Exploring the Active Lithium Loss in Anode-Free Lithium Metal Batteries: Mechanisms, Challenges, and Strategies
Xuzhi Duan and Jinran Sun contributed equally to this study.
Abstract
Anode-free lithium metal batteries (AFLMBs), also known as lithium metal batteries (LMBs) with zero excess lithium, have garnered significant attention due to their substantially higher energy density compared to conventional lithium metal anodes, improved safety characteristics, and lower production costs. However, the current cycling stability of AFLMBs faces formidable challenges primarily caused by significant lithium loss associated with the deposition of lithium metal. Therefore, this review focuses on the crucial aspects of lithium metal nucleation and growth on the anode side. Respectively, aiming to provide an in-depth understanding of the deposition mechanisms, comprehensively summarize the corresponding scientific influencing factors, and analyze specific strategies for addressing these issues through the integration of relevant exemplary cases. Importantly, this review endeavors to offer a profound explication of the scientific essence and intricate mechanisms that underlie the diverse modification strategies. This review possesses the inherent capacity to greatly facilitate the progress and enlightenment of research in this field, offering a valuable resource for the researchers.
1 Introduction
Since their introduction in the 1990s [1], lithium-ion batteries (LIBs) have become integral to our lives, thriving commercially for over three decades. Against the backdrop of the widespread adoption of new energy vehicles, there is a growing demand for higher energy density in batteries. The anode plays a crucial role in influencing the energy density of batteries. The conventional graphite anode has a capacity of 372 mAh g−1, resulting in an energy density of only 160 Wh kg−1 when paired with a LiFePO4 composition in the battery, which falls far short of meeting the current demand for battery energy density towards new energy vehicles [2, 3]. There is an urgent necessity to develop next-generation batteries with higher energy density (exceeding 500 Wh kg−1) [4, 5].
Anode-free lithium metal batteries (AFLMBs), as presented in Figure 1A, have emerged as a contender in meeting the escalating demands of the electric vehicle industry, which exhibits a dominant advantage in terms of energy density (LiNi0.8Mn0.1Co0.1O2||Cu-575 Wh kg−1) [7]. Compared with conventional LIBs and LMBs, AFLMBs exhibit significant advantages in both gravimetric energy density (GED) and volumetric energy density (VED) as illustrated in Figure 1B, especially in VED (1514 Wh L−1, 60% higher energy density compared to traditional LiBs) [6, 8]. Furthermore, AFLMBs possess advantages in terms of manufacturing cost and safety due to the unique configuration, which solely relies on a Cu foil as the current collector (CC) on the anode side. As for the LMBs, to ensure both production safety and the effectiveness of the lithium foil, the utilization of lithium foils typically necessitates strict control of the dew point in the production environment with high maintenance costs. In contrast, the anode-free design effectively circumvents these issues. Furthermore, the absence of additional lithium storage on the anode side enables a significant reduction in risks associated with reactive lithium metal, such as thermal runaway [9]. Research has indicated that AFLMBs may still undergo thermal runaway under conditions of thermal abuse. However, compared to LMBs of equivalent capacity, the extent of runaway is relatively less severe, rendering them safer in comparison [10].
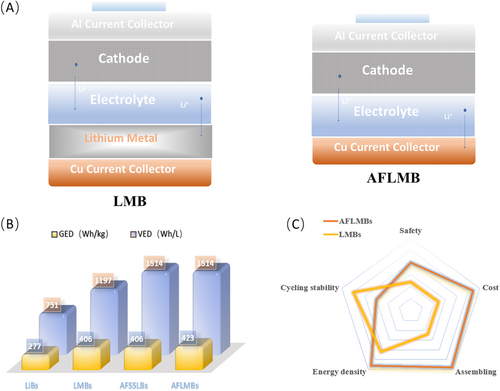
Nevertheless, under the condition that the lithium source in the anode-free battery system is limited (originating from the cathode), AFLMBs face more severe challenges in terms of cycling stability compared to conventional lithium batteries, making it difficult to fully leverage the advantages in high energy density for practical applications [11]. Figure 1C represents the comparison between LMBs and AFLMBs in terms of safety, cost, assembly, energy density and cycle stability. In the Cu CC side, the initial plating of high surface area lithium metal morphologies, such as dendritic and mossy structures, give rise to the formation of a thick solid electrolyte interphase (SEI) with high specific area [12], which consumes active lithium and electrolyte, resulting in low initial Coulombic efficiency. Moreover, the continuous unregular deposition of lithium metal during plating leads to volumetric expansion, exerting stress on the native SEI and making it prone to rupture and subsequent reformation. And the non-compact plating morphologies not only aggravate the formation of dead lithium but also exacerbate the deterioration of the anode interface, thereby resulting in a repetitive cycle of active lithium and electrolyte consumption [6, 13, 14].
It is unmistakably clear that the depletion of active lithium in AFLMBs is intricately intertwined with three pivotal factors: the nucleation of deposited lithium metal, the stability of SEI, and the extent of volumetric expansion accompanying the deposition of lithium metal. We systematically summarize the crucial modification directions relevant to the anode side for enhancing the performance of AFLMBs. The morphology of lithium metal plating, primarily determined by the nucleation process, hinges significantly on the properties of the CC, which serves as the crucial substrate for nucleation. The purposes of CC design primarily focus on reducing the nucleation overpotential to optimize the nucleation process of lithium metal. After initial nucleation, the subsequent growth morphology of lithium metal is also controlled by the stability of the SEI. Optimizing properties like ionic conductivity and mechanical stability of SEIs involves constructing an artificial solid electrolyte interface (ASEI) or adjusting the components, concentration, and morphology of the electrolyte, effectively extending the cycle life of AFLMBs. Furthermore, variations in testing conditions can significantly impact the morphology and growth process of lithium plating, consequently influencing the energy density and lifetime of AFLMBs. In contrast to other reviews concerning anode-free systems that predominantly concentrate on comprehensive summaries of specific modification methodologies, this review places emphasis on providing an in-depth elucidation of the scientific essence and mechanisms underlying various modification strategies as seen in Figure 2. By incorporating concrete examples, it aims to illustrate the scientific coherence behind the diverse modification schemes, offering readers a concise and clear direction for anode-related modifications in AFLMBs. Ultimately, the primary objective of this review is to stimulate the progress and innovation of emerging designs for AFLMBs.

