretion levels of chemokines/cytokines and angiogenic factors from BCCs and the amount of BCCs that are transmigrated and/or penetrating in a time point. Therefore, the changes in BBB-BMEC-TJs observed by confocal analysis could vary depending on the BCC characteristics and the levels of insult on the BBB. When the impairment is minimal, the changes in TJ protein expression are minimal. However, when the cytotoxic effects on the BBB are prominent, the effects on TJ complexes are significant and the expression levels of BMEC-TJ protein are reduced, such as in HIV-Gp120-mediated effects in the BBB.32
New blood vessel formation plays an important role in breast tumor growth and metastasis.39, 40 Tumor growth is preceded by the development of new blood vessels, which provide a pathway for metastasis and nutrients essential for growth. It was reported in several human cancers that increased expression of Ang-2 in tumor cells is closely correlated to the progression, invasiveness and metastasis.40, 41 Although expression of Ang-2 was reported in primary BCCs by RT-PCR and immunostaining,42 we were not able to detect in the BCCs used in our study expression of Ang-2 by RT-PCR analysis. Immunostaining and Western blotting also failed to detect secretion of Ang-2 in BCCs including tumor cells that “home” to the brain, the MDA-MB-231-BrM2 and CN34-BrM2 cells (data not shown).
We propose here a novel link between SP secreted by BCCs and their interaction with HBMECs, which induced changes in BMEC-TJ ZO-1 protein in HBMECs and increased expression of the chemokine TNF-α and of Ang-2 in HBMECs. SP may serve as an important and early factor in a chain of molecular events leading to BBB disruption and penetration of BCCs to the brain by mediating transmigration of BCCs across HBMEC-TJs.
In addition, SP may also play an important role in mediating the responsiveness of the microvascular endothelium to proinflammatory cytokines and loss of quiescence, brought by the actions of SP, and followed up by secretion of TNF-α and Ang-2, resulting in destabilization of HBMECs as a major component of the BBB (Fig. 5). Of note, TNF-α is a known chemokine to decrease BBB permeability43 and Ang-2 is known to destabilize microvasculature.35 Disruption of the BBB by SP may also enhance the entry of inflamed monocytes and macrophages across the BBB and lead to activation of microglial and astrocytes, which further lead to secretion of proinflammatory cytokines.
Inflammation and angiogenesis are shown to be associated with pathological disorders.37 TNF-α is a major inflammatory cytokine that also regulates angiogenesis.42 TNF-α induces TEK tyrosine kinase endothelial receptor (Tie-2) and Ang-2 expression in endothelial cells.42, 43 Ang-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation.43 Ang-2 promotes adhesion by sensitizing endothelial cells toward TNF-α and modulating TNF-α-induced expression of endothelial cell adhesion molecules42 (Fig. 5). Thus, TNF-α and Ang-2 may serve as regulators of endothelial cell inflammatory responses and act as a switch of vascular responsiveness exerting a permissive role for the activity of proinflammatory cytokines, further contributing to inflammation, angiogenesis and tumor metastasis in brain. To the best of our knowledge, this is the first report of a novel link between Ang-2 and SP and TNF-α inflammatory cytokine.
Taken together, this investigation may provide additional and novel insight into the adhesion, migration and transmigration of BCCs to the brain, and has identified a new pathway of SP/Ang-2 axis that might be involved in enhancing breast tumor metastasis to the brain by disruption of HBMEC-TJs integrity. BCCs that infiltrate the brain support their own metastasis by expressing SP and highlight the relevance of SP as a component of the BCC metastasis invasion capabilities to the brain. Therefore, using specific inhibitors for SP may lead to better therapeutic strategies for treatment modalities targeted to the BMEC and the BBB by focusing on inhibition of BCC colonization in the brain.
Acknowledgements
The authors thank Lili Wang, Esther Lee and Benjamin Chen for their help in typing and editing the manuscript, and for the quantitative analysis of ZO-1 and claudin-5 performed by Lili Wang.




