Amorphous Metal Metaphosphate for Oxygen Reduction
Min Zhou, Jinghui Guo, and Ruihu Lu contributed equally.
ABSTRACT
Efficient and cost-effective catalysts for oxygen reduction reaction (ORR) are crucial for the commercialization of metal-air batteries. In this study, we utilized theoretical calculations to guide the material synthesis strategy for preparing catalysts. Using density functional theory (DFT) calculations, we systematically explored the ORR performance of metal metaphosphates (A-M(PO3)2, B-M(PO3)2, M = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn) with both amorphous and crystalline structures. Amorphous A-Mn(PO3)2 showed optimal adsorption energy and the lowest ORR overpotential of 0.32 eV. Phytic acid was employed as a phosphorus source, and the chelating structure of phytic acid molecules and metal ions was broken through the “metal ion pre-adsorption and spatial confinement strategy” of carbon materials with electron-rich centers. Following high-temperature calcination, we successfully prepared a series of amorphous metal metaphosphate composite catalysts for the first time. In 0.1 M KOH electrolyte, both amorphous Mn(PO3)2-C/C3N4/CQDs (carbon quantum dots) and Mn(PO3)2-C/C3N4/CNTs (carbon nanotubes) exhibited excellent ORR catalytic activity, with half-wave potentials of 0.85 V and 0.80 V, respectively. A linear correlation between theoretical overpotentials and experimental half-wave potentials was discovered through comparison. This work could open a new avenue to the discovery of highly efficient non-precious metal-based catalysts with amorphous structures.
1 Introduction
In light of the rapid depletion of fossil fuels alongside the escalating gravity of ecological predicaments, electrochemical energy storage and conversion technology emerges as a pivotal avenue towards fostering the advancement of environmentally sound energy apparatus [1-3]. Zinc-air batteries (ZABs) have gained significant attention in the past decade as the most promising candidates due to their low cost (10$ kW−1 h−1), high theoretical capacity (1086 Wh kg−1), and environmental friendliness [4-7]. However, the kinetics of the cathodic oxygen reduction reaction (ORR) are extremely slow, requiring expensive platinum (Pt) as a catalyst to promote the reaction, greatly increasing manufacturing costs and limiting the large-scale application of ZABs [8-10]. Therefore, there is an urgent need to develop alternatives to Pt-based catalysts that enhance intrinsic ORR activity, optimize structural characteristics, promote the accessibility of active sites, and minimize mass transfer effects [11-14].
Transition metal phosphates (TMPs) have attracted tremendous attention in metal-ion batteries and photo/electrocatalysis due to their high theoretical specific capacitance, chemical stability, and nontoxicity [15-20]. Compared to conventional oxide materials with octahedral geometry, TMPs have larger ligands and crystal structures with lower symmetry [21-23]. Phosphate/pyrophosphate compounds exhibit various geometries, such as tetrahedral (Td), octahedral (Oh), and trigonal bipyramidal (TBP). Their flexible coordination environment can induce structural distortion by changing local positions, facilitating the adsorption of O2/OH− species on active sites, stabilizing the state of transition metal ions, and ultimately enhancing catalytic activity during the reaction [24-26]. Kim et al. [27] reported four cobalt-based phosphates with various coordination, namely Li2CoP2O7, NaCoPO4, LiCoPO4, and Na2CoP2O7. Due to the surface reorganization by the pyrophosphate ligand and favorable binding with water molecules, Na2CoP2O7 exhibits the highest distorted tetragonal geometry. The flexible coordination of the phosphate group can stabilize the intermediate state of transition metals by easily altering its local position, ensuring the effective redox transformation of transition metals. Moreover, the phosphate group can act as a proton acceptor, facilitating the oxidation of transition metal atoms, and proton-coupled electron transfer has been reported in recent years. Zhou et al. [28] reported that phosphate groups could stabilize the Co–N centers and deliver protons to enable proton-coupled electron transfer, facilitating the ORR process. However, the most reported tetrahedral phosphate exhibits deficient electronic conductivity and activity, severely constraining its capacity for advancing catalytic efficacy.