2 The Impact on Nucleation
Cu foil has been extensively employed as a CC for anode in LMBs due to its high electrical conductivity, excellent mechanical stability, and cost-effectiveness [15-17]. Cu and Li belong to a heterogeneous system. And for AFLMBs, it is necessary to deposit lithium metal directly onto the Cu foil rather than lithium itself [18]. Therefore, it is crucial to accurately understand the nucleation and growth mechanism of lithium on Cu as well as its influencing factors.
2.1 Nucleation Mechanism
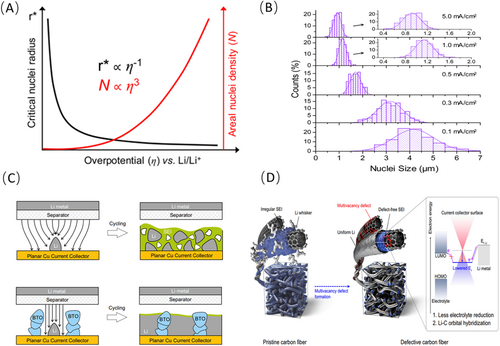
The inverse relationship between nuclei size and electrochemical overpotential is immediately apparent. Furthermore, for spherical nuclei, this relationship follows a cubic pattern with areal nuclei density and overpotential as illustrated in Figure 3A [19].
2.2 The Control of Local Current Density
The current density is an intuitive factor influencing the overpotential [28-31]. Cui et al. directly adjusted the overpotential by increasing/reducing the current density, thereby observing the morphology of lithium nucleation that was deposited on Cu [19]. At lower current density, initial lithium nuclei were sparsely distributed on the surface of the working electrode, eventually forming a dense arrangement as more charge passed through. However, the actual nucleation density remained relatively constant, exhibiting only a gradual expansion of the lithium nuclei. In contrast, at higher current density, the deposition morphology differed significantly from that at lower current density. Overpotential for lithium nucleation increases, leading to a reduction in Gibbs free energy and the formation of high-density and relatively small lithium nuclei as depicted in Figure 3B. This aligns well with the thermodynamic analysis presented above [32, 33]. Numerous literature reports have shown that dense, small lithium nuclei elevate the probability of electrolyte interaction with lithium metal [34-36]. Small lithium nuclei are prone to more parasite reaction, which leads to active lithium consumption [28, 29, 31, 37]. Up to date, the general agreement is that reducing current density to obtain the deposited with low specific surface area is favorable in AFLMB with limited active lithium source.
The design of 3D structured CCs has become a hotspot in AFLMBs, which provide larger specific surface areas to effectively reduce local current density and ensure uniform lithium nucleation with large size [38-44]. Commonly used 3D CCs include porous Cu [45], layered graphite oxide [46], and dendritic three-dimensional Cu [47]. In addition to the 3D structure of CC, augmenting the surface of CC resistance is also being explored as a method to decrease current density. Increasing the surface resistance of the CC, such as with Al2O3 coatings, reduces available nucleation sites for Li-metal, promoting sparse nucleation of Li-nuclei [48]. Besides, modifying the Cu surface with high dielectric coatings also can effectively reduce the overpotential. For example, combining high dielectric constant materials like BaTiO3 with 3D CC structures effectively reduces current density and uniforms local electric field gradients as presented in Figure 3C [26]. Kim et al. proposed an approach involves adopting a multi-vacancy defect-rich carbon 3D CC design concept [27]. This design not only reduces current density, but also effectively mitigates the side reactions between lithium and the electrolyte via lowering the Fermi energy level (Figure 3D). However, it is worth pointing out that the challenge of inherent side reactions between lithium metal and the electrolyte persists in these 3D CC structures. Achieving a balance between reducing local current density via 3D CC design and avoiding the more electrode interface side reactions induced by the high surface area the electrode itself remains a challenge which requires further research.
2.3 The Design of Lithiophilic CC
A weaker affinity between the substrate and lithium leads to a higher deposition overpotential [49-51]. Broadly speaking, at room temperature, Cu exhibits poor affinity with lithium. The deposition of lithium onto Cu surface necessitates overcoming a high nucleation barrier [52, 53]. Alternatively, metals that alloy with lithium exhibit minimal nucleation barriers. For instance, Au not only reacts with Li to form multiple LixAu alloy phases as shown in Figure 4A, but also has a solubility zone of Au inside Li metal when the atomic ratio of Li is near 100%, giving a solubility of ~0.7 at.% [19]. The surface layer of this solid solution shares an identical crystal structure with pure lithium metal (βLi), thus acting as a substrate for subsequent lithium plating and effectively diminishing the nucleation resistance. Therefore, the modification of the CC by establishing a lithophilic layer represents a strategy to enhance the cycling stability and longevity of AFLMBs.
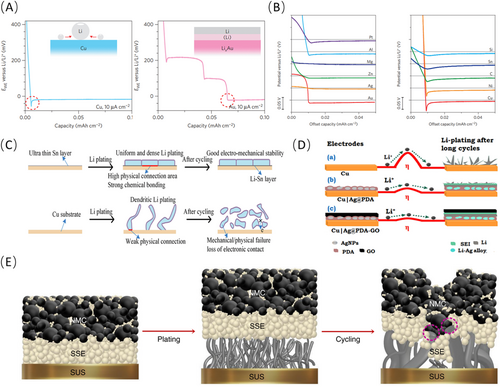
There are a variety of materials that have reduced nucleation overpotentials due to their significant lithophilicity as outlined in Figure 4B [58]. Researchers have strategically deposited Sn onto Cu surface to establish the affinity with lithium metal [59], facilitating the deposition of thicker lithium layers that adhere firmly to the substrate, contrasting sharply with lithium detachment observed on bare Cu surfaces as presented in Figure 4C [55]. Likewise, employing magnetron sputtering techniques to coat Cu surface with Zn yields a Cu99Zn layer [60], renowned for its exceptional wetting characteristics with molten lithium metal. To further enhance lithiophilicity, Lin et al. engineered a sophisticated multilayered metallic coating comprising Ga, In, and Sn, meticulously applied onto Cu CCs [61]. In stark contrast to pristine Cu CCs, these advanced multilayered metallic CCs instigate lithium-ion storage via alloy reactions, engendering the formation of an epitaxial induction layer, thereby markedly bolstering the cycling performance of AFLMBs. Moreover, numerous lithium alloys, including Li-Mg [62], Li-Pb [63], Li-Si [64], Li-Ag [65], Li-B [66], and Li-Al [67], have been leveraged as modified layer to mitigate nucleation overpotential and finely modulate the morphology of lithium plating.
In addition to metallic layers, various lithiophilic composite materials and compounds have been utilized in CCs. Multi-stage potential reduction typically refers to a process in which the potential is not decreased to the target value in a single step but rather is gradually lowered through multiple distinct stages. The composite component's multi-stage potential reduction enhances its efficiency and broadens its applicability in nucleation processes, prolonging battery cycle life. Wondimkun et al. employed a two-step spin-coating technique to fabricate lithiophilic hybrid CCs. This method significantly reduces the nucleation barrier for lithium by promoting uniform initial nucleation through the synergistic effects of lithiophilic Ag nanoparticles and polydopamine (Ag@PDA) [56]. Furthermore, an outer layer of graphene oxide (GO) acts as a buffer for lithium-ion flux in AFLMBs, which also enhances its wetting properties with the electrolyte. As a result, a lower nucleation overpotential is observed as evidenced in Figure 4D. In a related study, researchers coated Al2O3/polyacrylonitrile (PAN) composite layer (AOP) on Cu, which also can effectively reduce the nucleation potential of lithium [48].
This mechanism is also applicable to solid-state systems. By introducing metals with alloying characteristics as artificial interfacial layers, such as Li-Mg [68] and Li-Ag [69], the stability of the solid-solid interface can be enhanced. Lee's team reported an Ag-C nanocomposite layer as the anode of an anode-free solid-state lithium battery (AFSSLB) as demonstrated in Figure 4E, where Ag is soluble in lithium, facilitating uniform lithium deposition on the CC. Carbon plays the role of a separator, preventing contact reaction between lithium and the solid-state electrolyte (SSE), thus enhancing the durability of AFSSLBs [57]. During the procedure of plating, lithium is electrochemically inserted into graphite and subsequently reacts with Ag to form Li-Ag alloy [70]. At higher plating current densities, lithium embedding in graphite surpasses the reaction rate with Ag, delaying Li-Ag phase formation and resulting in more lithium metal plating on the CC. This work also offers crucial guidance for SSE interface engineering, and exploring similar metal composite layers with different alloys may have significantly different influences in enhancing the performance and stability of AFSSLBs.
3 Interface Stability Impact on Growth Morphology
- 1.
Initial Formation Phase: During the initial plating process, the fresh lithium metal reacts with the electrolyte to establish the initial SEI layer. According to the frontier orbital theory, when the anode's chemical potential exceeds the electrochemical stability window of the electrolyte, electrons migrate from the anode, leading to the reduction of electrolyte and the initial formation of SEI as depicted in Figure 5A [76, 80-82].
- 2.
Further Growth Stage: The SEI continues to evolve and adapt as the battery undergoes subsequent plating and stripping cycles. Throughout this process, lithium ions traverse the SEI layer, while the chemical composition and structure of the SEI evolve dynamically. The ongoing evolution of this phase significantly influences the cycling stability performance of the battery [83].
- 3.
Stabilization Phase: Once the interfacial phase layer reaches a certain thickness and stability, it enters an initial stable state, maintaining a relatively consistent composition and structure in the subsequent cycles [84].

From the above analysis, it is absolutely clear that the initial formation as well as the ongoing restructuring of SEI during lithium plating/stripping cycles, represents a significant factor contributing to the irreversible loss of active lithium and electrolyte depletion in anode-free systems [85]. Consequently, in this section, we primarily elucidate the scientific theoretical of the key properties of SEI, which influence the morphology of lithium deposition and the corresponding active lithium loss. The common optimization strategies toward SEI also are summarized by incorporating specific examples. In addition to SEI, other issues pertaining to the anode interface that affect the deposition of lithium metal have also been comprehensively summarized and analyzed.
3.1 Mechanical Stability of SEI
3.1.1 Restraining Crack via High E Inorganic Materials
Notably, critical stress refers to the stress level at which a material will fracture and fail due to crack propagation, and it is an important parameter for assessing the mechanical strength of a material. Griffith's theory of fracture provides an intuitive explanation of the relationship between critical stress and Young's modulus of a material. The higher Young's modulus, the higher the critical stress that the material can withstand. Consequently, in the AFLMB, when subjected to the same stress resulting from the deposition of lithium metal with equivalent capacity, SEI with a higher Young's modulus and surface energy is less prone to fracture, indicating better interface stability. It is worth pointing out that the ruptured SEI cannot inhibit lithium dendrite penetration, leading to uncontrolled lithium dendrite growth, exacerbating the loss of active lithium and triggering a series of side reactions.