Over the past decade, amorphous metal-based catalysts have emerged as efficient, low-cost, and environmentally friendly catalytic materials with great potential for practical applications [29, 30]. These materials exhibit superior electrocatalytic behavior, which can be attributed to their unique structural features. First, the absence of valence-state restrictions allows for a wide range of catalyst compositions to be selected and continuously adjusted to optimize the electronic structure of the catalytic active center [31]. Second, the inherent disorder in the structural arrangement, small volume expansion, abundant defects and vacancies, and additional active sites are highly favorable for rapid ion diffusion, which greatly improves electrocatalytic performance [32]. Thirdly, highly unsaturated surface atomic coordination endows amorphous catalysts with stronger activation ability toward reaction molecules and a higher density of active sites, resulting in better catalytic activity and selectivity [33]. Finally, the structural flexibility advantage allows amorphous materials to adapt to high-activity crystalline phases during electrocatalysis and self-regulate according to electrocatalytic conditions [34]. This provides a means for volume and surface-restricted electrocatalysis, while crystalline materials only provide space for surface catalysis. In summary, the unique structural features of amorphous metal-based catalysts make them a promising candidate for various electrocatalytic applications. Currently, due to the high cation dissolution rate, amorphous phases still exhibit inherent poor conductivity and low stability. In addition, the synthesis of metastable amorphous materials often involves methods such as rapid quenching, physical/chemical vapor deposition, etc. Therefore, there are still significant challenges in achieving low-cost and controllable synthesis of amorphous metal phosphates.
Here, we utilized theoretical calculations to guide the material synthesis strategy for preparing catalysts. Using density functional theory (DFT) calculations, we systematically explored the ORR performance of metal metaphosphates (A-M(PO3)2, B-M(PO3)2, M = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn) with both amorphous and crystalline structures. Amorphous A-Mn(PO3)2 showed optimal adsorption energy and lowest ORR overpotential of 0.32 eV. Phytic acid (PA) was employed as a phosphorus source, and the chelating structure of phytic acid molecules and metal ions was broken through the “metal ion pre-adsorption and spatial confinement strategy” of carbon materials with electron-rich centers. Following high-temperature calcination, we have successfully synthesized a series of amorphous metal metaphosphate composite catalysts (M(PO3)2-C/C3N4/CNTs, M = Mn, Co, Fe, and Zn) with zero-dimensional/one-dimensional/two-dimensional (0D/1D/2D) multiscale structures using metal ion pre-adsorption and spatial confinement strategies of electron-rich carbon materials. The introduced carbon materials can be 0D carbon quantum dots (CQDs), 1D carbon nanotubes (CNTs), 2D graphene oxide (GO), 2D C3N4, or 3D carbon spheres (CS). In 0.1 M KOH electrolyte, both amorphous Mn(PO3)2-C/C3N4/CQDs and Mn(PO3)2-C/C3N4/CNTs exhibited excellent ORR catalytic activity, with half-wave potentials of 0.85 V and 0.80 V, respectively. A linear correlation between theoretical overpotentials and experimental half-wave potentials was discovered through comparison. As the air electrode in ZABs, it exhibited higher power density and superior long-term stability compared to commercial Pt/C. This work could open a new avenue to the discovery of highly efficient non-precious metal-based catalysts with amorphous structures.
2 Results and Discussion
2.1 Theoretical Calculations
We investigated the effect of amorphization of metal metaphosphate on improving ORR activity through first-principle calculates using DFT, as shown in Figure 1A. We first modeled ten kinds of bulk and amorphous metaphosphate with varied 3d transition metals from Sc to Zn (Supporting Information: Table S1) and optimized them. The d-band center, developed by Nørskov et al., is a useful descriptor to evaluate the reactivity of transition metals [35, 36]. The d-band centers of transition metals in metaphosphate were calculated in Figure 1B. When moving further to the right in the element period, the transitional metals in bulk metaphosphate exhibit a downshifted trend, indicating lower reactivity from Sc to Zn. After amorphization, the same transition metals showed a drop in the d-band center and consequently weaker interaction with adsorbates. This suggests that amorphization can be a general regulation to tailor the adsorption ability for catalyst design. Furthermore, we performed the ORR process on transition metal sites according to the computational hydrogen electrode (CHE) models (Supporting Information: Figures S1–S2) [37]. Among them, A-Mn(PO3)2 performed the lowest energy barrier (0.32 eV) for the transformation from *OH to H2O. We observed a correlation between OH adsorption and d-electrons, with the formula of y = 0.06d2 + 0.84d + 3.45 (Figure 1C). This result was corroborated by the observation that amorphous Mn metaphosphate endows the lowest value of OH adsorption, close to 1.23 eV, reaching an optimal theoretical OH adsorption. Meanwhile, we calculated the free energy change alone in the ORR process and built a volcano-shaped relation between ΔG*OOH, ΔG*OH, and ORR activity in Figure 1D. We found a linear relation between ΔG*OOH and ΔG*OH: ΔG*OOH = 0.58ΔG*OH + 3.38 with an R-square of 0.76, similar to the scaling relation on metal oxides. Among all metal sites, A-Mn(PO3)2 possesses the highest ORR activity, consistent with the prediction of the d-band center in Figure 1C.