Building on this foundation, researchers have recognized the significance of high Young's modulus for controlling lithium metal growth, leading to a focus on inorganic components within the SEI with high Young's modulus (LiF: 135.3 GPa; Li2O: 78 GPa; Li2CO3: 54.8 GPa) [77, 89]. Several strategies have been derived to enhance the contents of high Young's modulus components within the SEI, primarily through electrolyte modulation or artificial interface design.
LiF is an example of a compound with an exceptionally high Young's modulus. A LiF-rich SEI can be obtained by designing the liquid electrolyte with fluorinated solvents, such as fluorinated ethylene carbonate (FEC) [90-92]. It is noteworthy that, due to its high viscosity, FEC cannot serve as a primary solvent and is typically combined with diethyl carbonate (DEC) or ethyl methyl carbonate (EMC) [91, 93, 94]. Additionally, the concept of highly concentrated lithium fluoride salt has been widely explored aiming to induce F-rich SEIs in AFLMBs systems [95, 96]. The aggregation of salt anions raise their reduction potential, and when combined with a solvent with a low reduction potential, it leads to the preferential formation of high-quality SEIs derived from the salt as demonstrated in Figure 5B. For instance, LiF-rich SEIs can be successfully formed by reducing lithium bis(fluorosulfonyl)imide (LiFSI) salt through increased LiFSI concentration in the carbonate electrolyte [77], as demonstrated by the development of high-concentration double-salt electrolytes by Jeff Dahn's team (2 M lithium difluoro(oxalato)borate (LiDFOB) and 1.4 M lithium tetrafluoroborate (LiBF4) in DEC:FEC = 4:1) [97, 98]. Moreover, the introduction of certain additives (e.g., LiPO2F2) [99] into the electrolyte system can also lead to the formation of LiF-rich inorganic SEI components following reductive decomposition, further enhancing mechanical stability.
In addition to electrolyte design, the preparation of a functional SEI with high contents of LiF also can be achieved through ASEI design. However, fabricating dense films poses challenges because of the rigidity of materials. A common design strategy is the incorporation of high Young's modulus inorganic components into an organic matrix, which offers ease of handling and good film-forming properties. For instance, Li and his co-workers developed a layer of organic–inorganic composite ASEI on the surface of lithium metal through in situ polymerization and LiDFOB additive [100]. Upon contact with lithium metal, the internal LiDFOB of this ASEI undergoes decomposition, forming LiF-rich inorganic components near the surface of lithium metal, thereby enhancing the stability of the ASEI. Although this strategy is highly feasible, the introduction of organic materials actually hinders the formation of dense high-modulus films, limiting their ability to fully suppress dendrite growth. Encouragingly, Sun et al. developed a composite ASEI comprising LiF and lithium phosphorus nitride (LiPON) heterostructures via co-sputtering, resulting in a dense inorganic film with high fracture toughness as demonstrated in Figure 5C [78]. It is worth adding that other materials with high mechanical strength also have been applied in ASEI, such as the nano-diamond thin film with a modulus of 200 GPa [101]. However, it is essential to consider whether non-SEI components can effectively fulfill the ion transport function of the SEI when employing them in AFLMBs.
3.1.2 Utilizing Elastic Polymers to Accommodating Volume Deformation
In addition to the strategy of increasing the Young's modulus of the SEI to enhance its fracture toughness. The elasticity allows them to undergo reversible deformation and recover their original shape after the applied stress is removed [102-106].
It is worth noting that the mechanical stability of polymer can be significantly influenced by their crystallinity. Lower crystallinity typically implies higher amorphousness or disorder, which benefits the elasticity and deformability of the polymer material. Huang's team reported that the introduction of LiI as an additive into PEO-based electrolytes can induce cross-linking via iodide ion interaction with hydrogen atoms on PEO chain segments as depicted in Figure 5D, reducing crystallinity degree and increasing mechanical strength and toughness of the anode interface [79].
The rigid inorganic SEI with high fracture toughness and the flexible organic polymer SEI with excellent deformability are currently widely reported as effective in mitigating issues of battery short circuits and active lithium loss caused by lithium dendrites. It is worth discussing that both have their own advantages and disadvantages, necessitating comprehensive consideration in practical applications. The inorganic SEI, under the precondition of membrane integrity, can achieve the full protection of deposited active lithium metal. However, once localized stress exceeds its critical threshold due to uneven deposition of lithium metal, the membrane is highly prone to rupture, losing its ability to inhibit dendrites and secondary reactions. Furthermore, the inorganic SEI imposes strict requirements on membrane quality (thickness uniformity, compositional uniformity, and membrane density), with inherently high fabrication difficulty and relatively stringent preparation conditions, often requiring various physical thin-film deposition techniques such as atomic layer deposition and magnetron sputtering, leading to higher practical application costs. Conversely, artificial SEIs composed of various organic polymers possess the advantages of easy implementation and low cost. Nevertheless, due to the inherent properties of polymers, it is challenging to effectively block the penetration of electrolytes within the range of SEI thickness requirements (generally in the nanometer scale, to ensure efficient ion transport at the interface), thereby providing only partial insulation and protection against interfacial side reactions. Besides, the inorganic SEI films exhibit excellent electrochemical stability, while organic SEIs predominantly composed of polymers require consideration of their electrochemical stability window and compatibility with the current battery system.