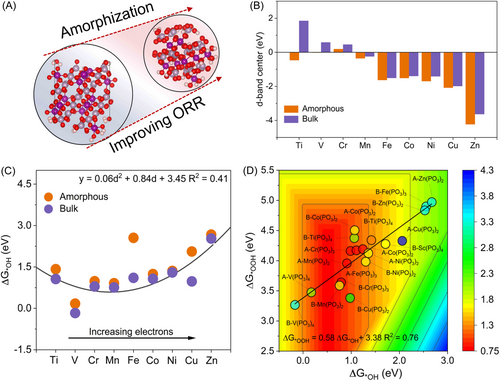
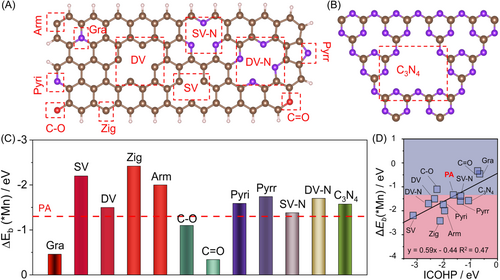
Based on theoretical calculations and predicted outcomes, we anticipate synthesizing amorphous metaphosphate by designing a rational experimental plan and verifying its ORR catalytic activity. PA is a natural organic phosphorus compound and a significant source of phosphoric acid, containing six phosphorus oxygen groups [38-40]. It can form relatively stable metal phytate complexes with metal ions, and crystalline metal pyrophosphates can be obtained by thermally decomposing metal phytate salts with regular coordination structures [41, 42]. In this study, we propose using a carbon material substrate with negative electron centers to pre-adsorb metal ions on its surface, thereby modifying the regular coordination structure with phytic acid. Meanwhile, larger carbon materials may disrupt the original structure over a long range, potentially resulting in the formation of irregular metal phytates. To verify this hypothesis, we performed theoretical calculations to analyze the possible adsorption active sites at the strong-functional groups (SFP) on the carbon material substrate, including single vacancy (SV), di-vacancy (DV), zigzag edges (Zig), armchair edge (Arm), single vacancy nitrogen (SV-N), di-vacancy nitrogen (DV-N), pyridine nitrogen (Pyri), pyrrole nitrogen (Pyrr), graphitic nitrogen (Gra), C–O, C=O, and C3N4 edge site defects (Figure 2A,B). The results showed that, except for Gra, C–O, and C=O, the interaction between Mn atoms and SFP was stronger than that between Mn atoms and PA (Figure 2C). Specifically, the C- and N-functional groups tended to bind strongly with Mn atoms. Furthermore, Figure 2D demonstrated a high correlation between the binding energy of Mn atoms (ΔE*Mn) and the integrated-crystal orbital Hamilton population (ICOHP) between Mn and SFP adsorption sites, providing further evidence of the strong interaction between Mn atoms and C- and N-functional groups. Based on the above results, we also constructed a model of A-Mn(PO3)2 and B-Mn(PO3)2 on C3N4 (Supporting Information: Figure S3). The adsorption energies of A-Mn(PO3)2 and B-Mn(PO3)2 on C3N4 were computed as −4.64 and −2.65 eV, confirming the superior activity of A-Mn(PO3)2 over B-Mn(PO3)2 (Supporting Information: Figure S4). We further calculated different charge density distributions to verify the electron transfer from the A-Mn(PO3)2 and B-Mn(PO3)2 to C3N4 (Supporting Information: Figure S5). An accumulation of electron density was observed at the A-Mn(PO3)2, coupled with a depletion of electron density in B-Mn(PO3)2. It evidenced the strong electronic interaction at the interface, enhancing the structure stability of the support catalysts. These theoretical calculation results suggest that the introduction of carbon materials can pre-adsorb metal ions on their surfaces, creating a spatial confinement effect that influences the coordination structure of metal ions with phytic acid and leads to the formation of irregular metal phytate salts, which may ultimately result in the formation of amorphous pyrophosphates.
2.2 Synthesis and Structural Characterization
Based on the theoretical calculation results mentioned above, we used phytic acid as a source of phosphorus. To disrupt the regular coordination structure of metal phytates, we formed a spatial confinement effect by pre-adsorbing metal ions on carbon materials. Finally, we prepared metal metaphosphate composites with amorphous structures after freeze-drying and pyrolyzing. In the synthesis procedure of the amorphous Mn(PO3)2-C/C3N4/CNTs catalyst, as shown in Figure 3A, 1D CNTs and 2D C3N4 were chosen as carbon carriers for metal ion adsorption. The phase and crystal structure of Mn(PO3)2-C/C3N4/CNTs were investigated by X-ray powder diffraction (XRD), as presented in Figure 3B. The XRD pattern shows two broad peaks at 26° and 44°, which correspond to the (002) and (101) planes of graphitic carbon, respectively. No other sharp diffraction peaks are observed, indicating that the sample is predominantly amorphous in structure. The scanning and transmission electron microscopy (SEM and TEM) images confirm that Mn(PO3)2-C/C3N4/CNTs possess a 3D porous network structure (Figure 3C,D and Supporting Information: Figure S6). The hybrid material exhibits a coexistence of closely interconnected 2D g-C3N4 with 1D CNTs, which not only facilitates contact with the electrolyte but also accelerates charge/mass transport during the catalytic reaction.