3.2 The Ionic Conductivity of SEI
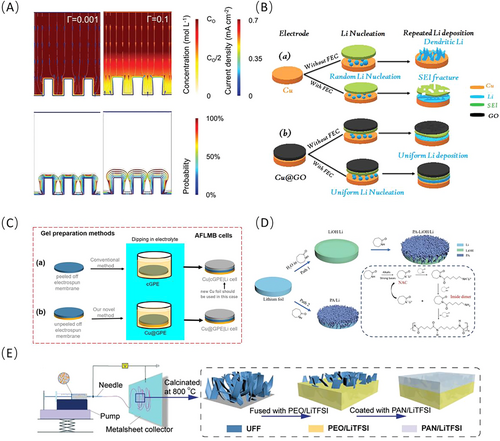
The ionic conductivity of the SEI profoundly influences lithium plating during charging. In an experiment conducted by Liu et al. [107], a parameter denoted as Γ was introduced, and Γ represents the ratio of the SEI ionic conductivity to that of the liquid electrolyte, with the latter set at 1.5 × 10−2 S cm−1 at room temperature. At very low conductivities (Γ = 0.001), lithium ions in the SEI are depleted, resulting in low current density on the substrate surface. As conductivity increases, lithium-ion depletion in the SEI decreases and the concentration gradient between the liquid electrolyte and SEI diminishes. Beyond Γ = 0.1, only little changes are observed, with high current densities concentrated at the top of the column. At low SEI ionic conductivities, electrodeposited lithium shows a tooth-like morphology with low electrodeposition probability as highlighted in Figure 6A. Conversely, at high conductivities (Γ ≥ 0.1), lithium exhibits a bulb-like morphology with higher electrodeposition probability. In summary, lithium-ion depletion probably leads to a reduction in current density on the substrate surface, as current density depends on the availability and mobility of lithium ions. Simply put, the fewer lithium ions available, the lower the current generated, resulting in reduced current density. These findings suggest that a higher ionic conductivity of the SEI is necessary to achieve efficient and uniform lithium plating on substrates covered with ASEI [15].
3.2.1 Inorganic-Dominated SEI Design
It seems that enhancing the proportion of high-conductivity components within the SEI is a paramount strategy for ensuring effective ion transport. Notably, the fast ion conductor Li3N (10−3 S cm−1) have garnered significant research interest as exceptional ion conductivity compared with the traditional SEI components (LiF = 10−11 S cm−1; Li2CO3 = 10−10 S cm−1; Li2O = 10−10 S cm−1) [14]. The rapid lithium conduction mechanism of Li3N involves three steps: (1) Adsorption: Initially, lithium ions are adsorbed onto the surface of Li3N. It exhibits high lithium-ion conductivity, a relatively high chemical potential, and a high surface activity with numerous active sites, making it a thermodynamically favorable process. As a result, the reaction can proceed rapidly. (2) Migration: The lithium ions then migrate within the Li3N material, moving along the channels of its lattice structure to other regions of the battery. (3) Release: Finally, the lithium ions are released from the Li3N material, entering the anode of the battery [111-113]. Presently, the primary method for constructing Li3N-rich SEIs involves the addition of N-containing additives, [114-116] such as KNO3 [117]. In addition to Li3N, Li3P also possesses high ionic conductivity (10−2 S cm−1) [118-120]. Nevertheless, the design of a Li3P-SEI for achieving stable lithium metal deposition in anode-free or LMB systems remains an unexplored research area, with no reported strategies available at present.
Regarding the issue of SEI conductivity, it appears that directly designing an ASEI may be simpler and more efficient than indirect design through the electrolyte. Besides the inorganic components from SEI, ASEIs composed of other type inorganic materials also have been widely utilized in LMBs, such as GO [121, 122] and mesoporous SiO2 [123]. Among them, GO stands out for its ultra-high ionic conductivity (10−2 S cm−1) [124] and electrical insulation [125], making it a suitable candidate for ASEIs in AFLMBs [108, 126]. In particular, the binder-free ultrathin GO developed by Wodimkun et al. offers a simple preparation method and efficient performance. By spin-coating GO onto Cu CCs, as depicted in Figure 6B, the lithium plating morphology becomes smooth, uniform, and dendrite-free. As a result, the capacity retention of full batteries assembled with NCM111 cathodes nearly double compared to bare Cu [108].
In addition to single material, composite structures are also considered important for effectively enhancing the conductivity of ASEI, which mainly includes inorganic-inorganic composites and organic-inorganic composites. Numerous theoretical studies have indicated significantly lower energy barriers for ion transport of the boundaries between inorganic species in the SEI, such as Li2O and LiF, compared to the single materials themselves. This suggests that the fabrication of mixed inorganic thin films with abundant interfaces holds potential as a strategy for obtaining high-conductivity ASEI. However, due to challenges related to film formation, inorganic composites require highly precise processing techniques, and there is currently limited research reported in this area. The artificial hybrid inorganic LiF-LiPON films developed by Cui's team [78] effectively reduce the ion transport barrier at the interface between the two phases, offering valuable insights for future research.
3.2.2 Organic-Dominated SEI Design
Compared with inorganic material, organic-based SEI layers offer several advantages, including tunable properties, facile deposition methods, and well compatibility [127, 128]. For example, electrostatically spun membranes based on polyvinylidene fluoride-hexafluoropropylene (PVDF-HFP) have been utilized as ASEI for use in anode-free systems as illustrated in Figure 6C. The formed pure β-phase PVDF-HFP with an impressive conductivity of 3.59 mS cm−1, in which the F elements are aligned toward the Cu surface, which lead to a uniform Li-ion flux due to the strong binding between Li and electronegative C-F functional groups [109].
Furthermore, the combination of organic coatings with inorganic layers can synergistically enhance their respective advantages. This hybrid approach can effectively address the challenges faced by pure organic layers in completely blocking the corrosion of lithium metal by the electrolyte. Polar groups, carrying a charge or dipole moment, interact with approaching lithium ions through electrostatic attraction or repulsion. This interaction guides the lithium ions toward or away from specific sites, thereby influencing their migration pathways and diffusion rates within the material. Cheng et al. [110] devised ASEI as an ordered bilayer structure, comprising an inner layer of inorganic LiOH and an outer layer of organic polyamide (PA). LiOH serves to prevent parasitic reactions between lithium and the electrolyte, while the abundant polar groups in PA regulate the Li+ flux on the electrode surface, thereby facilitating dendrite-free lithium plating as demonstrated in Figure 6D. The optimized LMB demonstrates plating/stripping stability of over 1000 hours at an ultra-high current density of 20 mA cm−2, realizing the vision of prolonged cycling and rapid charging for AFLMBs.
3.3 Ensuring Stability of Solid-Solid Contacts
The previous sections mainly discussed the crucial influence of SEI on lithium deposition morphology. However, in addition to that, unlike liquid-based battery systems [129], the mechanical stability of the SSE itself in solid-state batteries (SSBs) systems also affects the deposition morphology (dendrite) of lithium metal [130, 131]. It was proposed that the dendrite formation and the dendrite induced short-circuiting process in SSBs into two distinct stages: initiation and propagation [132]. The initiation of dendrites occurs through the deposition of lithium at near-interface pores (defects) connected to the lithium metal/solid electrolyte interface. During the process of lithium extrusion after pore filling, high current densities cause significant internal stress in lithium as a viscoplastic fluid, leading to the fracture of the electrolyte. High current densities can result in the rapid deposition of lithium on the electrode surface, often leading to inhomogeneous growth such as dendrites or other irregular structures. These uneven deposits cause localized volume expansion and deformation, generating internal stresses within the lithium metal. On the one hand, the expansion process of lithium dendrites involves the wedge opening of the SSE by a growing dendrite from the tail of the dendritic crack during deposition. The initiation of dendrites depends on the local fracture strength of the grain boundaries in the SSE, the size and distribution density of the pores, and the current density. On the other hand, the expansion process of dendrites is influenced by the macroscopic fracture toughness of the SSE, the distribution of dendrites within cracks, the current density, and the areal capacity during the charging process [133]. Thus, the mechanical stability of the SSE itself plays a crucial role in the growth behavior of dendrites in SSBs.
Combining inorganic ceramic electrolyte and polymer electrolyte composites has been proven to be an effective approach for enhancing mechanical stability [134]. Oxide garnet (Li6.75La3Zr1.75Ta0.25O12, LLZTO) was coated with a polymer electrolyte (LLZTO/PEO-CPE) [135] onto the cathode surface via spin-coating. Additionally, a composite SSE prepared by adding the sulfide electrolyte (Li6PS5Cl, LPSCl) with a polyvinylidene fluoride (PVDF) binder and LiF salt additives to a succinonitrile (SN) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) eutectic solution to overcome void presence in LPSCl [136]. In addition to simple blending, performing detailed structural design of composite materials helps fully leverage their advantages. Designing the composite electrolyte into a bilayer or 3D porous structure can leverage both polymer electrolyte and inorganic ceramic electrolyte advantages. Gong et al. proposed an ultrathin and lightweight bilayer SSE, where the ceramic filler enhances mechanical strength while the bilayer polymer electrolyte stabilizing lithium plating and high-voltage cathodes, enhancing ionic conductivity and regulating lithium plating [137]. In summary, composite SSE development provides vital support for realizing AFSSLBs. By combining inorganic ceramic electrolytes and organic polymer electrolytes and designing them into bilayer and 3D porous structures, the limitations of a single SSE can be overcome, achieving excellent ionic conductivity and mechanical properties as illustrated in Figure 6E. As of present, the development of flexible electrolyte membranes is approaching industrial levels, yet there still exists significant room for improvement and optimization in terms of their mechanical strength, ion conductivity, and electrochemical stability.
4 Testing Protocol Changes
In addition to the interface engineering and CC modification discussed previously, various testing conditions can significantly influence the cycling performance of AFLMBs [14, 85, 138], such as applied pressure [139], charge/discharge rate, and cut-off voltage exert profound effects [140]. A comprehensive delineation of both external and internal testing conditions is imperative for optimizing AFLMB performance.
4.1 Mechanical External Pressure
In the practical application of AFLMBs, the influence of pressure on electrochemical properties warrants consideration [139, 141]. During battery cycling, from the perspective of anode, factors that predominantly influence the variation of internal pressure encompass the reversible expansion induced by lithium plating and stripping during the cycling process, as well as the irreversible expansion arising from SEI growth and the accumulation of dead lithium. Remarkably, substantial research has demonstrated that the performance of batteries which is closely related to the lithium deposition behavior can be modulated by controlling the applied pressure. Moreover, the magnitude and uniformity of mechanical pressure markedly affect the deposition morphology and distribution of the lithium metal on the anode side, thereby determining the lithium loss in the battery [142]. Given that there exist certain distinctions between solid-state and liquid-state systems, this section will delineate the influence mechanisms of pressure separately in these two systems.