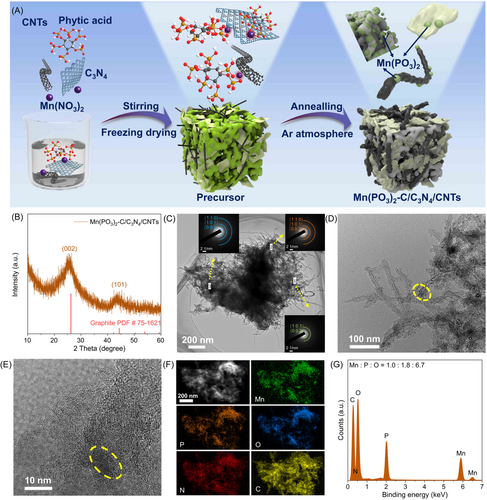
Crystallinity can be further confirmed through the selected area electron diffraction (SAED) patterns collected under TEM. Figure 3C (inset) shows that the electron diffraction (SAED) patterns from three different regions present the same broadened concentric rings, indicating that the Mn(PO3)2 in the hybrid exists in an amorphous phase. The distinguishable diffraction rings correspond to the (002), (101), and (110) planes of the graphitic carbon phase, which is consistent with the XRD result. Additionally, the high-resolution TEM (HRTEM) image in Figure 3E shows that Mn(PO3)2 nanoparticles with a size of 10 nm present no obvious crystal lattice. The high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image and corresponding elemental mapping images in Figure 3F further confirm the uniform distribution of Mn, P, and O elements on the carbonaceous framework. Additionally, the energy dispersive X-ray (EDX) spectrum reveals an atomic ratio of 1:1.8:6.7 for Mn, P, and O, respectively, which is highly consistent with the theoretical value. Moreover, we optimize the Mn(PO3)2 loading by varying the concentration of manganese nitrates (0.5, 1, 1.5, and 2 mmol). As the feed ratio of manganese salts increased, the crystal structure transitioned from amorphous to crystalline from the XRD pattern in Supporting Information: Figure S7, which could be ascribed to the limited adsorption sites on the carbon materials. In comparison, Mn(PO3)2-C sample can be prepared without added carbon materials by forming manganese phytate complexes using manganese ions, followed by calcination treatment, with the carbon content derived from the pyrolysis of PA. As shown in Supporting Information: Figure S8a, the diffraction peaks located at 20.4°, 27.5°, and 29.1° are assigned to the , (310), and planes of the monoclinic Mn(PO3)2 phase (JCPDF No. 00-029-0892), respectively. The HRTEM image shown in Supporting Information: Figure S8f displays clear fringes with an interplanar spacing of 0.186 nm, which corresponds to the () planes of Mn(PO3)2. The aforementioned results indicate that Mn(PO3)2-C samples, prepared without the addition of carbon materials, possess a monoclinic crystal structure. To validate the pre-adsorption strategy of carbon materials, we investigated other carbon materials with varying structural scales, including 0D CQDs, 1D CNTs, 2D GO, 2D C3N4, and 3D CS. The phases and morphologies of the synthesized samples are presented in Supporting Information: Figures S9–S14, which show an amorphous or extremely low crystallinity structure. Furthermore, to provide additional evidence of the significant influence of pre-adsorption of carbon carriers on the crystallinity of metal metaphosphates, we incorporated 3D polystyrene (PS) microspheres with a diameter similar to that of 3D carbon spheres as additive materials during sample synthesis. Due to the presence of a benzene ring structure in 3D PS microspheres, these polymers cannot adsorb manganese ions on their surface. As a result, the regular coordination structure of manganese phytate remains intact and cannot be disrupted. The synthesized Mn(PO3)2-PS exhibits a typical monoclinic crystal structure (Supporting Information: Figure S11a3). To demonstrate the universality of the construction strategy, we synthesized Co(PO3)2-C/C3N4/CNTs, Fe(PO3)2-C/C3N4/CNTs, and Zn(PO3)2-C/C3N4/CNTs samples with an amorphous structure using a similar synthesis process (Supporting Information: Figure S14).