4.1.1 Liquid Electrolyte Based AFLMBs
It is clearly reported that the uniformity of mechanical pressure applied to the liquid electrolyte based AFLMBs battery significantly influence the distribution and morphology of lithium deposition. Taking coin cell as an example, when a single spacer is used and the internal pressure is non-uniform, the lithium metal tends to deposited in the central region of the Cu CC [139]. However, by using two spacers to improve the uniformity of pressure, a uniform deposition morphology distributed on the surface of the Cu foil can be achieved [142]. The phenomenon of pressure affecting lithium distribution has also been demonstrated in pouch-cells. It was observed that Li-ions (from cathode) tend to electrochemically deposit in the central region of anode during the charging process, leading to a faster increase in the spontaneous pressure generation rate in the central region. Ideally, the electrochemical deposition of Li (from cathode) should follow a process of uniform distribution. However, influenced by pressure, some Li+ that should have deposited at the edges now bypass to the central region of the anode and deposit there, resulting in a thicker deposition layer covering the central region of anode as demonstrated in Figure 7A [143]. Researchers also have conducted in-depth analyses based on solid-state nuclear magnetic resonance (NMR) techniques and found that the formation of dead lithium at low pressure is primarily associated with dendritic lithium. On the other hand, at high pressure, the formation of dead lithium may be related to mossy lithium morphology. This reveals the differences in the evolution of dead lithium in LMBs under different pressure conditions and sheds light on the reasons for the variation in cycling performance between high and low pressures [147]. A comprehensive analysis suggests that increasing the pressure aids in enhancing deposition compactness can reduce associated lithium losses and optimized the cycling performances [148]. As empirically demonstrated in practical pouch LMBs, the performance of these batteries improve gradually as the initial average mechanical pressure increases from 75 to 2200 kPa [149]. It is noteworthy that, as reported, higher pressure does not necessarily equate to superior performance. Excessively high pressure can instead lead to the deterioration of battery performance. This phenomenon may be attributed to variations in the amounts of electrolyte retained within the electrodes under elevated pressures or inducing-deformation of the polymer separator [142].
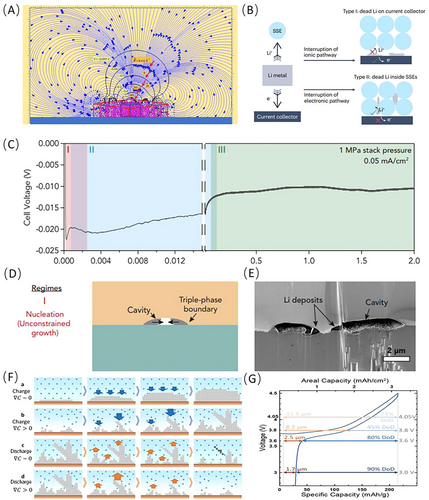
4.1.2 Solid-State AFLMBs
Furthermore, it is worth noting that the importance of pressure in solid-state lithium metal systems should not be underestimated, as it significantly influences lithium loss behavior. The dead lithium on the anode of SSBs primarily consists of two types: the dead lithium that remains on the CC, which losses ion transport pathways due to poor interface contact, and the dead lithium that remains within the electrolyte due to the loss of electronic transport pathways. The former is associated with poor interface contact that occurs under low pressure conditions, while the latter is closely related to lithium metal creep caused by excessive pressure and inappropriate pressure applied to the electrolyte as depicted in Figure 7B [144]. It is noteworthy that the applied preparation and stacking pressures on a specific SSB often vary depending on the type of SSE. The preparation pressure for low modulus halide SSEs (typically around 200–400 MPa) is significantly higher than that for sulfide and oxide SSEs (generally below 200 MPa) and brittle oxide SSEs in SSBs (usually below 10 MPa). The density of the SSE obtained under different pressure conditions directly affects the evolution of dead lithium in lithium metal.
In SSBs, the magnitude and spatial uniformity of the applied stacking pressure play a crucial role in determining the lithium deposition morphology, similar to the effects seen in liquid-state systems. Differently, studies have shown that the deposition morphology of lithium without stacking pressure mainly exhibits isolated island-like structures. During the deposition process, these island-like structures maintain an approximately spherical shape, with both the diameter and height increasing. In contrast, lithium metal at 5 MPa initially grows predominantly laterally along the surface and eventually grows upwards, exhibiting more interconnected protrusions in a larger proportion of the CC area, with a flatter appearance in the top region as illustrated in Figure 7C–E [145].
Based on the above analysis, it is abundant clear that pressure serves as a crucial bridge connecting the metal deposition morphology and the performance of AFLMBs. Controlling the pressure is essential for optimizing battery cycling. It is worth noting that considering the different optimal pressures reported for different electrolyte types, as well as the varying stacking pressures suitable for different types of SSEs, pressure regulation should be based on the realistic system.
4.2 Rate of Charge and Discharge
The cycling performance of AFLMB exhibits a significant correlation with the charge-discharge rate. Professor Dahn conducted a comparative study on the performance of AFLMB under different charge-discharge cycling conditions. The study concluded that the relative rate of charge and discharge is more important than the absolute current density. During lithium plating, the concentration gradient at the anode is directly proportional to the applied current. Therefore, under fast charging conditions, significant concentration gradients are formed on the lithium surface. Additionally, the unavoidable convection further interferes with ion diffusion, resulting in uneven distribution of lithium ions and the non-uniform localized current density. This leads to rapid growth of high surface area lithium. On the contrary, lower current density results in the formation of denser, low surface area lithium morphologies as shown in Figure 7F [146]. During lithium stripping, lithium in an environment with extremely low concentration gradient is uniformly stripped from the surface. It worth noting that the isolated inactive lithium may be formed due to the stripping of the bottom of lithium during the uniform stripping process. In contrast, at faster discharge rates, significant concentration gradients occur on the lithium surface. These concentration gradients increase the current density at the tips of lithium, leading to preferential stripping of the tips. This helps to remove uneven lithium deposition and results in a relatively uniform surface at the end of discharge. It can be concluded that faster discharge is advantageous for removing uneven and porous lithium deposition, while slow charging promotes the formation of ideal morphologies.
Therefore, the use of the asymmetric slower charge protocol was identified as an optimal strategy for reducing lithium loss in each cycle. It has been successfully demonstrated that the battery employing an asymmetric slow charging scheme exhibited the better performance, which retained 80% of its capacity after 80 cycles. Conversely, the symmetric charging and discharging scheme has 80% capacity retention only after 50 cycles.
4.3 Depth of Discharge and Integrated Optimization
In AFLMBs, the lithium source comes solely from the cathode, and there is no additional lithium reservoir. As a result, the lithium loss during the cycling process cannot be replenished promptly, leading to poorer cycling stability compared to LMBs. To address this issue, researchers have proposed a solution to create an additional lithium reservoir by controlling the discharge voltage. Figure 7G illustrates the relationship between discharge-cut-off voltage and the thickness of the created lithium reservoir [146]. While this approach can improve cycling performance, the limited discharge depth also decreases the energy density of the battery due to the restricted capacity of cycled lithium. Therefore, it is important to consider the trade-off between improved cycling stability and reduced energy density via this protocol. Given that, Dahn's team suggests alternating high-depth discharge cycles with low-depth discharge cycles to enhance energy density while retaining a portion of the lithium reservoir, thereby ensuring long-term cycle stability. In this protocol, even batteries under low discharge depth conditions can maintain a high energy density of over 700 Wh L−1.
4.4 Temperature as Well as the Formation Process
It has been revealed that there are significant differences in the cycling performance of pouch cells within the same system at different temperatures. Taking NCM532/Cu foil (LiDFOB/LiBF4 dual lithium salt electrolyte) cells as examples, the capacity retention after 50 cycles at 40°C was notably higher than the value obtained after 30 cycles at 30°C [150]. This study highlights the crucial role of temperature in the cycling behavior of AFLMBs. Temperature directly influences the activation energy of chemical reactions occurring within the battery, including the energy barrier for lithium nucleation and the electrolyte reduction reaction involved in the formation of the SEI. Consequently, temperature has a direct impact on lithium deposition on the anode. Even the influence of temperature on the SEI formation stage can have a persistent effect on subsequent cycles. Research indicates that when operating at 20°C, cells without hot formation exhibit porous and irregular lithium metal deposition on the anode. In contrast, lithium deposition subjected to the cell after hot formation displays a smooth and tightly packed columnar morphology and maintain better cycling stability even in low-temperature environments in subsequent cycles [150].
When designing a test protocol for a new anode-free battery, it is imperative to integrate several factors such as cut-off voltage, operating pressure, and current density to optimize performance.
5 Summary and Perspective
Based on the above analysis, it can be concluded that the nucleation and growth of lithium metal at the anode interface in AFLMB significantly determine the active lithium loss, thereby affecting key performance metrics such as energy density and cycling stability. The nucleation of lithium metal is mainly influenced by the CC, while the subsequent growth is closely related to the interface properties and stability on the anode side.
- a.
Designing the CC structure to reduce local current density.
- b.
Directly constructing a lithium-affinitive interface.
- a.
Building a high Young's modulus SEI to restrain crack formation.
- b.
Designing an elastic SEI to accommodate lithium deposition-induced deformation.
- c.
Developing a high conductivity SEI to achieve low surface area deposition morphology.
- d.
Constructing a stable electrolyte-electrode contact interface to minimize active lithium loss.
Future directions and industrialization process:
Future research and development on AFLMBs will prioritize achieving higher energy densities, improved cycle stability, enhanced safety, simplified assembly, and advanced production technologies alongside material selection. Consequently, the design of CCs and electrolytes remains a focal point of ongoing research.
The current strategies for CC design primarily involve material selection and optimization, as well as surface modification and interface engineering. While these approaches can significantly enhance performance, they also inevitably increase costs and complicate manufacturability. Therefore, it is crucial to consider the commercial viability of these advancements. Efforts should be directed toward reducing research and development costs, optimizing material synthesis and processing techniques, and ensuring that the modified collectors are feasible for large-scale industrial applications. In terms of SSEs, inorganic composite electrolytes are widely employed to address the challenges posed by unstable interfacial chemistry and volume changes at the solid-solid interface, owing to their excellent ionic conductivity and robust mechanical properties. However, the fabrication of high-quality inorganic composite electrolytes and the development of methods to produce them in thin-film form remain critical areas of focus for future research. Leveraging artificial intelligence (AI) for the screening of battery materials is poised to become a critical trend in the evolution of AFLMBs. By compiling comprehensive data on electrolyte properties—such as ionic conductivity, electrochemical stability window, and viscosity—unique models can be developed to optimize electrolytes. Advanced techniques, including the use of encoder-decoder frameworks, can compress high-dimensional discrete chemical spaces into low-dimensional continuous representations, thereby streamlining the selection of optimal candidate materials. Such methodologies, which are already widely employed in biological and medical small molecule screening, can be adapted for AFLMB development. These technological advancements not only contribute to improved energy density and cycle life but also hasten the commercialization process, enabling earlier real-world applications. In addition, we summarize the importance of various testing conditions for optimizing the lithium metal deposition morphology on the anode side. In summary, this review provides a systematic and organized presentation of the current key strategies and approaches for optimizing anode-free batteries by summarizing the scientific mechanisms related to various factors influencing lithium metal deposition and nucleation, along with typical case analyses. AFLMBs face numerous opportunities and challenges and represent an important opportunity for the development of advanced energy storage technologies. This article aims to provide ideas and inspiration for the development of next-generation AFLMBs with high energy density, long cycling stability, and high safety.
Acknowledgments
This research was supported by the Finance Science and Technology Project of the National Key R&D Program of China (No. 2022YFB3803400), National Natural Science Foundation of China (22179135), Postdoctoral Fellowship Program of CPSF (GZC20232806), Shandong Provincial Natural Science Foundation (ZR2024QB297, ZR2023JQ003, ZR2022ZD11), Qingdao Postdoctoral Funding Program (QDBSH20240102103), Taishan Scholars of Shandong Province (No. ts201511063), Taishan Scholars Program for Young Expert of Shandong Province (tsqn202103145), and Qingdao New Energy Shandong Laboratory (QIBEBT/SEI/QNESLS202304).
Conflicts of Interest
The authors declare no conflicts of interest.