The chemical composition of prepared samples was investigated using Fourier transform infrared (FTIR). As presented in Figure 4A, the distinct peaks at 578 cm−1 and 733 cm−1 were attributed to the phosphate group (PO3−) and the Mn−O stretching mode, respectively. Additionally, two absorption bands at 1099 and 1298 cm−1 were assigned to the P=O asymmetric and symmetric stretching vibration of the PO3− group, respectively [43, 44]. The wide band at around 3435 cm−1 was allocated to the O–H molecular stretching vibration mode, while the vibration band at 1580 cm−1 was ascribed to the H–O–H bending mode [45]. The FTIR spectrum of Mn(PO3)2-C/C3N4/CNTs exhibits more broad absorption peaks in comparison to that of Mn(PO3)2-C, which can be attributed to differences in their crystal structures. Figure 4B displays the Raman spectra featuring the characteristic bands of Mn(PO3)2 at 441.5 and 1159.0 cm−1, which are associated with the symmetric stretching vibration of bridge oxygen and the symmetric stretching vibration of PO3−, respectively [46, 47]. In addition, two prominent peaks at 1336 and 1590 cm−1 are observed in the Raman spectra, corresponding to the defect carbon (D) and sp2-hybridized graphitized carbon (G), respectively [48, 49]. The decrease in the area ratio of ID/IG in Mn(PO3)2-C/C3N4/CNTs (1.81) compared to Mn(PO3)2-C (2.19) can be attributed to the presence of additional CNTs and C3N4, which increase the proportion of graphitized carbon. This increase in graphitization enhances the conductivity and stability of the material during the catalytic process. To further understand the mechanism of Mn adsorption, we characterized the inductively coupled plasma-atomic emission spectroscopy (ICP-AES) of composite material named Mn/C3N4/CNTs, which contains C3N4 and CNTs with 1 mmol of MnCl2 (Supporting information: Table S2). By comparing the Mn content in Mn/C3N4/CNTs with other related samples such as Mn(PO3)2-C/C3N4, Mn(PO3)2-C/CNTs, and Mn(PO3)2-C/C3N4/CNTs, it is observed that Mn(PO3)2-C/C3N4/CNTs exhibits the highest Mn content, followed by Mn/C3N4/CNTs, Mn(PO3)2-C/CNTs, Mn(PO3)2-C/C3N4. It was indicated that the strong-functional groups on the carbon materials substrate could provide favorable sites for the adsorption of metal ions. This finding aligns with theoretical calculation results, reinforcing the experimental observations and providing a comprehensive understanding of the mechanisms underlying Mn adsorption in these composite materials.
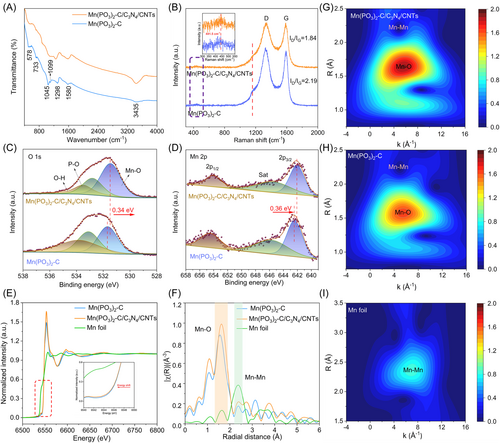
X-ray photoelectron spectroscopy (XPS) is employed to investigate the chemical composition and valence states of the prepared samples. Figure 4C shows the high-resolution O 1s spectra, with peaks at 531.4, 532.8, and 533.5 eV attributed to O–Mn, O–P, and adsorbed OH, respectively [50]. The binding energy of O–Mn bond in Mn(PO3)2-C/C3N4/CNTs is negatively shifted by approximately 0.34 eV compared to that of Mn(PO3)2-C. Additionally, the Mn 2p spectra in Figure 4D show that the binding energies of Mn 2p3/2 and Mn 2p1/2 in Mn(PO3)2-C/C3N4/CNTs are also negatively shifted by approximately 0.36 eV, as compared to those in Mn(PO3)2-C. As shown in Supporting Information: Figure S15, the phenomenon of binding energy negative shift was also observed in other synthesized amorphous samples, such as Mn(PO3)2-C/C3N4 and Mn(PO3)2-C/CNTs. These results can be attributed to the irregular atomic arrangements in amorphous structures, which result in an unsaturated coordination structure and lower binding energy. In Supporting Information: Figure S16, the peaks of N 1s spectrum in Mn(PO3)2-C/C3N4/CNTs at 398.2 eV and 401.2 eV are attributed to pyrrolic-N and graphitic-N, respectively [51]. The N species, which originate from C3N4, can act as the active center for ORR. The Brunner–Emmet–Teller (BET) surface area of Mn(PO3)2-C/C3N4/CNTs is as high as 412.8 m2 g−1 (Supporting Information: Figure S17). The large surface area of the catalyst not only expose more active sites but also promote contact between intermediates and the catalytic surface, resulting in a positive impact on the ORR process [52].
To gain more information about the electronic structure and local coordination environment in prepared samples, X-ray absorption spectroscopy (XAS) was performed at the Mn K edges. Figure 4E shows the Mn K-edge X-ray absorption near edge structure (XANES) spectra of Mn(PO3)2-C/C3N4/CNTs, Mn(PO3)2-C, along with Mn foil. The white line peak of Mn(PO3)2-C/C3N4/CNTs is shifted positively by 6.78 eV compared to Mn foil, and it coincides with that of Mn(PO3)2-C, strongly indicating that the Mn in Mn(PO3)2-C/C3N4/CNTs is primarily in the +2 valence state. Typically, the pre-edge peak is attributed to the electric dipole transition from the 1s to 3d states. Figure 4F presents the Fourier transformed (FT) extended X-ray absorption fine structure (EXAFS) spectra in R space at the Mn K-edge. For Mn foil, it exhibits Mn–Mn coordination with a peak at about 2.3 Å, corresponding to the Mn–Mn bond [53, 54]. For Mn(PO3)2-C and Mn(PO3)2-C/C3N4/CNTs, a dominant peak attributed to the Mn-O bond scattering in the first coordination shell is observed at almost 1.6 Å, while a very weak peak of the Mn–Mn bond in the second coordination shell is detected at about 2.4 Å [55]. The Mn–O bond length in amorphous Mn(PO3)2-C/C3N4/CNTs is slightly longer than that in crystalline Mn(PO3)2-C, which is attributed to the loose packing characteristics of the amorphous structure, similar to previous results [56]. To provide additional evidence for the aforementioned results, Figure 4G–I displays the wavelet transforms (WT) of the EXAFS, which can distinguish high-resolution backscattered atoms in k and R spaces. As shown in Figure 4G,H, both Mn(PO3)2-C/C3N4/CNTs and Mn(PO3)2-C exhibit a single intensity maximum at around 5.4 Å−1, confirming Mn-O coordination. Furthermore, the maximum intensity of Mn foil is observed near 6.9 Å−1, which is consistent with Mn–Mn coordination [54]. The XAS measurements indicate that the manganese atoms in Mn(PO3)2-C/C3N4/CNTs are bonded with oxygen and exhibit high dispersion, suggesting an amorphous nature with a short-range ordered and long-range disordered structure.
2.3 Electrocatalytic Performance Evaluation
The ORR electrocatalytic performances of Mn(PO3)2-C/C3N4/CNTs are first evaluated by linear sweep voltammetry (LSV) with a rotating disk electrode (RDE) in O2-saturated 0.1 M KOH solution. Figure 5A compares the LSV curves of Mn(PO3)2-C/C3N4/CNTs, Mn(PO3)2-C/CNTs, Mn(PO3)2-C/C3N4, Mn(PO3)2-C, and commercial Pt/C (20 wt.%). The Tafel slopes were derived from the ORR polarization curves to further explore the ORR kinetics. As illustrated in Figure 5B, the Mn(PO3)2-C/C3N4/CNTs exhibit the smallest Tafel slope of 56.90 mV dec−1, which is outperforming than that of Mn(PO3)2-C/C3N4 (61.65 mV dec−1), Mn(PO3)2-C/CNTs (70.28 mV dec−1), Mn(PO3)2-C (76.23 mV dec−1), and Pt/C (65.82 mV dec−1), thereby confirming its fast ORR kinetic process. Among prepared samples, Mn(PO3)2-C/C3N4/CNTs exhibit the high onset potential and half-wave potential of 0.92 and 0.80 V versus the reversible hydrogen electrode (vs. RHE), as well as the highest diffusion-limiting current density (JL) of 6.3 mA cm−2 (Figure 5C), which is superior to most reported metal phosphates in Supporting Information: Table S3. These values are very close to that of commercial Pt/C catalyst (Eonset = 0.97 V, E1/2 = 0.86 V, and JL = 5.5 mA cm−2), but much more positive than those of Mn(PO3)2-C (Eonset = 0.87 V, E1/2 = 0.73 V, and JL = 5.2 mA cm−2), Mn(PO3)2-C/CNTs (Eonset = 0.88 V, E1/2 = 0.76 V, and JL = 5.5 mA cm−2), Mn(PO3)2-C/C3N4 (Eonset = 0.89 V, E1/2 = 0.78 V, and JL = 6.0 mA cm−2). Furthermore, we tested the LSV plot with different feed ratio of manganese as shown in Supporting information: Figure S18, which has demonstrated the Mn(PO3)2-C/C3N4/CNTs display the most elevated onset potential and half-wave potential, along with the highest diffusion-limiting current density. Conversely, the crystalline excellence of Mn(PO3)2-C/C3N4/CNTs-2 mmol correlates with inferior ORR performance, suggesting that unsaturated coordination imparts amorphous catalysts with improved activation and higher density of active sites.
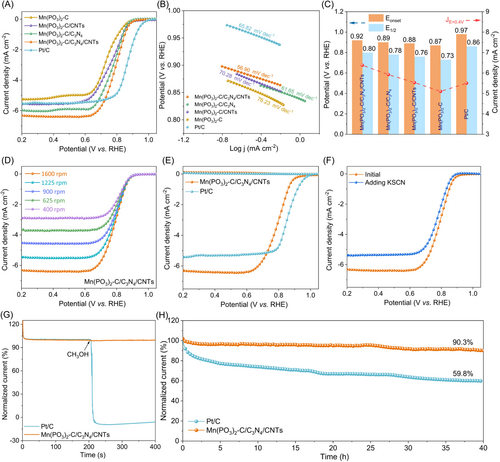
We also evaluated the ORR activity of samples synthesized using carbon materials with different structural scales, including 0D CQDs, 1D CNTs, 2D GO, 2D C3N4, and 3D CS. As shown in Supporting Information: Figure S19, Mn(PO3)2-C/C3N4/CQDs prepared by simultaneously adding CQDs and C3N4 exhibit the highest ORR activity, delivering a half wave potential of 0.85 V. This may be attributed to the unique 0D structure of CQDs, which have a larger specific surface area and are more likely to induce the formation of nonperiodic manganese phytate complexes. Additionally, we also evaluated the ORR activity of the synthesized Co, Fe, and Zn-based amorphous phosphate complexes, including Co(PO3)2-C/C3N4/CNTs, Fe(PO3)2-C/C3N4/CNTs, and Zn(PO3)2-C/C3N4/CNTs. As shown in Supporting Information: Figure S20a, Mn(PO3)2-C/C3N4/CNTs exhibits the highest catalytic activity, which is consistent with the previous calculation results. We compared the experimental performance with the theoretical predicted overpotential (Supporting Information: Figure S20b) and found a certain linear correlation between the theoretical and experimental data, which confirms the close consistency between the theoretical and experimental results. To further reveal the influence of carbon materials, we assessed the performance of sample prepared using PS microspheres without adsorption effects. As shown in Supporting Information: Figure S21, Mn(PO3)2-C/PS exhibits nearly identical properties to Mn(PO3)2-C, even though the high-temperature decomposition of PS provides a macroporous structure.
The electron-transfer kinetics of catalysts are further investigated by the LSV curves at various rotating speeds from 400 to 1600 rpm (Figure 5D and Supporting Information: Figures S22–23). The Kentucky–Levich (K-L) plots conducted from LSV curves at potential ranging from 0.3 to 0.45 V play good linearity, indicating a first-order ORR kinetics concerning O2 consumption. The electron transform number from the K-L plot is calculated to be 3.74, which evidenced the 4e− dominates the process of catalyst. To get better insight into the ORR mechanism, rotating ring-disk electrode (RRDE) measurements are carried out as shown in Figure 5E. The calculated H2O2 yield for Mn(PO3)2-C/C3N4/CNTs at potential ranging from 0.2 to 0.6 V versus RHE is less than 5%, which is comparable to that of Pt/C and lower than those of other samples (Supporting Information: Figure S24). The n value derived from RRDE measurements fell within the 3.72–3.76, which is close to Pt/C and in agreement with RDE results. The above results verify the four-electron dominant pathway of the ORR reaction for Mn(PO3)2-C/C3N4/CNTs. Furthermore, to further verify the active sites of Mn(PO3)2-C/C3N4/CNTs in ORR, thiocyanide ions (SCN−) are used as a probe to poison the metal sites due to its strong affinity to Mn [57]. As expected, after the addition of 10 mM KSCN to the surface of RDE (Figure 5F), the ORR catalytic activity underwent significant degradation. Notably, the Mn(PO3)2-C/C3N4/CNTs show much more negative E1/2 and greatly decreased diffusion-limiting current density. These results indicate that metal sites are the major catalytic active sites for ORR. Besides the excellent ORR activity, Mn(PO3)2-C/C3N4/CNTs also demonstrates superior methanol tolerance (Figure 5G). The ORR current density of Mn(PO3)2-C/C3N4/CNTs remains the same without obvious current variation after injecting 3 M methanol. Contrarily, the Pt/C catalysts show a sharp jump, because the ORR is immediately replaced by the methanol oxidation reaction. Moreover, Mn(PO3)2-C/C3N4/CNTs provided a smaller deterioration of only 9.7% for 40 h while commercial Pt/C displays a larger deterioration of 40.2% (Figure 5H). After conducting ORR measurements, the phase and composition of Mn(PO3)2-C/C3N4/CNTs were characterized (Supporting Information: Figures S25 and S26), which showed no obvious changes compared to its original state. The excellent ORR activity of the Mn(PO3)2-C/C3N4/CNTs catalyst can be attributed to the following factors: (i) the high catalytic activity of the amorphous Mn(PO3)2 structure; (ii) the multi-scale structures which endow the materials with high electron/ion/gas transport performance; (iii) the manganese phosphate nanoparticles that are anchored on carbon carriers, ensuring good structural stability of the catalyst.
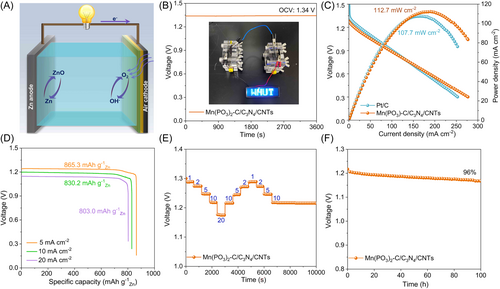
Motivated by the superior ORR performance of Mn(PO3)2-C/C3N4/CNTs, we have explored the practical applicability of Mn(PO3)2-C/C3N4/CNTs as an air electrode in a homemade ZABs. The battery consists of carbon cloth-supported Mn(PO3)2-C/C3N4/CNTs as the air cathode, Zn plate as the anode, and 6.0 M KOH + 0.2 M Zn(Ac)2 as the electrolyte (Figure 6A). Meanwhile, a comparison battery is fabricated using a commercial Pt/C catalyst as the air cathode. As presented in Figure 6B, two series-connected ZABs based on Mn(PO3)2-C/C3N4/CNTs can light the blue light-emitting diode (LED) (2.0 V) screens showing “WHUT”, representative of its promising application in ZABs. Figure 6C shows the typical discharge polarization curves and power density plots. The Zn-air battery with Mn(PO3)2-C/C3N4/CNTs catalyst demonstrates a maximum power density of 112.7 mW cm−2, which exceeds that of the Pt/C battery (107.7 mW cm−2). Moreover, the maximum power density increases as high as 130.2 mW cm−2 when O2 flow is injected into the system in Figure S27 (Supporting Information). The specific capacity is measured according to the consumption of Zn (Figure 6D). At 5 mA cm−2, the galvanostatic discharge curve of Mn(PO3)2-C/C3N4/CNTs exhibits the highest specific capacity of 865.3 mAh gZn−1, followed by the 830.2 mAh gZn−1 in 10 mA cm−2 and 803.0 mAh gZn−1 in 20 mA cm−2. These values are also outperformed by the Pt/C (786.2 mAh gZn−1 in 10 mA cm−2). Furthermore, the discharge curve at various current densities (1–20 mA cm−2) has been tested, indicating using Mn(PO3)2-C/C3N4/CNTs as air cathode in ZAB has a stable discharge plateau, high reversibility, small voltage fading, and outstanding discharge rate capability (Figure 6E). More impressively, when Mn(PO3)2-C/C3N4/CNTs as air cathode in ZAB can discharge 100 h with a slight decrease in Figure 6F.
3 Conclusion
In summary, we employed theoretical calculations to guide the material synthesis strategy for catalyst preparation. Phytic acid was used as a phosphorus source, and the chelating structure of phytic acid molecules and metal ions was disrupted through the “metal ion pre-adsorption and spatial confinement strategy” of carbon materials with electron-rich centers. Following high-temperature calcination, we successfully prepared a series of amorphous metal metaphosphate composite catalysts for the first time. The introduced carbon materials can be 0D CQDs, 1D CNTs, 2D GO, 2D C3N4, or 3D CS. In 0.1 M KOH electrolyte, both amorphous Mn(PO3)2-C/C3N4/CQDs and Mn(PO3)2-C/C3N4/CNTs exhibited excellent ORR catalytic activity, with half-wave potentials of 0.85 V and 0.80 V, respectively. A linear correlation between theoretical overpotentials and experimental half-wave potentials was discovered through comparison. When used as the air electrode in ZABs, it displayed higher power density and superior long-term stability compared to commercial Pt/C. This work opens up a new avenue for the discovery of highly efficient nonprecious metal-based electrocatalysts with amorphous structures.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (22273069), the Scientific Research Project of Education Department of Hubei Province (B2023158), the Open Fund of the Hubei Provincial Key Laboratory of Green Materials for Light Industry (202307A07), and the Natural Science Foundation of Xiaogan City (XGKJ2023010058). This research used resources of the Advanced Photon Source (beamline 12-BM), a US Department of Energy (DOE) Office of Science User Facility, operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




